Abstract
Recently, thiazolidinone derivatives have been widely studied as antiparasitic agents. Previous investigations showed that fused 4-thiazolidinone derivatives (especially thiopyranothiazoles) retain pharmacological activity of their synthetic precursors—simple 5-ene-4-thiazolidinones. A series of isothiochromeno[4a,4-d][1,3] thiazoles was investigated in an in vitro assay towards bloodstream forms of Trypanosoma brucei brucei. All compounds inhibited parasite growth at concentrations in the micromolar range. The established low acute toxicity of this class of compounds along with a good trypanocidal profile indicates that isothiochromenothiazole derivatives may be promising for designing new antitrypanosomal drugs.
1. Introduction
African Trypanosomiasis or sleeping sickness is one of the so-called world’s neglected diseases affecting population in sub-Saharan Africa. It is caused by the parasites Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense and transmitted by the tsetse fly. Despite the fact that the number of cases reported in recent years has significantly decreased [1], nearly 60 million people remain at risk of infection. No new drugs for the African trypanosomiasis treatment have been approved since the early 1990s. The latter along with the absence of a vaccine or drugs for prophylaxis, the toxicity and the insufficient efficiency activity of the existing drugs, attest to the development of new efficient and safe antitrypanosomal agents. American trypanosomiasis or Chagas disease is caused by Trypanosoma cruzi transmitted by the triatomine bug. About 7 million people are infected by this parasite, mainly in Latin America. Due to human migration, the potential spreading of Chagas disease to other continents is alarming (e.g., transmission by blood transfusion). Our therapeutic arsenal against Chagas disease is furthermore very limited, with toxic drugs. So, the need for new chemotherapeutics for the American trypanosomiasis treatment is topical [2]. Moreover, it is worth mentioning that the discovery of highly active antitrypanosomals is of special interest for the search for drugs against other parasitic diseases, such as Leishmaniasis or Malaria [3,4]. This is argued by some phylogenetical similarities between protozoan parasite targets, such as dihydropholate reductase [5] or topoisomerase I [6].
Thiazolidinone core has been widely used in medicinal chemistry as a relatively easily accessible and pharmacologically attractive scaffold [7,8,9]. Studies on 2,4-thiazolidinedione, rhodanine (2-thioxo-4-thiazolidinone), 2-alkyl(aryl)-substituted, and 2-R-amino(imino)-substituted 4-thiazolidinone subtypes were dedicated to the search for new antimicrobial, antidiabetic, anti-inflammatory and anticancer agents [10,11,12]. Recent investigations had proved 1,3-thiazoles as well as 4-thiazolidinone derivatives to be interesting and promising structures for the design of antitrypanosomal agents [2,13]. Thiazole core can be considered as a cyclic mimetic or bioisoster of the highly active antiTrypanosoma cruzi pharmacophore—thiosemicarbazone [14,15], which inhibits cruzain, an essential cysteine protease of Trypanosoma cruzi [16]. Moreover, some studies have showed that cyclization of thioureas/thiosemicarbazides into thiazole/thiazolidine derivatives is beneficial for Trypanosoma cruzi inhibition when compared to the antiparasitic activity of thiosemicarbazone derivatives [17]. 4-Thiazolidinone derivatives have been studied as potent trypanocidals since the 2000s, giving more active and less toxic lead compounds every year [18,19,20,21,22]. Our previous findings showed good antitrypanosomal activity of thiazolidinone-pyrazoline hybrids [23,24]. On the other hand, the trypanocidal effect of isothiocoumarin derivatives obtained on the base of 5-ene-4-thiazolidinones was too weak to warrant their further study [25]. Thiazolidinone derivatives, especially 5-ene-rhodanines, are often claimed as pan-assay interference compounds (PAINs) due to the conjugation of C5-exocyclic double with the C4 carbonyl group of thiazolidine core and possible Michael acceptor properties. However, this formal approach useful for large-scale screening campaigns is critically discussed in the literature [26]. In contrast, fused derivatives of 5-ene-4-thiazolidinones, namely thiopyranothiazoles retain the pharmacological profile of starting thiazolidinones and are apparently devoid of Michael acceptor functionality, constituting promising scaffolds for the development of antiparasitic agents. Earlier-established anticancer activity of isothiochromenothiazoles [27] and related thiopyranothiazoles [28,29,30] became one more argument for the study of their antitrypanosomal activity. Anticancer drugs such as bortezomib, aclarubicin, doxorubicin, and mitoxantrone were studied towards Trypanosoma brucei showing trypanocidal activities comparable to those of commercial antitrypanosomal drugs [31,32] within repurposing strategy. This gives more opportunities to find new antiparasitic compounds among already discovered agents or drugs with antiproliferative activity as well as to gain insight into the possible mechanisms of action of the latter, or even to find new biotargets essential for parasites’ survival. The aim of the present work was to study whether isothiochromeno[4a,4-d][1,3]thiazoles with anticancer activity and their analogs possess antitrypanosomal activity.
2. Materials and Methods
2.1. Chemistry
All chemicals were of the analytical grade, commercially available and used without further purification. The starting compound 1 was synthesized as described previously [33]. 1H and 13C NMR spectra were determined with Varian Mercury 400 (400 MHz/100 MHz) spectrometer, in DMSO-d6 using tetramethylsilane as an internal standard. Elemental analyses (C, H, N) was performed at the Perkin-Elmer 2400 CHN analyzer and were within ± 0.4% from the theoretical values. LC–MS spectra were recorded on a Finnigan MAT INCOS-50 using electrospray ionization (ESI) technique. The purity of the compounds was checked by thin-layer chromatography performed with Merck Silica Gel 60 F254 aluminum sheets.
2.2. General Procedure for the Synthesis of 3-N-Substituted (5aR,8R,9aR)-5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3]thiazol-2-ones (2–13)
Ethanol solution of potassium hydroxide (0.616 g, 0.011 mol) was added to the suspension of compound 1 (2.69 g, 0.01 mol) in 30 mL ethanol; after the mixture was intensively stirred, appropriate chloroacetamide (0.011 mol) and catalytically amount of KI (0.05 g) were added. Then the mixture was heated under reflux for 4–5 h and precipitated with water. Resulting precipitate was filtered off and recrystallized from acetonitrile (2, 5, 10, 11), ethanol:water mixture (1:1) (3, 4, 12, 13), toluene:hexane mixture (2:1) (9), isopropanol (6, 8), acetic acid:water mixture (1:1) (7).
2.2.1. 2-{5,5,8-Trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3]thiazol-2-on-3-yl}- acetamide (2)
Yield 83%, mp. 128–130 °C. 1Н NMR (400 MHz, DMSO-d6), δ: 0.89 (d, 3Н, J = 6.4 Hz, СН3СН), 0.98–1.03 (m, 3H), 1.29 (s, 3H, СН3), 1.32 (s, 3H, СН3), 1.48–1.60 (m, 1H), 1.68 (t, 1H, J = 11.0 Hz), 1.78 (d, 1H, J = 11.0 Hz), 1.90 (m, 2Н), 2.20 (t, 1H, J = 9.4 Hz), 4.00 (d, 1H, J = 17.4 Hz, СН2СО), 4.15 (d, 1H, J = 17.4 Hz, СН2СО), 7.15 (s, 1H, NH), 7.50 (s, 1H, NH); 13C NMR (100 MHz, DMSO-d6): 170.4, 167.8, 122.1, 106.0, 50.7, 48.4, 45.0, 42.4, 36.7, 34.9, 32.0, 27.6, 25.9, 23.4, 22.4. LC–MS (ESI) m/z 327 (M + H)+. C15H22N2O2S2. Calculated, % C—55.18, % H—6.79, % N—8.58; Found, % C—55.40, % H—6.90, % N—8.40.
2.2.2. N-(Phenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3]thiazol-2-on-3-yl}-acetamide (3)
Yield 93%, mp. 180–183 °C. 1Н NMR (400 MHz, DMSO-d6), δ: 0.90–1.05 (m, 6H, CH3CH, CH3), 1.20 (bs, 6H, 2*CH3), 1.45–1.55 (m, 1H), 1.65 (m, 1H), 1.75 (m, 1H), 1.90 (m, 2H), 2.34 (m, 1H), 4.30 (bs, 2Н, СН2СО), 6.90 (t, J = 8.4 Hz, 1Н, arom.), 7.20 (t, J = 8.4 Hz, 2Н, arom.), 7.45 (d, J = 8.4 Hz, 2Н, arom.), 10.20 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): 171.1, 164.7, 139.0, 129.3, 124.0, 119.5, 113.7, 106.2, 50.7, 48.7, 45.8, 42.4, 36.7, 34.9, 32.0, 27.6, 25.9, 23.4, 22.4. LCMS (ESI) m/z 401 (M + H)+. С21H26N2O2S2. Calculated, % C—62.65, % H—6.51, % N—6.96; Found, % C—62.80, % H—6.80, % N—6.70.
2.2.3. N-(4-Methylphenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3] thiazol-2-on-3-yl}-acetamide (4)
Yield 75%, mp. 180–182 °C. 1Н NMR (400 MHz, DMSO-d6), δ: 0.91 (d, 3Н, J = 6.3 Hz, СН3СН), 0.98–1.05 (m, 3H), 1.27 (s, 3H, СН3), 1.32 (s, 3H, СН3), 1.46–1.58 (m, 1H), 1.66 (t, 1H, J = 11.4 Hz), 1.76 (d, 1H, J = 11.4 Hz), 1.89 (m, 2Н), 2.25 (s, 3H, 4-CH3-C6H4), 2.33 (t, 1H, J = 11.1 Hz), 4.28 (d, 1H, J = 17.1 Hz, СН2СО), 4.41 (d, 1H, J = 17.1 Hz, СН2СО), 7.12 (d, 2H, J = 8.1 Hz, 3-Н, 5-Н, 4-Me-C6H4), 7.58 (d, 2H, J = 8.1 Hz, 2-H, 6-Н, 4-Me-C6H4), 10.20 (s, 1Н, NH). 13C NMR (100 MHz, DMSO-d6): 170.8, 164.5, 139.0, 129.2, 124.0, 119.4, 113.6, 106.2, 50.6, 48.8, 45.6, 42.4, 36.8, 34.9, 32.1, 27.7, 25.9, 23.5, 22.4, 21.2. C22H28N2O2S2. Calculated, % C—63.43, % H—6.77, % N—6.72; Found, % C—63.30, % H—6.90, % N—6.80.
2.2.4. N-(3-Methylphenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3] thiazol-2-on-3-yl}-acetamide (5)
Yield 69%, mp. 184–186 °C. 1Н NMR (400 MHz, DMSO-d6), δ: 0.93 (d, 3Н, J = 6.3 Hz, СН3СН), 0.99–1.08 (m, 3H), 1.29 (s, 3H, СН3), 1.33 (s, 3H, СН3), 1.52–1.63 (m, 1H), 1.67 (t, 1H, J = 10.8 Hz), 1.78 (d, 1H, J = 10.8 Hz), 1.88 (m, 2Н), 2.29 (s, 3H, 3-CH3-C6H4), 2.35 (t, 1H, J = 8.1 Hz), 4.30 (d, 1H, J = 17.1 Hz, СН2СО), 4.43 (d, 1H, J = 17.1 Hz, СН2СО), 6.90 (d, 1H, J = 7.5 Hz, 4-Н, 3-CН3-C6H4), 7.21 (t, 1H, J = 7.8 Hz, 5-H, 3-CН3-C6H4), 7.33 (d, 1H, J = 8.1 Hz, 6-H, 3-Me-C6H4), 7.43 (s, 1H, 2-H, 3-Me-C6H4), 10.23 (s, 1Н, NH). 13C NMR (100 MHz, DMSO-d6): 170.5, 164.9, 152.7, 149.5, 138.9, 136.5, 129.1, 120.0, 114.7, 108.3, 50.7, 48.6, 47.0, 42.4, 36.7, 34.9, 32.0, 27.6, 25.9, 23.4, 22.4, 21.6. C22H28N2O2S2. Calculated, % C—63.43, % H—6.77, % N—6.72; Found, % C—63.60, % H—6.80, % N—6.90.
2.2.5. N-(4-Isopropylphenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3] thiazol-2-on-3-yl}-acetamide (6)
Yield 68%, mp. 241–244 °C. 1Н NMR (400 MHz, DMSO-d6), δ: 0.90 (d, J = 6.8 Hz, 3H, CH3CH), 1.00–1.05 (m, 3H), 1.16 (s, 3H, CH3), 1.19 (s, 3H, CH3), 1.29 (d, J = 12.9 Hz, 2*СН3, 6Н), 1.45–1.55 (m, 1H), 1.65 (t, J = 12.0 Hz, 1H), 1.75 (d, J = 12.0 Hz, 1H), 1.90 (m, 2H), 2.34 (t, J = 8.0 Hz, 1H), 2.85 (m, 1Н, СНМе2), 4.28 (d, J = 18.0 Hz, 1Н, СН2СО), 4.38 (d, J = 18.0 Hz, 1Н, СН2СО), 7.18 (d, J = 8.4 Hz, 2Н, arom.), 7.45 (d, J = 8.4 Hz, 2Н, arom.), 10.19 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): 171.3, 164.0, 152.5, 150.0, 139.0, 136.3, 129.5, 119.1, 107.7, 50.6, 47.6, 42.8, 36.8, 35.0, 34.9, 32.2, 27.9, 26.1, 23.8, 23.6, 22.6. С24H32N2O2S2. Calculated, % C—64.83, % H—7.25, % N—6.30; Found, % C—64.60, % H—7.10, % N—6.40.
2.2.6. N-(4-Sulfonamido-phenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3]thiazol-2-on-3-yl}-acetamide (7)
Yield 79%, mp. 111–114 °C. 1Н NMR (400 MHz, DMSO-d6), δ: 0.88–0.92 (m, 3H, CH3CH); 0.97–1.03 (m, 3H), 1.27–1.32 (m, 6H, 2*CH3), 1.49–1.57 (m, 1H), 1.61–1.67 (m, 1H), 1.71–1.81 (m, 1H), 1.86–1.89 (m, 1H), 2.27–2.34 (m, 1H), 4.35 (d, J = 17.2 Hz, 1Н, СН2СО), 4.43 (d, J = 17.0 Hz, 1Н, СН2СО), 7.24 (s, 2H, NH2), 7,71 (d, 2H, J = 8,7 Hz, 2Н, C6H4), 7,77 (d, 2H, J = 8,4 Hz, 2H, C6H4), 10.61 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): 170.5, 165.4, 141.8, 139.2, 127.3, 122.0, 119.1, 106.3, 50.4, 48.7, 45.9, 42.6, 36.6, 34.8, 32.0, 27.7, 25.8, 23.6, 22.4. С21H27N3O4S3. Calculated, % C—52.37, % H—5.65, % N—8.72; Found, % C—52.50, % H—5.80, % N—8.60.
2.2.7. N-(4-Acetylamino-phenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3]thiazol-2-on-3-yl}-acetamide (8)
Yield 87%, mp. 259–263 °C. 1Н NMR (400 MHz, DMSO-d6), δ: 0.94 (d, J = 6.8 Hz, 3H, CH3CH), 1.00–1.18 (m, 3H), 1.28 (s, 3H, CH3), 1.32 (s, 3H, CH3), 1.45–1.55 (m, 1H), 1.66 (m, 1H), 1.75 (m, 1H), 1.88 (m, 2H), 2.02 (s, 3Н, СН3СО), 2.33 (m, 1H), 4.30 (d, J = 18.0 Hz, 1Н, СН2СО), 4.40 (d, J = 18.0 Hz, 1Н, СН2СО), 7.50 (bs, 4Н, arom.), 9.91 (s, 1Н, NH), 10.24 (s, 1Н, NH). 13C NMR (100 MHz, DMSO-d6): 170.5, 168.4, 164.3, 135.6, 134.2, 122.1, 119.9, 106.2, 50.7, 48.6, 45.7, 42.4, 36.7, 34.9, 32.0, 27.6, 25.9, 24.3, 23.4, 22.3. LCMS (ESI) m/z 460 (M + H)+. С23H29N3O3S2. Calculated, % C—60.10, % H—6.36, % N—9.14; Found, % C—60.00, % H—6.60, % N—8.90.
2.2.8. 4-[2-(5,5,8-Trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3]thiazol-2-on-3-yl)- acetylamino]-benzoic acid ethyl ester (9)
Yield 75%, mp. 196–198 °C. 1Н NMR (400 MHz, DMSO-d6), δ: 0.93 (d, 3Н, J = 6.4 Hz, СН3СН), 1.00–1.10 (m, 3H), 1.28 (s, 3H, СН3), 1.32 (s, 3H, СН3), 1.34 (t, 3Н, СН3СН2О), 1.48–1.60 (m, 1H), 1.67 (t, 1H, J = 11.2 Hz), 1.80 (d, 1H, J = 11.2 Hz), 1.90 (m, 2Н), 2.33 (t, 1H, J = 9.0 Hz), 4.48 (q, 2Н, СН3СН2О), 4.30 (d, 1H, J = 17.1 Hz, СН2СО), 4.42 (d, 1H, J = 17.1 Hz, СН2СО), 7.67 (d, 2H, J = 8.0 Hz, 3-Н, 5-Н, 4-COOEt-C6H4), 7.90 (d, 2H, J = 8.0 Hz, 2-H, 6-Н, 4-COOEt-C6H4), 10.53 (s, 1Н, NH). 13C NMR (100 MHz, DMSO-d6): 170.6, 165.7, 165.4, 143.3, 130.9, 125.1, 122.1, 119.0, 106.4, 61.0, 50.8, 48.8, 45.9, 42.4, 36.8, 34.9, 32.0, 27.6, 25.9, 23.5, 22.4, 14.7. C24H30N2O4S2. Calculated, % C—60.73, % H—6.37, % N—5.90; Found, % C—60.50, % H—6.50, % N—5.70.
2.2.9. N-(4-Chlorophenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3] thiazol-2-on-3-yl}-acetamide (10)
Yield 78%, mp. 206–208 °C. 1Н NMR (400 MHz, DMSO-d6), δ: 0.86 (d, J= 7.0 Hz, 3H,CH3CH), 0.91–1.03 (m, 3H), 1.23 (s, 3H, CH3), 1.27 (s, 3H, CH3), 1.40–1.49 (m, 1H), 1.54 (m, 1H), 1.71 (d, 1H, J = 11.7 Hz,), 1.80 (m, 2H), 2.27 (t, 1H, J = 7.8 Hz), 4.32 (d, 1H, J = 16.6 Hz, CH2CO), 4.48 (d, 1H, J = 16.7 Hz, CH2CO), 7.67 (d, 2H, J = 8.2 Hz, arom.), 7.80 (d, 2H, J = 8.1 Hz, arom.), 10.72 (s, 1H, NH). 13 C NMR (100 MHz, DMSO-d6): 170.8, 164.9, 138.7, 129.3, 124.3, 119.0, 113.5, 106.9, 50.6, 47.8, 42.7, 36.8, 35.1, 32.0, 27.6, 26.2, 25.0, 22.4. C21H25ClN2O2S2. Calculated, % C—57.72, % H—5.77, % N—6.41; Found, % C—57.50, % H—5.90, % N—6.60.
2.2.10. N-(3,4-Dichlorophenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3]thiazol-2-on-3-yl}-acetamide (11)
Yield 67%, mp. 210–212 °C. 1Н NMR (400 MHz, DMSO-d6), δ: 0.89 (d, J = 7.3 Hz, 3H,CH3CH), 0.89–0.95 (m, 3H), 1.26 (s, 3H, CH3), 1.32 (s, 3H, CH3), 1.42–1.50 (m, 1H), 1.60 (m, 1H), 1.72–1.76 (m, 1H), 1.86–1.90 (m, 2H), 2.31 (t, 1H, J = 8.0 Hz), 4.37 (d, 1H, J = 17.1 Hz, CH2CO), 4.52 (d, 1H, J = 17.3 Hz, CH2CO), 7.71(d, 1H, J = 8.3 Hz, arom.), 7.92 (d, 1H, J = 8.3 Hz, arom.), 8.01 (s, 1H, arom), 10.94 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): 170.5, 165.4, 139.0, 131.6, 131.3, 125.5, 122.0, 120.8, 119.6, 106.4, 50.8, 48.7, 45.8, 42.4, 36.7, 34.9, 32.0, 27.6, 25.9, 23.4, 22.4. C21H24Cl2N2O2S2. Calculated, % C—53.50, % H—5.13, % N—5.94; Found, % C—53.70, % H—5.20, % N—6.00.
2.2.11. N-(2-Trifluoromethylphenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno [4a,4-d][1,3]thiazol-2-on-3-yl}-acetamide (12)
Yield 91%, mp. 163–165 °C. 1Н NMR (400 MHz, DMSO-d6), δ: 0.90 (d, 3Н, J = 6.6 Hz, СН3СН), 0.95–1.08 (m, 3H), 1.27 (s, 3H, СН3), 1.34 (s, 3H, СН3), 1.45–1.60 (m, 1H), 1.67 (t, 1H, J = 11.3 Hz), 1.75 (d, 1H, J = 11.3 Hz), 1.90 (m, 2Н), 2.32 (t, 1H, J = 9.0 Hz), 4.36 (d, 1H, J = 17.4 Hz, СН2СО), 4.47 (d, 1H, J = 17.4 Hz, СН2СО), 7.47 (t, 1H, J = 7.8 Hz, 5-Н, 2-CF3-C6H4), 7.49 (d, 1H, J = 7.8 Hz, 3-H, 2-CF3-C6H4), 7.70 (t, 1H, J = 7.5 Hz, 4-H, 2-CF3-C6H4), 7.75 (d, 1H, J = 7.5 Hz, 6-H, 2-CF3-C6H4), 9.93 (s, 1Н, NH). 13C NMR (100 MHz, DMSO-d6): 170.5, 166.0, 135.0, 133.6, 130.1, 127.3, 126.8 (q, J = 185 Hz), 125.0, 123.0 (q, J = 19.8 Hz), 122.0, 106.3, 50.8, 48.5, 45.3, 42.4, 36.8, 34.9, 32.0, 27.6, 25.9, 23.4, 22.4. LCMS (ESI) m/z 471 (M + H)+. C22H25F3N2O2S2. Calculated, % C—56.15, % H—5.35, % N—5.95; Found, % C—56.30, % H—5.60, % N—6.10.
2.2.12. N-(3-Trifluoromethylphenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno [4a,4-d][1,3]thiazol-2-on-3-yl}-acetamide (13)
Yield 80%, mp. 182–184 °C. 1Н NMR (400 MHz, DMSO-d6), δ: 0.92 (d, 3Н, J = 6.2 Hz, СН3СН), 0.98–1.06 (m, 3H), 1.28 (s, 3H, СН3), 1.32 (s, 3H, СН3), 1.51–1.56 (m, 1H), 1.67 (t, 1H, J = 11.3 Hz), 1.75 (d, 1H, J = 11.3 Hz), 1.88 (m, 2Н), 2.34 (m, 1H), 4.37 (d, 1H, J = 17.4 Hz, СН2СО), 4.47 (d, 1H, J = 17.1 Hz, СН2СО), 7.43 (d, 1H, J = 7.3 Hz, 3-CF3-C6H4), 7.59 (d, 1H, J = 7.1 Hz, 2-CF3-C6H4), 7.72–7.74 (m, 1H, 3-CF3-C6H4), 8.09 (s, 1H, 3-CF3-C6H4), 10.74 (s, 1Н, NH). 13C NMR (100 MHz, DMSO-d6): 170.6, 165.5, 139.8, 130.7 (q, J = 20 Hz), 123.1 (q, J = 160 Hz), 122.0, 120.5, 114.6 (q, J = 5 Hz), 111.8, 109.3, 103.4, 50.8, 48.8, 44.0, 42.6, 36.8, 34.8, 32.0, 27.6, 25.9, 23.5, 22.4. LCMS (ESI) m/z 471 (M + H)+. C22H25F3N2O2S2. Calculated, % C—56.15, % H—5.35, % N—5.95; Found, % C—56.00, % H—5.50, % N—5.70.
2.3. Pharmacology
2.3.1. Antitrypanosomal Activity Assay
Bloodstream forms of Trypanosoma brucei strain 90-13 were cultured in HMI9 medium supplemented with 10% FCS (fetal calf serum) at 37 °C under an atmosphere of 5% CO2 [34]. In all experiments, log-phase parasite cultures were harvested by centrifugation at 3000× g and immediately used. Drug assays were based on the conversion of a redox-sensitive dye (resazurin) to a fluorescent product by viable cells as previously described [35,36]. Drug stock solutions were prepared in pure DMSO. Trypanosoma brucei bloodstream forms (103 cells/well) were cultured in 96-well plates either in the absence or in the presence of different concentrations of inhibitors in a final volume of 200 µL. After 72 h incubation, resazurin solution was added in each well at the final concentration of 45 µM and fluorescence was measured at 530 nm and 590 nm absorbance after a further 4 h incubation. The percentage of inhibition of parasite growth rate was calculated by comparing the fluorescence of parasites maintained in the presence of drug to that of in the absence of drug. DMSO was used as control. Concentration inhibiting 50% of parasite growth (IC50) was determined from the dose–response curve with drug concentrations ranging from 10 to 0.625 µg/mL and presented in µM.
2.3.2. Acute Toxicity In Vivo
The experiments were conducted on white male mice weighing 23–25 g. Compounds were dissolved in saline solution (0.9% NaCl) with 1–2 drops of Polysorbate 80 (Tween-80®). After dissolution, they were administered to mice via intraperitoneal route. The LD50 was evaluated for 4 or 5 different doses each on 6 animals and calculated by the Litchfield and Wilcoxon method [37].
3. Results and Discussion
3.1. Chemistry
Isothiochromeno[4a,4-d][1,3]thiazoles 1–13 were synthesized (compounds 2, 4, 5, 9, 11, 13-resynthesized) according to the known synthetic protocol [27,38]. Starting (5aR,8R,9aR)-5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-octahydro-2H-isothiochromeno[4a,4-d][1,3]thiazole-2-one 1 was obtained in the Knoevenagel-hetero-Diels–Alder reaction of 3,7-dimethyloct-6-enal (citronellal) and 4-thioxo-2-thiazolidinone (isorhodanine) in the presence of ethylenediamine diacetate in acetonitrile medium at room temperature [33]. Target compounds 2–13 were synthesized in the alkylation reactions of 1 potassium salt by different chloroacetamides in ethanol. The reaction goes in situ through the stage of potassium salt formation which was not isolated (Scheme 1).
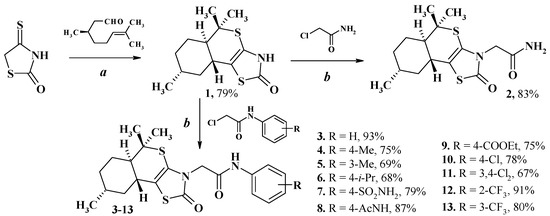
Scheme 1.
Target isothiochromeno[4a,4-d][1,3]thiazoles synthesis. Reagents and conditions: (a) isorhodanine (1.0 equiv.), EDDA (0.15 equiv.), 3,7-dimethyloct-6-enal (1.3 equiv.), MeCN, rt, 24 h, 79% [33]; (b) appropriate chloroacetamide (1.1 equiv.), KOH (1.1 equiv.), KI (cat. amount), EtOH, reflux, 4–5 h, 67–93%.
Structure and purity of compounds were confirmed by NMR spectroscopy and chromatography data (experimental part). Stereoconfiguration of compounds were assigned based on our previous data (spectral data, X-ray analysis) [33].
3.2. Pharmacology
Synthesized isothiochromeno[4a,4-d][1,3]thiazoles inhibited the growth of Trypanosoma brucei bloodstream forms with IC50 values in the micromolar range (Table 1) comparable to Nifutimox (IC50 = 2.39 µM). Increasing activity from the starting compound 1 (IC50 = 29.3 µM) as well as 2 (IC50 = 44.4 µM) proves the necessity of aryl substituents in the N3 position of the main core for the trypanocidal activity. Substituents in the aryl fragment also influence the antitrypanosomal activity. For example, halogen atoms in the aryl moiety significantly increase the inhibition activity of derivatives 10–13 (IC50 ranging from 2.97 to 8.50 µM). Interestingly, previously tested and structurally related thiopyranothiazoles with a norbornane moiety and the same fragments in the N3 position of the thiazolidine core [29] showed the same levels of IC50 values against Trypanosoma brucei. Probably, an acetamide moiety with different substituents in the aryl ring has an impact on the spatial arrangement of atoms that increases the molecules affinity to the yet unknown biotarget. On the other hand, an ester moiety in the aryl ring of 9 also highly contributes to the trypanocidal activity (IC50 = 1.55 µM).

Table 1.
Antitrypanosomal and anticancer activity of target isothiochromeno[4a,4-d]thiazol-2-ones.
Establishment of any anticancer and antitrypanosomal activity correlation for isothiochromenothiazoles was also an issue of the study. Previously, some of the synthesized isothichromenothiazoles were studied for their anticancer activity within a Developmental Therapeutic Program protocol (National Cancer Institute, Bethesda, MD, USA) [27]. Among isothiochromeno[4a,4-d]thiazoles tested at a concentration of 10−5 М, selectivity towards melanoma cell line UACC-257 was observed for N-(2-trifluoromethylphenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-oktahydro-2H-isothiochromeno[4a,4-d]thiazol-2-on-3-yl}-acetamide 12 (GP = 0.76%) and N-(p-tolyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-oktahydro-2H-isothiochromeno[4a,4-d]thiazol-2-on-3-yl}-acetamide 4 (GP = 5.23%). These two derivatives also inhibited growth of more than 60% of cells of ovarian cancer line OVCAR-8. Change of the trifluoromethyl moiety in the benzene ring to 3,4-dichlorobenzene did not contribute to anticancer activity which was rather low. Worth mentioning is that the absence of arylidene ring in the acetamide substituent in the N3-position significantly decreased the antitumor potential as well as the antiparasitic activity. This attests to the crucial role of aryl substituents in the N3 position of the isothiochromeno[4a,4-d]thiazole core for the antiproliferative activity. N-(3-Methylphenyl)-2-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-oktahydro-2H-isothiochromeno[4a,4-d]thiazol-2-on-3-yl}-acetamide 5 was tested in a range of concentrations from 0.1 to 100 µM and showed the highest growth inhibitory levels (GI50) against cell lines of leukemia, non-small-cell lung cancer and prostate cancer [27]. One more argument in favor of a dual action for the tested derivatives is a significant decrease of trypanocidal potential and the absence of anticancer activity (inhibition of cancer cells growth less than by 20%) for N-{5,5,8-trimethyl-3,5,5a,6,7,8,9,9a-oktahydro-2H-isothiochromeno[4a,4-d]thiazol-2-on-3-yl}-acetamide 2.
For derivatives with the highest trypanocidal activity, 9, 10, and 13 and the starting compound 1, the acute toxicity (mice) was studied and their LD50 were determined [35,36]. The stock solutions of the compounds used in this study were prepared immediately before usage and increasing amounts of substances (100–1000 mg/kg) were injected intraperitoneally. The LD50 values were calculated according to Litchfield and Wilcoxon. These derivatives show a little acute toxicity in mice with LD50 values within the range of 240–480 mg/kg.
4. Conclusions
A series of isothiochromeno[4a,4-d][1,3]thiazole derivatives was synthesized and investigated in an in vitro assay against Trypanosoma brucei brucei. Compounds showed inhibitory activity towards the parasite growth at concentrations in the micromolar range. Structure–activity relationships reveal the positive influence of N3-substituent for the trypanocidal activity. The same trend was found for the anticancer activity of compounds. This is an additional argument in favor of the search for compounds with dual action (antitrypanosomal and anticancer). Along with this, low acute toxicity of compounds indicates that isothiochromenothiazoles may be used in further directed design of antitrypanosomal agents.
Author Contributions
R.L., A.K., and P.G. conceived and designed the project and paper; D.K. and A.K. performed the synthesis of target compounds; P.G. tested compounds for antitrypanosomal activity; I.N. studied acute toxicity of compounds in vivo; R.L. and A.K. wrote the paper. All authors read and approved the final manuscript.
Funding
The project was partly supported by the State Fund for Fundamental Research of Ukraine (F76/72-2017, F76/13-2018) and the Ministry of Education and Science of Ukraine (M/181-2017; 92-2018), Ukrainian-France program “Dnipro” M/188-2015; M/71-2016).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Trypanosomiasis, Human African (Sleeping Sickness)/Fact Sheets. Available online: http://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 28 June 2018).
- Kryshchyshyn, A.; Kaminskyy, D.; Grellier, P.; Lesyk, R. Trends in research of antitrypanosomal agents among synthetic heterocycles. Eur. J. Med. Chem. 2014, 85, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Khan, M.O.; Austin, S.E.; Croft, S.L.; Yardley, V.; Rock, P.; Douglas, K.T. Antitrypanosomal, antileishmanial, and antimalarial activities of quaternary arylalkylammonium 2-amino-4-chlorophenyl phenyl sulfides, a new class of trypanothione reductase inhibitor, and of N-acyl derivatives of 2-amino-4-chlorophenyl phenyl sulfide. J. Med. Chem. 2005, 48, 8087–8097. [Google Scholar] [CrossRef] [PubMed]
- Bakunov, S.A.; Bakunova, S.M.; Wenzler, T.; Ghebru, M.; Werbovetz, K.A.; Brun, R.; Tidwell, R.R. Synthesis and antiprotozoal activity of cationic 1,4-diphenyl-1H-1,2,3-triazoles. J. Med. Chem. 2009, 53, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, I.H. Inhibitors of dihydrofolate reductase in Leishmania and trypanosomes. Biochim. Biophys. Acta 2002, 1587, 249–257. [Google Scholar] [CrossRef]
- Bodley, A.L.; Shapiro, T.A. Molecular and cytotoxic effects of camptothecin, a topoisomerase I inhibitor, on trypanosomes and Leishmania. Proc. Natl. Acad. Sci. USA 1995, 92, 3726–3730. [Google Scholar] [CrossRef] [PubMed]
- Lesyk, R.B.; Zimenkovsky, B.S.; Kaminskyy, D.V.; Kryshchyshyn, A.P.; Havrylyuk, D.Y.; Atamanyuk, D.V.; Subtel’na, I.Y.; Khylyuk, D.V. Thiazolidinone motif in anticancer drug discovery. Experience of DH LNMU medicinal chemistry scientific group. Biopolym. Cell 2011, 27, 107–117. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. Recent developments with rhodanine as a scaffold for drug discovery. Expert Opin. Drug Discov. 2017, 12, 1233–1252. [Google Scholar] [CrossRef] [PubMed]
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. 5-Ene-4-thiazolidinones—An efficient tool in medicinal chemistry. Eur. J. Med. Chem. 2017, 140, 542–594. [Google Scholar] [CrossRef] [PubMed]
- Tomašić, T.; Peterlin Mašič, L. Rhodanine as a scaffold in drug discovery: A critical review of its biological activities and mechanisms of target modulation. Expert Opin. Drug Discov. 2012, 7, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.S.; Vora, D.K.; Ramaa, C.S. Thiazolidine-2,4-diones: Progress towards multifarious applications. Bioorg. Med. Chem. 2013, 21, 1599–1620. [Google Scholar] [CrossRef] [PubMed]
- Metwally, M.A.; Farahat, A.A.; Abdel-Wahab, B.F. 2-Amino-4-thiazolidinones: Synthesis and reactions. J. Sulfur Chem. 2010, 31, 315–349. [Google Scholar] [CrossRef]
- Gomes, P.A.; Oliveira, A.R.; Cardoso, M.V.; Santiago, E.F.; Barbosa, M.O.; de Siqueira, L.R.; Moreira, D.R.; Bastos, T.M.; Brayner, F.A.; Soares, M.B.; et al. Phthalimido-thiazoles as building blocks and their effects on the growth and morphology of Trypanosoma cruzi. Eur. J. Med. Chem. 2016, 111, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes Moreira, D.R.; de Oliveira, A.D.; Teixeira de Moraes Gomes, P.A.; de Simone, C.A.; Villela, F.S.; Ferreira, R.S.; da Silva, A.C.; dos Santos, T.A.; Brelaz de Castro, M.C.; Pereira, V.R.; et al. Conformational restriction of aryl thiosemicarbazones produces potent and selective anti-Trypanosoma cruzi compounds which induce apoptotic parasite death. Eur. J. Med. Chem. 2014, 75, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Espíndola, J.W.; Cardoso, M.V.; Filho, G.B.; Oliveira E Silva, D.A.; Moreira, D.R.; Bastos, T.M.; Simone, C.A.; Soares, M.B.; Villela, F.S.; Ferreira, R.S.; et al. Synthesis and structure-activity relationship study of a new series of antiparasitic aryloxyl thiosemicarbazones inhibiting Trypanosoma cruzi cruzain. Eur. J. Med. Chem. 2015, 101, 818–835. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, G.; Boiani, L.; Cerecetto, H.; Fernández, M.; González, M.; Denicola, A.; Otero, L.; Gambino, D.; Rigol, C.; Olea-Azar, C.; Faundez, M. In vitro activity and mechanism of action against the protozoan parasite Trypanosoma cruzi of 5-nitrofuryl containing thiosemicarbazones. Bioorg. Med. Chem. 2004, 12, 4885–4893. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.V.; de Siqueira, L.R.; da Silva, E.B.; Costa, L.B.; Hernandes, M.Z.; Rabello, M.M.; Ferreira, R.S.; da Cruz, L.F.; Moreira, D.R.; Pereira, V.R.; et al. 2-Pyridyl thiazoles as novel anti-Trypanosoma cruzi agents: Structural design, synthesis and pharmacological evaluation. Eur. J. Med. Chem. 2014, 86, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.C.; de Lima, R.S.; Moreira, D.R.; Cardoso, M.V.; Gouveia de Brito, A.C.; Farias Dos Santos, L.M.; Hernandes, M.Z.; Kiperstok, A.C.; de Lima, R.S.; Soares, M.B. Synthesis, docking, and in vitro activity of thiosemicarbazones, aminoacyl-thiosemicarbazides and acylthiazolidones against Trypanosoma cruzi. Bioorg. Med. Chem. 2006, 14, 3749–3757. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.C.; Moreira, D.R.; Cardoso, M.V.; Hernandes, M.Z.; Alves Pereira, V.R.; Silva, R.O.; Kiperstok, A.C.; Lima, M.S.; Soares, M.B. Synthesis, Cruzain docking, and in vitro studies of aryl-4-oxothiazolylhydrazones against Trypanosoma cruzi. ChemMedChem 2007, 2, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, M.Z.; Rabello, M.M.; Leite, A.C.; Cardoso, M.V.; Moreira, D.R.; Brondani, D.J.; Simone, C.A.; Reis, L.C.; Souza, M.A.; Pereira, V.R.; et al. Studies toward the structural optimization of novel thiazolylhydrazone-based potent antitrypanosomal agents. Bioorg. Med. Chem. 2010, 18, 7826–7835. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.M.; Hernandes, M.Z.; Rabello, M.M.; da Cruz, L.F.; Ferreira, R.S.; de Simone, C.A.; Meira, C.S.; Guimaraes, E.T.; da Silva, A.C.; dos Santos, T.A.; et al. Structural design, synthesis and structure-activity relationships of thiazolidinones with enhanced anti-Trypanosoma cruzi activity. ChemMedChem 2014, 9, 177–188. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Filho, G.B.; de Oliveira Cardoso, M.V.; Espíndola, J.W.; Ferreira, L.F.; de Simone, C.A.; Ferreira, R.S.; Coelho, P.L.; Meira, C.S.; Magalhaes Moreira, D.R.; Soares, M.B.; et al. Structural design, synthesis and pharmacological evaluation of 4-thiazolidinones against Trypanosoma cruzi. Bioorg. Med. Chem. 2015, 23, 7478–7486. [Google Scholar] [CrossRef] [PubMed]
- Havrylyuk, D.; Zimenkovsky, B.; Vasylenko, O.; Day, C.W.; Smee, D.F.; Grellier, P.; Lesyk, R. Synthesis and biological activity evaluation of 5-pyrazoline substituted 4-thiazolidinones. Eur. J. Med. Chem. 2013, 66, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Havrylyuk, D.; Zimenkovsky, B.; Karpenko, O.; Grellier, P.; Lesyk, R. Synthesis of pyrazoline–thiazolidinone hybrids with trypanocidal activity. Eur. J. Med. Chem. 2014, 85, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kaminskyy, D.; Kryshchyshyn, A.; Nektegayev, I.; Vasylenko, O.; Grellier, P.; Lesyk, R. Isothiocoumarin-3-carboxylic acid derivatives: Synthesis, anticancer and antitrypanosomal activity evaluation. Eur. J. Med. Chem. 2014, 75, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.B.; Nissink, J.W.M. Seven Year Itch: Pan-Assay Interference Compounds (PAINS) in 2017—Utility and Limitations. ACS Chem. Biol. 2017, 13, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Kryshchyshyn, A.; Zimenkovsky, B.; Lesyk, R. Synthesis and anticancer activity of isothiochromeno[3,4-d]thiazole derivatives. Ann. Univ. Mar. Curie Sklodowska DDD Pharm. 2008, 21, 247–251. [Google Scholar] [CrossRef]
- Kryshchyshyn, A.; Atamanyuk, D.; Lesyk, R. Fused thiopyrano[2,3-d]thiazole derivatives as potential anticancer agents. Sci. Pharm. 2012, 80, 509–529. [Google Scholar] [CrossRef] [PubMed]
- Kryshchyshyn, A.P.; Atamanyuk, D.V.; Kaminskyy, D.V.; Grellier, P.; Lesyk, R.B. Investigation of anticancer and anti-parasitic activity of thiopyrano[2,3-d]thiazoles bearing norbornane moiety. Biopolym. Cell 2017, 33, 183–205. [Google Scholar] [CrossRef]
- Kryshchyshyn, A.; Roman, O.; Lozynskyi, A.; Lesyk, R. Thiopyrano[2,3-d]thiazoles as New Efficient Scaffolds in Medicinal Chemistry. Sci. Pharm. 2018, 86, 26. [Google Scholar] [CrossRef] [PubMed]
- Steverding, D.; Wang, X. Trypanocidal activity of the proteasome inhibitor and anti-cancer drug bortezomib. Parasit. Vectors 2009, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Deterding, A.; Dungey, F.; Thompson, K.; Steverding, D. Anti-trypanosomal activities of DNA topoisomerase inhibitors. Acta Trop. 2005, 93, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Matiychuk, V.; Lesyk, R.; Obushak, M.; Gzella, A.; Atamanyuk, D.; Ostapiuk, Y.; Kryshchyshyn, A. A new domino-Knoevenagel-hetero-Diels-Alder reaction. Tetrahedron Lett. 2008, 49, 4648–4651. [Google Scholar] [CrossRef]
- Smith, W.G. 1 Pharmacological Screening Tests. Prog. Med. Chem. 1961, 1, 1–33. [Google Scholar]
- Lethu, S.; Bosc, D.; Mouray, E.; Grellier, P.; Dubois, J. New protein farnesyltransferase inhibitors in the 3-arylthiophene 2-carboxylic acid series: Diversification of the aryl moiety by solid-phase synthesis. J. Enzym. Inhib. Med. Chem. 2013, 1, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cruz, F.; Serra, S.; Delogu, G.; Lapier, M.; Diego Maya, J.; Olea-Azar, C.; Santana, L.; Uriarte, E. Antitrypanosomal and antioxidant properties of 4-hydroxycoumarins derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 5569–5573. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, J.T.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar] [PubMed]
- Kryshchychyn, A.; Lesyk, R. Synthesis and antitumor activity study of new isothiochromeno[4a,4-d]thiazole derivatives. Pharm. Rev. 2010, 2, 6–10. (In Ukrainian) [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).