Anionic Polyelectrolyte Hydrogel as an Adjuvant for Vaccine Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hydrogel Particles
2.3. Characterization of Hydrogel Particles
2.4. Immunization
2.5. Antibody Purification and Detection
2.6. Histopathological Characterization
2.7. Particle Toxicity Test
2.8. Statistical Analysis
3. Results
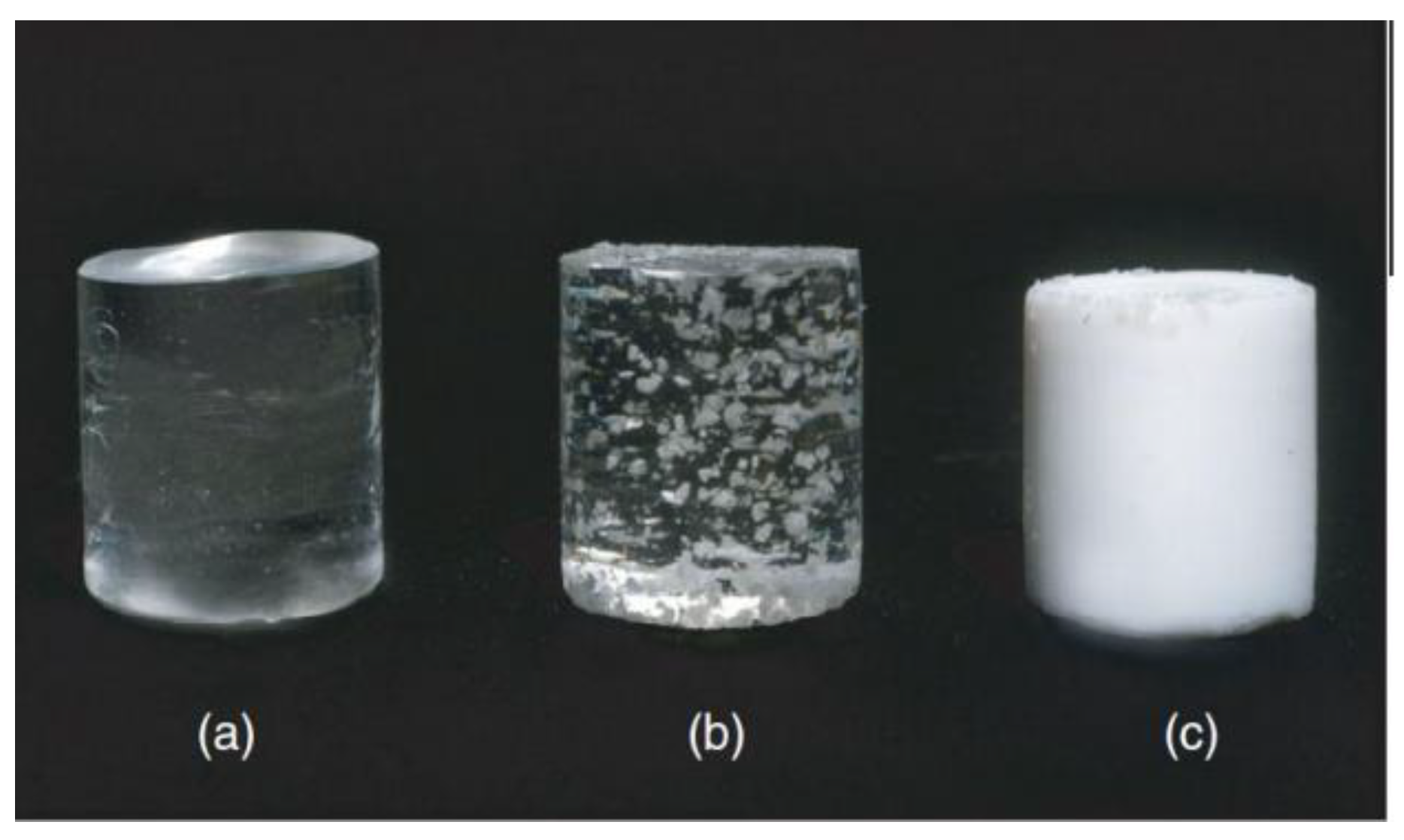
3.1. The Synthesis, Structural, and Colloidal-Chemical Properties of Polyelectrolyte Based Cured Hydrogel Particles
3.2. Adjuvant Properties of PHG
3.3. Histology Observations
3.4. Body Weight and Coefficient of Organs
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | acrylic acid |
| AIBN | initiator azobisisobutyronitrile |
| BA | butyl acrylate |
| BSA | bovine serum albumin |
| CFA | complete Freund’s adjuvant |
| DLS | dynamic light scattering |
| GMA | glycidyl methacrylate |
| 1H NMR | proton nuclear magnetic resonance |
| IFA | incomplete Freund’s adjuvant |
| NPs | nanoparticles |
| PBS | phosphate buffered saline |
| PHG | anionic polyelectrolyte hydrogel |
| TBS | Tris-buffered saline |
| TEGDMA | Triethylene glycol dimethacrylate |
References
- Powell, B.S.; Andrianov, A.K.; Fusco, P.C. Polyionic vaccine adjuvants: Another look at aluminum salts and polyelectrolytes. Clin. Exp. Vaccine Res. 2015, 4, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, M.; Navarro-Locsin, G.; Tan, H.K.; Yamanaka, N.; Sonsuwan, N.; Wang, P.C.; Dung, N.T.; Restuti, R.D.; Hashim, S.S.; Vijayasekaran, S. A review of the burden of disease due to otitis media in the Asia-Pacific. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Memish, Z.A. Meningococcal disease and travel. Clin. Infect. Dis. 2002, 34, 84–90. [Google Scholar] [CrossRef] [Green Version]
- LeMoignic, P. Application to man of vaccines consisting of emulsions in fatty substances (lipo-vaccines). C. R. Soc. Biol. 1916, 79, 352–354. [Google Scholar]
- Glenny, A.T.; Pope, C.G.; Waddington, H.; Wallace, U. Immunological Notes: XVII–XXIV. J. Pathol. Bacteriol. 1926, 29, 31–40. [Google Scholar] [CrossRef]
- Gupta, R.K.; Rost, B.E.; Relyveld, E.; Siber, G.R. Adjuvant properties of aluminum and calcium compounds. Pharm. Biotechnol. 1995, 6, 229–248. [Google Scholar]
- Freund, G.; Cassals, J.; Hoster, E.P. Sensitization and antibody formation after injection of tubercle bacilli and paraffin oil. Procidings Soc. Exp. Biol. Med. 1937, 37, 509–513. [Google Scholar] [CrossRef] [Green Version]
- Powers, J.G.; Nash, P.B.; Rhyan, J.C.; Yoder, C.A.; Miller, L.A. Comparison of immune and adverse effects induced by AdjuVac and Freund’s complete adjuvant in New Zealand white rabbits (Oryctolagus cuniculus). Lab. Anim. 2007, 36, 51–58. [Google Scholar] [CrossRef]
- Stuart–Harris, C.J. Adjuvant influenza vaccines. Bull World Health Organ. 1969, 4, 617–621. [Google Scholar]
- De Souza Apostólico, J.; Lunardelli, V.A.S.; Coirada, F.C.; Boscardin, S.B.; Rosa, D.S. Adjuvants: Classification, modus operandi, and licensing. J. Immunol. Res. 2016, 2016, 1459394. [Google Scholar] [CrossRef] [Green Version]
- O’Hagan, D.T.; Ott, G.S.; Gregorio, E.D.; Seubert, A. The mechanism of action of MF59—An innately attractive adjuvant formulation. Vaccine 2012, 30, 4341–4348. [Google Scholar] [CrossRef]
- Bal, S.M.; Ding, Z.; Kersten, G.F.; Jiskoot, W.; Bouwstra, J.A. Micronidle-based transcutaneous immunization in mice with N-trimethyl chitosan adjuvanted diphteria toxoid formulations. Pharm. Res. 2010, 9, 1837–1847. [Google Scholar] [CrossRef] [Green Version]
- Pharmacy Times: May 2016 Skin & Eye Health. Available online: https://www.pharmacytimes.com/publications/issue/2016/may2016/R776_may2016 (accessed on 23 May 2016).
- Cunha, J.P. Fluad (Influenza Virus Vaccine, Surface Antigen, Inactivated, Adjuvanted with MF59C.1) side effects drug center. Rxlist 2017. Available online: https://www.rxlist.com/fluad-side-effects-drug-center.htm (accessed on 7 March 2017).
- Kabanov, V.A. From synthetic polyelectrolytes to polymer-subunit vaccines. Pure Appl. Chem. 2004, 76, 1659–1677. [Google Scholar] [CrossRef]
- Schwarz, B.; Uchida, M.; Douglas, T. Biomedical and catalytic opportunities of virus-like particles in nanotechnology. Adv. Virus Res. 2018, 97, 1–60. [Google Scholar]
- Braun, D.; Cherdron, H.; Ritter, H. Polymer Synthesis: Theory and Practice: Fundamentals, Methods, Experiments; Springer Science & Business Media: Berlin, Germany, 2013; p. 333. [Google Scholar]
- De Castro, M.L.; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 16, 2383–2389. [Google Scholar] [CrossRef]
- Schimmel, G. Elektronenmikroskopische Methodik; Springer: Berlin/Heidelberg, Germany, 1969; p. 244. [Google Scholar]
- Kuo, J. (Ed.) Electron Microscopy: Methods and Protocols; Humana Press: New York, NY, USA, 2014; p. 799. [Google Scholar]
- Pishak, V.P. Histology with the Basics of Histological Technology; Kondor: Kyiv, Ukraine, 2008; p. 400. [Google Scholar]
- Salman, H.H.; Garmazo, C.; de Smidt, P.C.; Russel-Jones, G.; Irache, J.M. Evaluation of bioadhesive capacity and immunoadjuvant of vitamin B(12)-Gantrez nanoparticles. Pharm. Res. 2008, 25, 2859–2868. [Google Scholar] [CrossRef] [Green Version]
- Salman, H.H.; Irache, J.M.; Garmazo, C. Immunoadjuvant capacity of flagellin and mannosamine-coated poly(anhydride) nanoparticles in oral vaccination. Vaccine 2009, 27, 4784–4790. [Google Scholar] [CrossRef]
- Stieneker, F.; Kreuter, J.; Lower, J. High antibody titres in mice with polymethylmethacrylate nanoparticles as adjuvant for HIV vaccines. AIDS 1991, 5, 431–435. [Google Scholar] [CrossRef]
- Maubant, S.; Banissi, C.; Beck, S.; Chauvat, A.; Carpentier, A. Adjuvant properties of Cytosine-phosphate-guanosineoligodeoxynucleotide in combination with various polycations in an ovalbumin-vaccine model. Nucleic Acid Ther. 2011, 21, 231–240. [Google Scholar] [CrossRef]
- Vasiliev, Y.M. Chitosan-based vaccine adjuvants: Incomplete characterization complicates preclinical and clinical evaluation. Expert Rev. Vaccines 2015, 14, 37–53. [Google Scholar] [CrossRef]
- Brunner, R.; Jensen-Jarolim, E.; Pali-Schöll, I. The ABC of clinical and experimental adjuvants—A brief overwiew. Immunol. Lett. 2010, 1, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Kit, Y.; Bilyy, R.; Stoika, R.; Zaichenko, A. Immunogenicity and adjuvant properties of novel biocompatible nanoparticles. In Biocompatible Nanomaterials: Synthesis, Characterization and Applications; Kumar, S.A., Thiagarajan, S., Wang, S.-F., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 209–223. [Google Scholar]
- Kozak, M.R.; Oliynyk, A.V.; Zaichenko, O.S.; Vlizlo, V.V. Adjuvant properties of polymer based on acrylic acid. Ukrains’ Kyi Biokhimichnyi Zhurnal 2013, 85, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.L.; Soema, P.C.; Slütter, B.; Ossendorp, F.; JiskootHum, W. PLGA particulate delivery systems for subunit vaccines: Linking particle properties to immunogenicity. Vaccin. Immunother. 2016, 12, 1056–1069. [Google Scholar] [CrossRef]
- Gutierro, I.; Hernandez, R.M.; Igartua, M.; Gascon, A.R.; Pedraz, J.L. Size dependent immune response after subcutaneous, oral and intranasal administration of BSA loaded nanospheres. Vaccine 2002, 21, 67–77. [Google Scholar] [CrossRef]
- Kozak, M.; Oliynyk, A.; Moskvin, M.; Ostapiv, D.; Mitina, N.; Zaichenko, O.; Vlizlo, V. Anionic polyelectrolyte hydrogels: Influence on antibodies production and enzyme activity. ECPB 2017, 79, 11–16. [Google Scholar] [CrossRef]
- Ayman, M.; Badr, D.A.E.A.; Shebl, R.I.; Mohamed, A.F. Comparative evaluation of alum and MPL as adjuvants toxicity and related immune potential of adjuvanted rabies vaccine. Gastroenterology 2017, 1, 112–123. [Google Scholar]
- Esquivel, N.; García, Y.; Lores, B.; Gutiérrez, M.; Rodríguez, C. Characterization of aged male BALB/ccenp mice as a model of dementia. Lab. Anim. Res. 2020, 36, 7. [Google Scholar] [CrossRef] [Green Version]







| Composition of the Monomer Mixture, Mole Fraction, % | Composition of the Polymer Hydrogel, Mole Fraction, % | Average Particle Size, nm (from TEM) | Hydrodynamic. Diameter of Particles (Dispersion in H2O), nm (from DLS) | Zeta-Potential, mV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GMA | BA | TEGDMA | AA | GMA | BA | TEGDMA | AA | |||
| k’ | l’ | m’ | n’ | k | l | m | n | |||
| 10 | 15 | 1 | 74 | 14 | 12 | 4 | 70 | 400 ± 130 | 600 ± 250 | −49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozak, M.; Mitina, N.; Zaichenko, A.; Vlizlo, V. Anionic Polyelectrolyte Hydrogel as an Adjuvant for Vaccine Development. Sci. Pharm. 2020, 88, 56. https://doi.org/10.3390/scipharm88040056
Kozak M, Mitina N, Zaichenko A, Vlizlo V. Anionic Polyelectrolyte Hydrogel as an Adjuvant for Vaccine Development. Scientia Pharmaceutica. 2020; 88(4):56. https://doi.org/10.3390/scipharm88040056
Chicago/Turabian StyleKozak, Mariya, Nataliya Mitina, Alexandr Zaichenko, and Vasyl Vlizlo. 2020. "Anionic Polyelectrolyte Hydrogel as an Adjuvant for Vaccine Development" Scientia Pharmaceutica 88, no. 4: 56. https://doi.org/10.3390/scipharm88040056
APA StyleKozak, M., Mitina, N., Zaichenko, A., & Vlizlo, V. (2020). Anionic Polyelectrolyte Hydrogel as an Adjuvant for Vaccine Development. Scientia Pharmaceutica, 88(4), 56. https://doi.org/10.3390/scipharm88040056






