Ex Vivo and In Vivo Study of Some Isoquinoline Precursors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Methods
2.1.1. Synthesis of 1-(3,4-Dimethoxyphenyl)propan-2-amine 3
2.1.2. Preparation of 1-(3,4-Dimethoxyphenyl)propan-2-amides 4; Typical Procedure
2.2. Smooth Muscle Activity
2.2.1. Ex Vivo Experiments on Gastric SMPs from Rat Wistar
2.2.2. Method of Studying a Mechanical Activity of Isolated SM Preparation
2.3. In Vivo Methods of Studying Cognitive Functions Learning and Memory
2.3.1. Shuttle-Box Active Avoidance Test
2.3.2. Step-Down Passive Avoidance Test
2.4. Housing and Nutrition
2.5. Ethics Statement
2.6. Statistical Analysis
3. Results
3.1. Effects of 4d on Smooth Muscle Activity
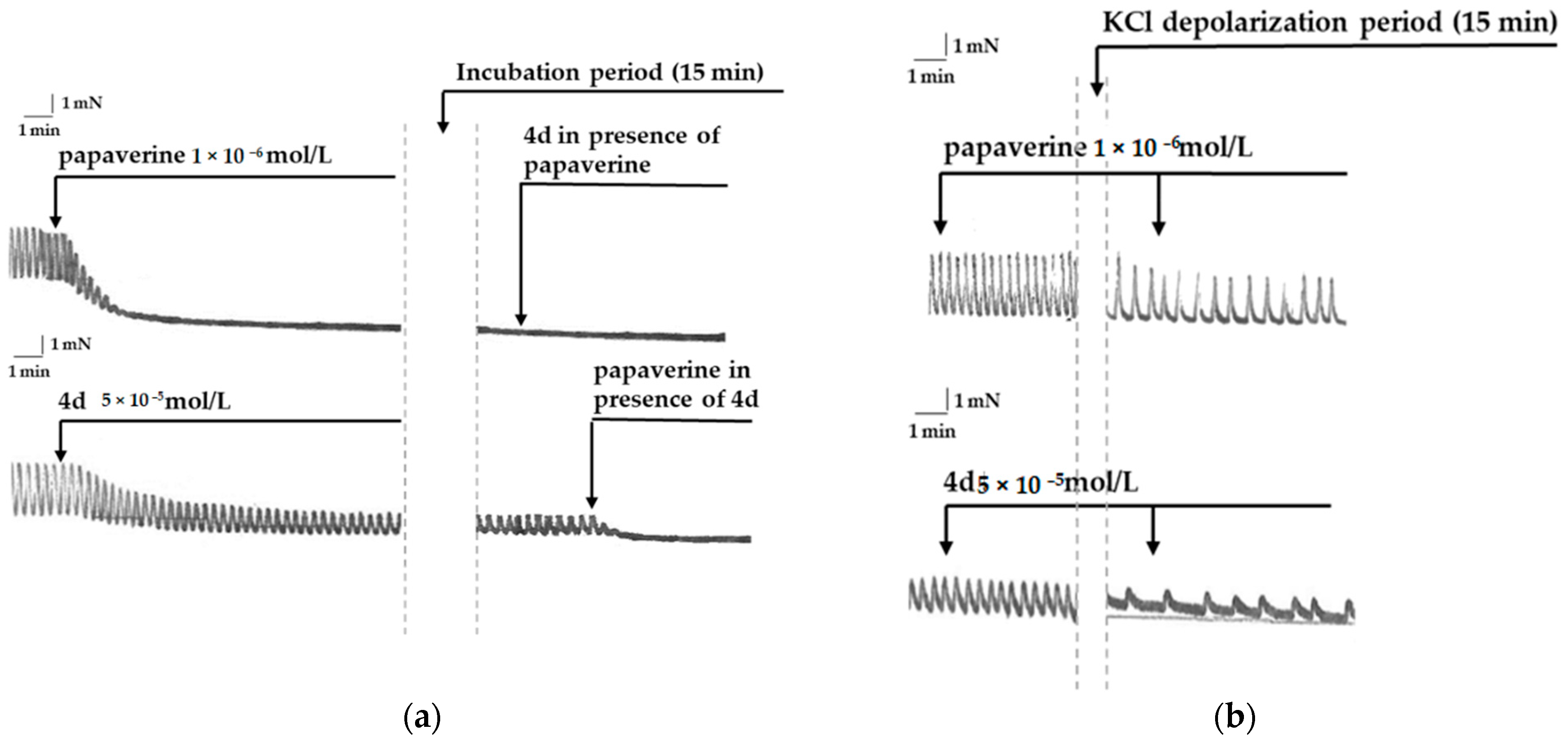
3.1.1. Mutual Influence between Papaverine and 4d
3.1.2. Smooth Muscle Effects of 4d and Papaverine in Conditions of KCl Depolarization
3.2. Effects of 4d on Cognitive Functions Learning and Memory in Rats
3.2.1. Shuttle-Box Active Avoidance Test
3.2.2. Step-Down Passive Avoidance Test
4. Discussion
4.1. In Silico Predictions and Synthesis
4.2. Ex vivo Experiments on SM Activity
4.3. In Vivo Methods of Studying Cognitive Functions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jacob, J.; Varghese, N.; Rasheed, S.P.; Agnihotri, S.; Sharma, V.; Wakode, S. Recent advances in the synthesis of isoquinoline and its analogue: A review. World J. Pharm. Pharm. Sci. 2016, 5, 1821. [Google Scholar] [CrossRef]
- Chulia, S.; Ivorra, M.D.; Martinez, S.; Elorriaga, M.; Valiente, M.; Noguera, M.A.; Lugnier, C.; Advenier, C.; D’Ocon, P. Relationships between structure and vascular activity in a series of benzylisoquinolines. Br. J. Pharmacol. 1997, 122, 409–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Xu, J.; Mach, R.; Tu, Z. Synthesis of fluorine-containing papaverine analogs as PDE10A inhibitors. J. Nucl. Med. 2009, 50, 1880. [Google Scholar]
- Cocolas, G.H. Textbook of Organic Medicinal and Pharmaceutical Chemistry; Delgado, J.N., Remers, W.A., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1998; pp. 505–551. [Google Scholar]
- Lindner, E. Structure Activities and Pharmacological Properties of the Opium Alkaloids. In The Chemistry and Biology of Isoquinoline Alkaloids; Phillipson, J.D., Roberts, M.F., Zenk, M.H., Eds.; Proceedings in Life Sciences; Springer: Berlin/Heidelberg, Germany, 1985; pp. 36–46. [Google Scholar] [CrossRef]
- Schmeller, T.; Wink, M. Utilization of Alkaloids in Modern Medicine. In Alkaloids; Springer: Boston, MA, USA, 1998; pp. 435–459. [Google Scholar]
- Reneerkens, O.A.H.; Rutten, K.; Steinbusch, H.W.M.; Blokland, J.R. Selective phosphodiesterase inhibitors: A promising target for cognition enhancement. Psychopharmacology 2009, 202, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.M.; Pan, Y. Advances in chemical constituents of isoquinoline alkaloids from Nelumbo nucifera and their smooth muscle relaxation effect. Zhongguo Zhong Yao Za Zhi 2019, 44, 3924–3934. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Yang, G.M.; Sun, J.; Pan, Y.; Zhang, J.L.; Xiao, M.; Zhu, M.S. Isolation and identification of a tribenzylisoquinoline alkaloid from Nelumbo nucifera Gaertn, a novel potential smooth muscle relaxant. Fitoterapia 2018, 124, 58–65. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Nishimura, K.; Itoh, A.; Tanahashi, T.; Nakajima, H.; Oshiro, H.; Sun, S.; Toda, T.; Yamada, J. Serotonergic mechanisms are involved in antidepressant-like effects of bisbenzylisoquinolines liensinine and its analogs isolated from the embryo of Nelumbo nucifera Gaertner seeds in mice. J. Pharm. Pharmacol. 2015, 67, 1716–1722. [Google Scholar] [CrossRef]
- Liu, D.; Meng, X.; Wu, D.; Qiu, Z.; Luo, H. A Natural Isoquinoline Alkaloid With Antitumor Activity: Studies of the Biological Activities of Berberine. Front. Pharmacol. 2019, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Manogaran, P.; Beeraka, N.M.; Padma, V.V. The Cytoprotective and Anti-cancer Potential of Bisbenzylisoquinoline Alkaloids from Nelumbo nucifera. Curr. Top. Med. Chem. 2019, 19, 2940–2957. [Google Scholar] [CrossRef]
- Qing, Z.-X.; Yang, P.; Tang, Q.; Cheng, P.; Liu, X.-B.; Zheng, Y.-j.; Liu, Y.-S.; Zeng, J.-G. Isoquinoline alkaloids and their antiviral, antibacterial, and antifungal activities and structure-activity relationship. Curr. Org. Chem. 2017, 21, 1920–1934. [Google Scholar] [CrossRef]
- Jumayev, I.; Usmanov, P.; Rustamov, S.; Zhurakulov, S. Comparative Inotropic Effects of the Some Isoquinoline Alkaloids. Biomed. Pharmacol. J. 2020, 13, 325–333. [Google Scholar] [CrossRef]
- Patil, S.; Tawari, S.; Mundhada, D.; Nadeem, S. Protective effect of berberine, an isoquinoline alkaloid ameliorates ethanol-induced oxidative stress and memory dysfunction in rats. Pharmacol. Biochem. Behav. 2015, 136, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Pöch, G.; Umfahrer, W. Differentiation of intestinal smooth muscle relaxation caused by drugs that inhibit phosphodiesterase. Naunyn Schmiedeberg’s Arch. Pharmacol. 1976, 293, 257–268. [Google Scholar] [CrossRef]
- Pöch, G.; Kukovetz, W.R.; Holzmann, S.; Paietta, E. Effect of relaxants on phosphodiesterase activity and interaction with Ca2+ in smooth muscle. Naunyn Schmiedeberg’s Arch. Pharmacol. 1974, 285, R63. [Google Scholar]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, D.M.; Vásquez, F.J.J.; Osornio, M.R.; Osornio, M.C.R.; Suárez, S.O.; Retana Márque, S. Serotonin and noradrenaline content and release in the dorsal hippocampus during learning and spatial memory in prenatally stressed rats. Acta Neurobiol. Exp. 2020, 80, 400. [Google Scholar] [CrossRef]
- Reis, J.; Cagide, F.; Valencia, M.E.; Teixeria, J.; Bagetta, D.; Pérez, C.; Uriarte, E.; Oliviera, P.J.; Ortuso, F.; Alcaro, S.; et al. Multi-target-directed ligands for Alzheimer’s disease: Discovery of chromone-based monoamine oxidase/cholinesterase inhibitors. Eur. J. Med. Chem. 2018, 158, 781. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, B.; Tykocki, N.R.; Watts, S.W.; Cobbett, P.J. Measurement of smooth muscle function in the isolated tissue bath-applications to pharmacology research. J. Vis. Exp. 2015, 95, 52324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, I.; Nikolova, S.; Aladjov, D.; Stefanova, I.; Zagorchev, P. Synthesis and Contractile Activity of Substituted 1,2,3,4-Tetrahydroisoquinolines. Molecules 2011, 16, 7019–7042. [Google Scholar] [CrossRef]
- Stefanova, I.; Argirova, M.; Krustev, A. Influence of model melanoidins on calciumdependent transport mechanisms in smooth muscle tissue. Mol. Nutr. Food Res. 2007, 51, 468–472. [Google Scholar] [CrossRef]
- Argirova, M.; Stefanova, I.; Krustev, A. New biological properties of coffee melanoidins. Food Funct. 2013, 4, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Sagorchev, P.; Lukanov, J.; Beer, A.-M. Effects of 1.8-cineole (eucalyptol) on the spontaneous contractile activity of smooth muscles fibre. J. Med. Plants Res. 2015, 9, 486–493. [Google Scholar] [CrossRef] [Green Version]
- Beer, A.M.; Lukanov, J.; Sagorchev, P. Effect of Thymol on the spontaneous contractile activity of the smooth muscles. Phytomedicine 2007, 14, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Gledacheva, V.N.; Stefanova, I.D.; Slavchev, V.I.; Ardasheva, R.G.; Kristev, A.D.; Nikolova, S.A.; Saracheva, K.E.; Dimitrova, D.S. Impact of a Newly Synthesized Molecule (2-chloro-N-(1-(3,4-dimethoxyphenyl) propan-2-yl)-2-phenylacetamide) on the Bioelectrogenesis and the Contractile Activity of Isolated Smooth Muscles. Folia Med. 2020, 62, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Gledacheva, V.; Pencheva, M.; Nikolova, S.; Stefanova, I. Ability of 2-Chloro-N-(1-(3,4-dimethoxyphenyl)propan-2-yl)-2-phenylacetamide to Stimulate Endogenous Nitric Oxide Synthesis. Appl. Sci. 2022, 12, 4473. [Google Scholar] [CrossRef]
- Ekins, S.; Mestres, J.; Testa, B. In silico pharmacology for drug discovery: Methods for virtual ligand screening and profiling. Br. J. Pharmacol. 2007, 152, 9. [Google Scholar] [CrossRef] [Green Version]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS: Prediction of activity spectra for biologically active substances. Bioinformatics 2000, 16, 747. [Google Scholar] [CrossRef]
- Mahesh, S.; Tang, K.-C.; Raj, M. Amide Bond Activation of Biological Molecules. Molecules 2018, 23, 2615. [Google Scholar] [CrossRef] [Green Version]
- Seavill, P.W.; Wilden, J.D. The preparation and application of amides using electrosynthesis. Green Chem. 2020, 22, 7737. [Google Scholar] [CrossRef]
- Brading, A.F.; Burdyga, T.V.; Scripnyuk, Z.D. The effects of papaverine on the electrical and mechanical activity of the guinea-pig ureter. J. Physiol. 1983, 334, 79. [Google Scholar] [CrossRef]
- Sunagane, N.; Uruno, T.; Kubota, K. Mechanism of relaxant action of papaverine. Effect on caffeine-induced contraction of guinea pig taenia coli. Jpn. J. Pharmacol. 1982, 32, 785. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kanaide, H.; Nakamura, M. Complete overlap of caffeine- and K+ depolarization-sensitive intracellular calcium storage site in cultured rat arterial smooth muscle cells. J. Biol. Chem. 1986, 261, 15709. [Google Scholar] [CrossRef]
- Webb, R.C. Smooth muscle contraction and relaxation. Adv. Physiol. Educ. 2003, 27, 201. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, D.; Scarpani, E. Neurodegenerative Diseases: Clinical Aspects, Molecular Genetics and Biomarkers; Springer: London, UK, 2015. [Google Scholar] [CrossRef] [Green Version]
- Plazas, E.; Avila, M.; Munoz, D.R.; Cuca, L.E. Natural isoquinoline alkaloids: Pharmacological features and multi-target potential for complex diseases. Pharmacol. Res. 2022, 177, 106126. [Google Scholar] [CrossRef]
- Poddar, M.; Chakraborty, A.; Banerjee, S. Neurodegeneration: Diagnosis, prevention, and therapy. In Oxidoreductase; Mansour, M.A., Ed.; IntechOpen: London, UK, 2021; Available online: https://www.intechopen.com/chapters/74857 (accessed on 1 April 2022). [CrossRef]
- Cai, Z.; Wang, C.; Yang, W. Role of berberine in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2016, 12, 2509–2520. [Google Scholar] [CrossRef] [Green Version]
- Yuan, N.; Cai, C.; Wu, M.; Su, H.; Li, M.; Lu, J. Neuroprotective effects of berberine in animal models of Alzheimer’s disease: A systematic review of pre-clinical studies. BMC Complement. Altern. Med. 2019, 19, 109. [Google Scholar] [CrossRef]
- Grauer, S.M.; Pulito, V.L.; Navarra, R.L.; Kelly, M.P.; Kelley, C.; Graf, R.; Langen, B.; Logue, S.; Brennan, J.; Jiang, L.; et al. Phosphodiesterase PDE10A inhibitor activity in preclinical models of the positive, cognitive, and negative symptoms of schizophrenia. J. Pharmacol. Exp. Ther. 2009, 331, 574–590. [Google Scholar] [CrossRef] [Green Version]
- Siuciak, J.A.; Chapin, D.S.; Harms, J.F.; Lebel, L.A.; McCarthy, S.A.; Chambers, L.; Shrikhande, A.; Wong, S.; Menniti, F.S.; Schmidt, C.J. Inhibition of the striatum-enriched phosphodiesterase PDE10A: A novel approach to the treatment of psychosis. Neuropharmacology 2006, 51, 386–396. [Google Scholar] [CrossRef]
- Giralt, A.; Saavedra, A.; Carreton, O.; Arumi, H.; Tyebji, S.; Alberch, J.; Perez-Navarro, E. PDE10 inhibition increases GluA1 and CREB phosphorylation and improves spatial and recognition memories in a Huntington’s disease mouse model. Hippocampus 2013, 23, 684–695. [Google Scholar] [CrossRef]
- Bliss, T.; Collingridge, G. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]








| Applied Agent after KCl Depolarization | SM Activity Parameters | Base Values | % Change | n | p |
|---|---|---|---|---|---|
| 4d | Tone | −1.35 mN | 58.96 | 11 | 0.032 |
| Frequency | 3.19 min−1 | 47.33 | 0.012 | ||
| Amplitude | 0.05 mN | 52.61 | 0.042 | ||
| Papaverine | Tone | −3.57 | 81.21 | 10 | 0.070 |
| Frequency | 2.9 min−1 | 85.55 | 0.057 | ||
| Amplitude | 0.03 mN | 80.09 | 0.060 |
| 4 | R | mp, °C | Yield [%] |
|---|---|---|---|
| a | CH3 | 83–85 | 81 |
| b | C6H5 | 103–104 | 80 |
| c | CH2C6H5 | 120–123 | 90 |
| d | CH(Cl)C6H5 | 87–89 | 72 |
| e | 2-Cl-C6H5 | 88–90 | 91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milusheva, M.; Gledacheva, V.; Batmazyan, M.; Nikolova, S.; Stefanova, I.; Dimitrova, D.; Saracheva, K.; Tomov, D.; Chaova-Gizdakova, V. Ex Vivo and In Vivo Study of Some Isoquinoline Precursors. Sci. Pharm. 2022, 90, 37. https://doi.org/10.3390/scipharm90020037
Milusheva M, Gledacheva V, Batmazyan M, Nikolova S, Stefanova I, Dimitrova D, Saracheva K, Tomov D, Chaova-Gizdakova V. Ex Vivo and In Vivo Study of Some Isoquinoline Precursors. Scientia Pharmaceutica. 2022; 90(2):37. https://doi.org/10.3390/scipharm90020037
Chicago/Turabian StyleMilusheva, Miglena, Vera Gledacheva, Margarita Batmazyan, Stoyanka Nikolova, Iliyana Stefanova, Darinka Dimitrova, Kremena Saracheva, Desislav Tomov, and Veneta Chaova-Gizdakova. 2022. "Ex Vivo and In Vivo Study of Some Isoquinoline Precursors" Scientia Pharmaceutica 90, no. 2: 37. https://doi.org/10.3390/scipharm90020037
APA StyleMilusheva, M., Gledacheva, V., Batmazyan, M., Nikolova, S., Stefanova, I., Dimitrova, D., Saracheva, K., Tomov, D., & Chaova-Gizdakova, V. (2022). Ex Vivo and In Vivo Study of Some Isoquinoline Precursors. Scientia Pharmaceutica, 90(2), 37. https://doi.org/10.3390/scipharm90020037










