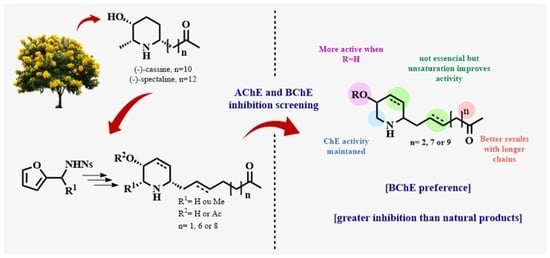
2.1. Synthetic Methods
General: Dichloromethane (DCM) and triethylamine (Et3N) were pretreated with calcium hydride and distilled before use. Ethyl acetate, acetonitrile, methanol, chloroform and toluene were treated with 4 Å molecular sieves for at least 24 h before use and stored under nitrogen-purged atmosphere. BF3·OEt was distilled prior to use. All other solvents and commercial reagents were used as supplied without further purification unless stated otherwise. Reactions were monitored by thin-layer chromatography (silica gel 60 F254 in aluminum foil), and visualization was achieved under UV light (254 nm) followed by staining in potassium permanganate (KMnO4), Dragendorff stain or p-anisaldehyde stain (p-ASD). Silica gel 60 (200–400 Mesh) was used for purifications by standard flash column chromatography. NMR spectra were recorded on a Bruker Avance DPX 250 MHz (250 MHz 1H, 63 MHz 13C), Bruker Avance III 400 (400 MHz 1H, 101 MHz 13C) or Bruker Avance III 500 (500 MHz 1H, 126 MHz 13C) unit (Bruker Co., Billerica, Massachusetts, USA). The chemical shifts are expressed in parts per million (ppm) relative to the residual solvent signal as an internal reference: (1) CDCl3 1H RMN = 7.26, 13C RMN = 77.16; (2) methanol-d4: 1H RMN = 3.31, 13C RMN = 49.00. Multiplicities are reported with the following symbols: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet and multiples thereof. High-resolution mass spectra (ESI) were acquired on an Xevo Q-Tof mass spectrometer (Waters, Manchester, UK) equipped with a nanoESI-type ionization source. IR spectra were recorded using a Thermo Scientific Nicolet IS5 spectrometer, using Thermo Scientific ID3 ATR (Thermo Fisher Scientific, Waltham, Massachusetts, USA) Melting points were recorded on a MP50 Mettler Toledo (Columbus, Ohio, USA) melting point apparatus and are uncorrected.
N-(furan-2-ylmethyl)-2-nitrobenzenesulfonamide (5a) [
24]. Commercially available furfurylamine (0.70 mL, 8.0 mmol, 1.0 eq) was dissolved in THF:H
2O (
v/
v 1:1, 80.0 mL), followed by the addition of NaHCO
3 (2.00 g, 24.0 mmol, 3.00 eq) and 2-nitrobenzenesulfonyl chloride (NsCl) (2.20 g, 9.60 mmol, 1.20 eq) at room temperature. The reaction mixture was stirred for 2 h and extracted with EtOAc (3 × 50.0 mL). The combined organic phases were washed with saturated NaCl solution, dried over Na
2SO
4, filtered and concentrated under reduced pressure. The crude product was purified by flash column chromatography (SiO
2, hexanes/EtOAc 0% to 30%, 10% increases) yielding
5a as a white solid (2,0g, 7,3 mmol, 92% yield).
TLC: (hexanes: EtOAc = 7:3), Rf = 0,4 (UV, KmnO4 or p-ASD)
1H NMR (500 MHz, CDCl3) δ 8.07 - 7.99 (m, 1 H), 7.86 - 7.79 (m, 1 H), 7.72 - 7.63 (m, 2 H), 7.07 (dd, J = 0.9, 1.8 Hz, 1 H), 6.16 - 6.07 (m, 2 H), 5.86 (t, J = 5.8 Hz, 1 H), 4.35 (d, J = 6.1 Hz, 2 H)
13C NMR (126 MHz, CDCl3) δ 149.2, 147.8, 142.7, 134.1, 133.5, 132.9, 131.2, 125.4, 110.4, 108.7, 40.8
IR (cm−1, thin film, ATR) 3298, 1530, 1360, 1323, 1157, 1044, 856, 700
HRMS (ESI) calculated for C11H10N2O5Sna [M+Na]+: 305.0208; found 305.0226
N-(1-(furan-2-yl)ethyl)-2-nitrobenzenesulfonamide (5b):
Commercially available furfurylamine (1.00 mL, 12.0 mmol, 1.20 eq) and benzophenone (1.80 g, 10.0 mmol, 1.00 eq) were dissolved in toluene (24.0 mL). Then, BF3·OEt2 (123 µL, 1.00 mmol, 0.10 eq) was added, the round bottom flask was adapted with a Dean–Stark trap and the reaction mixture was heated under reflux overnight. After this period, the solvent was removed under reduced pressure, producing a brown-yellow solid. Recrystallization with methanol (heat to 45 °C and cool to 0 °C) furnished N-(diphenylmethylene)-1-(furan-2-yl)methanamine (S-I, CAS: 56542-90-6) as white crystals (1.85 g, 7.00 mmol, 71%).
1H NMR (400 MHz, CDCl3) δ 7.69 - 7.63 (m, 2 H), 7.53 - 7.44 (m, 3 H), 7.42 - 7.30 (m, 4 H), 7.24 (s, 2 H), 6.33 (br s, 1 H), 6.23 (br s., 1 H), 4.55 (s, 2 H)
13C NMR (101 MHz, CDCl3) δ 170.2, 153.9, 141.8, 139.7, 136.5, 130.4, 128.8, 128.7, 128.2, 128.0, 110.4, 106.5, 51.2
S-I (1.0 g, 3.8 mmol, 1.0 eq) was solubilized in THF (38 mL, 0.1 M) under a nitrogen atmosphere and the mixture was cooled to −78 °C. A solution of n-BuLi 1.6 M in hexanes (2.50 mL, 5.75 mmol, 1.50 eq) was added dropwise and the solution was stirred for 40 min. Iodomethane (360 µL, 5.75 mmol, 1.50 eq) was added to the dark red solution which was allowed to stir for 1 h at 0 °C. After this period, the reaction turned a dark yellow color and it was quenched with saturated aqueous NaHCO3 solution and extracted with Et2O. The combined organic phases were washed with saturated NaCl solution, dried over Na2SO4, filtered and concentrated under reduced pressure. The crude residue was used without purification in the next step.
The residue from the previous step was dissolved in a mixture of acetone (19 mL) and HCl 1M (19 mL) at 0 °C. The mixture was stirred overnight and then extracted with Et2O. The aqueous phase was neutralized with solid K2CO3 until pH 7–8 and THF (19 mL), NaHCO3 (968 mg, 11.5 mmol, 3.00 eq) and 2-nitrobenzenesulfonyl chloride (1.0 g, 4.6 mmol, 1.2 eq) were added. The reaction was stirred for 5 h or until TLC showed complete conversion of the starting material. The reaction mixture was extracted with EtOAc (3 × 20.0 mL) and the combined organic phases were washed with saturated NaCl solution, dried over Na2SO4, filtered and concentrated under reduced pressure. The material was purified by flash column chromatography (SiO2, hexanes/EtOAc 0% to 40%, 10% increases) yielding 5b as a white solid (795 mg, 2.68 mmol, 70% yield).
TLC: (hexanes: EtOAc = 7:3), Rf = 0.5 (UV, p-ASD)
MP: 62.5–64.6 °C
1H NMR (250 MHz, CDCl3) δ 8.05 - 7.89 (m, 1 H), 7.86 - 7.75 (m, 1 H), 7.71 - 7.55 (m, 2 H), 7.01 - 6.91 (m, 1 H), 6.11 - 5.96 (m, 2 H), 5.81 (d, J = 8.8 Hz, 1 H), 4.83 - 4.60 (m, 1 H), 1.53 (d, J = 7.0 Hz, 3 H)
13C NMR (63 MHz, CDCl3) δ 153.4, 147.5, 142.0, 134.3, 133.3, 132.9, 131.0, 125.3, 110.1, 106.6, 48.3, 20.9
IR (cm−1, thin film, ATR) 1538, 1415, 1355, 1338, 1167, 1157, 735
HRMS (ESI) calculated for C12H12N2O5sNa [M+Na]+: 319.0365; found 319.0309
6-allyl-1-((2-nitrophenyl)sulfonyl)-1,6-dihydropyridin-3(2H)-one (7a): To a solution of compound 5a (1.4 g, 5.0 mmol, 1.0 eq) in THF:H2O (v/v 4:1, 50.0 mL), was added NaHCO3 (842 mg, 10.0 mmol, 2.00 eq), NaOAc (410 mg, 5.00 mmol, 1.00 eq) and N-bromosuccinimide (899 mg, 5.00 mmol, 1.00 eq) at 0 °C. The reaction was kept at this temperature under magnetic stirring for 30 min or until total consumption of starting material was achieved (TLC analysis). After this period, the reaction was quenched by addition of saturated NaHCO3 solution (20.0 mL), saturated with Na2S2O3 (20.0 mL) and extracted with EtOAc (3 × 20.0 mL). The combined organic phases were washed with saturated NaCl solution, dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was subjected to the next step without purification.
The material obtained previously was dissolved in dry MeCN (50 mL, 0,1 M) under an N2 atmosphere and cooled to −30 °C. Then, allyltrimethylsilane (2.45 mL, 15.0 mmol, 3.00 eq) was added followed by Sn(oTf)2 (322 mg, 0.750 mmol, 0.150 eq), and the reaction was kept at this temperature under magnetic stirring for 60 min or until total consumption of starting material was achieved (TLC analysis). After this period, the reaction was quenched by the addition of saturated NaHCO3 solution (20 mL) and extracted with EtOAc (3 × 20 mL). The combined organic phases were washed with saturated NaCl solution, dried over Na2SO4, filtered and concentrated under reduced pressure. The material was purified by column chromatography (SiO2, hexanes/EtOAc 0% to 50%, 10% increases) yielding 7a as a light yellow solid (972 mg, 3.00 mmol, 60% yield, 2 steps).
TLC: (hexanes: EtOAc = 7:3), Rf = 0,33 (UV, kMnO4)
MP: 108–111 °C
1H NMR (400 MHz, CDCl3) δ 8.00 (dd, J = 1.7, 7.6 Hz, 1 H), 7.69 (dquin, J = 1.7, 7.5 Hz, 2 H), 7.65 - 7.60 (m, 1 H), 7.04 (dd, J = 5.1, 10.5 Hz, 1 H), 6.03 - 5.93 (m, 1 H), 5.82 (tdd, J = 7.2, 10.0, 17.1 Hz, 1 H), 5.24 - 5.10 (m, 2 H), 4.80 - 4.71 (m, 1 H), 4.32 (d, J = 18.6 Hz, 1 H), 4.02 (d, J = 18.6 Hz, 1 H), 2.64 - 2.51 (m, 2 H)
13C NMR (126 MHz, CDCl3) δ 191.0, 149.8, 147.9, 134.3, 132.5, 132.4, 132.2, 131.2, 127.0, 124.6, 119.7, 54.0, 49.7, 37.4
IR (cm−1, thin film, ATR) 1693, 1542, 1439, 1358, 1261, 1165, 1126, 1048, 993, 920, 852, 778, 743, 730, 675
HRMS (ESI) calculated for C14H15N2O5S [M+H]+: 323.0702; found 323.0699
6-allyl-2-methyl-1-((2-nitrophenyl)sulfonyl)-1,6-dihydropyridin-3(2H)-one (7b): To a solution of compound 5b (593 mg, 2.00 mmol, 1.00 eq) in THF:H2O (v/v 4:1, 20 mL) was added NaHCO3 (337 mg, 4.00 mmol, 2.00 eq), NaOAc (164 mg, 2.00 mmol, 1.00 eq) and N-bromosuccinimide (360 mg, 2.00 mmol, 1.00 eq) at 0 °C. The reaction was kept at this temperature under magnetic stirring for 30 min or until total consumption of starting material was achieved according to TLC. After this period, the reaction was quenched by addition of saturated NaHCO3 solution (10 mL), saturated with Na2S2O3 (10mL) and extracted with EtOAc (3 × 20 mL). The combined organic phases were washed with saturated NaCl solution, dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was subjected to the next step without purification.
The material obtained in the previous step was solubilized in dry MeCN (20 mL, 0,1 M) under an N2 atmosphere and cooled to −30 °C. Then, allyltrimethylsilane (1.3 mL, 8.0 mmol, 4.0 eq) was added followed by Sn(oTf)2 (125 mg, 0.30 mmol, 0.15 eq), and the reaction was kept at this temperature under magnetic stirring for 60 min or until total consumption of starting material was achieved (TLC analysis). After this period, the reaction was quenched by addition of saturated NaHCO3 solution (20 mL) and extracted with EtOAc (3 × 20 mL). The combined organic phases were washed with saturated NaCl solution, dried over Na2SO4, filtered and concentrated under reduced pressure. The material was purified by column chromatography (SiO2, hexanes/EtOAc 0% to 50%, 10% increases) yielding 7b as a solid (414 mg, 1.23 mmol, 61% yield, 2 steps).
TLC: (hexanes: EtOAc = 7:3), Rf = 0,5 (UV, p-ASD)
MP: 90.8–91.8 °C
1H NMR (500 MHz, CDCl3) δ 7.98 (d, J = 7.5 Hz, 1 H), 7.76 - 7.65 (m, 2 H), 7.62 (d, J = 8.8 Hz, 0 H), 7.06 (dd, J = 5.0, 10.7 Hz, 1 H), 5.98 (dd, J = 1.3, 10.7 Hz, 0 H), 5.94 - 5.84 (m, 1 H), 5.26 - 5.14 (m, 2 H), 4.77 - 4.64 (m, 1 H), 4.37 (q, J = 7.3 Hz, 1 H), 2.79 (td, J = 6.8, 13.4 Hz, 1 H), 2.65 - 2.50 (m, 1 H), 1.59 (d, J = 7.5 Hz, 4 H)
13C NMR (126MHz, CDCl3) δ 194.1, 148.9, 148.1, 134.2, 133.0, 132.6, 132.1, 131.4, 124.8, 124.8, 119.5, 57.4, 54.1, 42.1, 21.6
IR (cm−1, thin film, ATR) 1675, 1535, 1358, 1170
6-allyl-1-((2-nitrophenyl)sulfonyl)-1,2,3,6-tetrahydropyridin-3-ol (8a): Compound 7a (754 mg, 2.35 mmol, 1.00 eq) was dissolved in methanol (45 mL), then CeCl3·7H2O (1,2 g, 3.0 mmol, 1.3 eq) was added. After a homogeneous solution was formed, the reaction mixture was cooled to −78 °C and NaBH4 (148 mg, 3.50 mmol, 1.50 eq) was added. After 20 min, TLC analysis showed complete conversion of starting material and the reaction mixture was allowed to reach room temperature. The reaction was quenched by addition of HCl 0,5M solution (20 mL) and extracted with DCM (3 × 20 mL). The combined organic phases were washed with saturated NaCl solution, dried over Na2SO4, filtered and concentrated under reduced pressure. The material was purified by column chromatography (SiO2, hexanes/EtOAc 0% to 50%, 10% increases) yielding 8a as a white solid (560 mg, 2,20 mmol, 74% yield).
TLC: (hexanes: EtOAc = 1:1), Rf = 0,46 (UV, kMnO4 or p-ASD)
MP: 82–85 °C
1H NMR (500 MHz, CDCl3) δ 8.04 (dd, J = 1.7, 7.5 Hz, 1 H), 7.69 (dquin, J = 1.7, 7.4 Hz, 2 H), 7.64 - 7.61 (m, 1 H), 5.84 - 5.78 (m, 1 H), 5.78 - 5.66 (m, 2 H), 5.06 (dd, J = 1.6, 17.1 Hz, 1 H), 5.04 - 4.97 (m, 1 H), 4.39 (br s., 1 H), 4.17 - 4.09 (m, 1 H), 4.05 (dd, J = 6.1, 13.7 Hz, 1 H), 2.98 (dd, J = 9.9, 13.7 Hz, 1 H), 2.45 - 2.35 (m, 2 H)
13C NMR (126 MHz, CDCl3) δ 147.9, 134.0, 133.8, 133.5, 132.0, 130.8, 130.3, 129.0, 124.4, 118.6, 62.9, 54.0, 45.3, 38.9
IR (cm−1, thin film, ATR) 3496, 1539, 1330, 1163, 1151, 939, 741
HRMS (ESI) calculated for C14H15N2O5SK [M+K]+: 363.0417; found 363.0411
6-allyl-2-methyl-1-((2-nitrophenyl)sulfonyl)-1,2,3,6-tetrahydropyridin-3-ol (8b): Compound 7b (414 mg, 1.23 mmol, 1.00 eq) was dissolved in methanol (25 mL), then CeCl3·7H2O (602 mg, 1.60 mmol, 1.30 eq) was added. After a homogeneous solution was formed, the reaction mixture was cooled to −78 °C and NaBH4 (78 mg, 1.8 mmol, 1.5 eq) was added. After 1 h, TLC analysis showed complete conversion of starting material and the reaction mixture was allowed to reach room temperature. The reaction was quenched by addition of HCl 0,5M solution (10 mL) and extracted with DCM (3 × 15 mL). The combined organic phases were washed with saturated NaCl solution, dried over Na2SO4, filtered and concentrated under reduced pressure. The material was purified by column chromatography (SiO2, hexanes/EtOAc 0% to 70%, 10% increases) yielding 8b as an oil (302 mg, 0.89 mmol, 72% yield).
TLC: (hexanes: EtOAc = 1:1), Rf = 0,40 (UV and p-ASD)
1H NMR (250 MHz, CDCl3) δ 8.11 - 7.96 (m, 1 H), 7.75 - 7.65 (m, 2 H), 7.65 - 7.54 (m, 1 H), 5.97 - 5.74 (m, 2 H), 5.64 - 5.50 (m, 1 H), 5.20 - 5.11 (m, 1 H), 5.09 (s, 1 H), 4.40 - 4.24 (m, 1 H), 4.24 - 4.12 (m, 1 H), 4.09 (br s, 1 H), 2.78 - 2.57 (m, 1 H), 2.40 (ddd, J = 8.7, 9.5, 13.5 Hz, 1 H), 1.75 (d, J = 5.1 Hz, 1 H), 1.28 (d, J = 6.7 Hz, 3 H)
13C NMR (63 MHz, CDCl3) δ 148.0, 134.4, 133.8, 133.8, 131.9, 131.2, 127.2, 126.8, 124.5, 118.5, 65.6, 53.5, 50.6, 42.2, 14.8
IR (cm−1, thin film, ATR) 3530, 1542, 1371, 1169, 1139, 1125, 1020, 996, 757
HRMS (ESI) calculated for C15H19N2O5S [M+H]+: 339.1009; found 339.1001
Methyl ketones synthesis
General procedure A (alkylation)
Acetyl acetoacetate (0.61 mL, 4.8 mmol, 1.2 eq) was added to a solution of NaOEt, prepared from ethanol (4.0 mL) and Na (110 mg, 4.80 mmol, 1.20 eq). Bromo alkene (4.0 mmol, 1.0 eq) was added to the solution and kept stirring under reflux for 12 h. After this period, the reaction was allowed to reach room temperature, neutralized with HCl 6 M and, after addition of water, extracted with EtOAc (3×). The combined organic phases were washed with saturated NaCl solution, dried over Na2SO4, filtered and concentrated under reduced pressure. The material was purified by column chromatography (SiO2, hexanes/EtOAc 0% to 4%, 2% increases).
Ethyl 2-acetyldec-9-enoate (S-IV): The title compound was prepared according to general procedure A using 8-bromo-1-octene (0.7 mL, 4.0 mmol, 1.0 eq). Yield of 71%, colorless oil.
TLC: (hexanes: EtOAc = 9:1), Rf = 0,43 (p-ASD)
1H NMR (400 MHz, CDCl3) δ 5.78 (tdd, J = 6.7, 10.3, 17.0 Hz, 1 H), 4.97 (qd, J = 1.6, 17.1 Hz, 1 H), 4.91 (dd, J = 1.4, 10.2 Hz, 1 H), 4.23 - 4.14 (m, 2 H), 3.38 (t, J = 7.4 Hz, 1 H), 2.20 (s, 3 H), 2.07 - 1.96 (m, 2 H), 1.90 - 1.75 (m, 2 H), 1.39 - 1.28 (m, 6 H), 1.28 - 1.23 (m, 5 H).
13C NMR (101 MHz, CDCl3) δ 203.3, 169.9, 139.0, 114.2, 61.2, 59.9, 33.6, 29.1, 28.7 (3x) 28.1, 27.3, 14.1)
IR: (cm−1, thin film, ATR) 2928, 2856, 1740, 1716, 1241, 1148, 909
Ethyl 2-acetyldodec-11-enoate (S-V): The title compound was prepared according to general procedure A using 10-bromo-decene (0.83 mL, 4.00 mmol, 1.00 eq). Yield was 72%, colorless oil.
TLC: (hexanes: EtOAc = 9:1), Rf = 0,43 (p-ASD)
1H NMR 1H NMR (500MHz, CDCl3) δ 5.77 (tdd, J = 6.7, 10.3, 17.0 Hz, 1 H), 4.95 (qd, J = 1.7, 17.1 Hz, 1 H), 4.89 (td, J = 1.1, 10.1 Hz, 1 H), 4.21 - 4.12 (m, 2 H), 3.36 (t, J = 7.5 Hz, 1 H), 2.19 (s, 3 H), 2.04 - 1.95 (m, 2 H), 1.88 - 1.73 (m, 2 H), 1.39 - 1.29 (m, 2 H), 1.29 - 1.18 (m, 13 H)
13C NMR 13C NMR (126 MHz, CDCl3) δ 203.2, 169.9, 139.0, 114.1, 61.2, 59.9, 33.7, 29.2, 29.2, 29.2, 29.0, 28.8, 28.6, 28.1, 27.3, 14.0
General procedure B (Krapcho decarboxylation)
A solution of ketoester (1.00 eq) in DMSO (sufficient for 0.05 M) was treated with ground NaCl (3 eq) and H2O (32 eq) and heated between 170–180 °C for 18 h. After this period, the reaction mixture was cooled, water was added and extracted with EtOAc. The combined organic phases were washed with saturated NaCl solution, dried over Na2SO4, filtered and concentrated under reduced pressure. The material was purified by column chromatography (SiO2, hexanes/EtOAc 5% to 10%, 1% increases).
Undec-10-en-2-one (B) (CAS 36219-73-5) [
25]: the title compound was prepared according to general procedure B using
S-IV (103 mg, 0.43 mmol, 1.00). Yield was 69% (50.0 mg, 0.23 mmol), colorless oil.
TLC: (hexanes: EtOAc = 9:1), Rf = 0,53 (p-ASD)
1H NMR 1H NMR (500MHz, CDCl3) δ 5.79 (dd, J = 10.3, 17.1 Hz, 1 H), 5.01 - 4.89 (m, 2 H), 2.40 (t, J = 7.5 Hz, 2 H), 2.12 (s, 3 H), 2.05 - 1.99 (m, 2 H), 1.59 - 1.51 (m, 2 H), 1.39 - 1.34 (m, 2 H), 1.33 (br s., 1 H), 1.31 - 1.21 (m, 6 H)
13C NMR (126 MHz, CDCl3) δ 209.2, 139.0, 114.1, 43.7, 33.7, 29.8, 29.2, 29.1, 28.9, 28.8, 23.8
Tridec-12-en-2-one (C) (CAS 60437-21-0) [
26]: the title compound was prepared according to general procedure B using
S-V (609 mg, 2.57 mmol, 1.00). Yield was 80% (609 mg, 2.57 mmol), colorless oil.
TLC: (hexanes: EtOAc = 9:1), Rf = 0,50 (p-ASD)
1H NMR (400 MHz, CDCl3) δ 5.79 (tdd, J = 6.7, 10.3, 17.0 Hz, 1 H), 4.97 (qd, J = 1.7, 17.1 Hz, 1 H), 4.94 − 4.87 (m, 1 H), 2.40 (t, J = 7.5 Hz, 2 H), 2.12 (s, 3 H), 2.07 - 1.97 (m, 2 H), 1.61 - 1.49 (m, 2 H), 1.42 - 1.30 (m, 3 H), 1.26 (s, 11 H)
13C NMR (101MHz, CDCl3) δ 209.3, 139.2, 114.1, 43.8, 33.8, 29.8, 29.4, 29.4, 29.3, 29.1, 29.1, 28.9, 23.8
General Procedure C (Cross-metathesis reaction)
To a mixture of hydroxypiperidine (8a or 8b, 1.0 eq) and unsaturated methyl ketone (5.0 eq) in DCM (sufficient for 0.05 M) was added Hoveyda–Grubbs II catalyst 7.5 mol%, portion-wise. The reaction mixture was kept under reflux for 24 h, allowed to reach room temperature and treated with DMSO (3,75 eq) for 12 h under magnetic stirring. The solvent was removed under reduced pressure and the residue was purified by column chromatography (SiO2, hexanes/Et2O 0% to 100%, 10% increases).
7-(5-hydroxy-1-((2-nitrophenyl)sulfonyl)-1,2,5,6-tetrahydropyridin-2-yl)hept-5-en-2-one (E/Z mixture) (9a): the title compound was prepared according to general procedure C using 8a (130 mg, 0.40 mmol, 1.00 eq), commercially available 5-hexen-2-one (234 µL, 2.00 mmol, 5.00 eq) and Hoveyda–Grubbs II catalyst (19.40 mg, 0.030 mmol, 0.075 eq). Yield was 81% (128 mg, 0.324 mmol).
TLC: (hexanes: EtOAc = 1:1), Rf = 0,17 (UV or p-ASD)
1H NMR (400MHz, CDCl3) δ 8.06 - 8.00 (m, 1 H), 7.73 - 7.65 (m, 2 H), 7.65 - 7.60 (m, 1 H), 5.82 - 5.74 (m, 1 H), 5.74 - 5.67 (m, 1 H), 5.47 - 5.33 (m, 2 H), 4.41 - 4.33 (m, 1 H), 4.06 - 3.95 (m, 2 H), 2.56 - 2.44 (m, 2 H), 2.44 - 2.19 (m, 5 H), 2.16 - 2.10 (m, 3 H) (Major isomer)
13C NMR (101MHz, CDCl3) δ 209.2, 148.0, 134.1, 133.7, 132.6, 132.0, 130.9, 130.7, 128.8, 126.2, 124.4, 62.8, 54.1, 45.5, 42.7, 37.8, 30.3, 26.6
IR (cm−1, thin film, ATR) 3421, 1708, 1543, 1371, 1164, 971
HRMS (ESI) calculated for C18H22N2O6SK [M+K]+: 433.0836; found 433.0804
12-(5-hydroxy-1-((2-nitrophenyl)sulfonyl)-1,2,5,6-tetrahydropyridin-2-yl)dodec-10-en-2-one (E/Z mixture) (9b): the title compound was prepared according to general procedure C using 8a (114 mg, 0.35 mmol, 1.00 eq), methyl ketone B (294 mg, 1.75 mmol, 5.00 eq) and Hoveyda–Grubbs II catalyst (17.0 mg, 0.026 mmol, 0.075 eq). Yield was 68% (111.0 mg, 0.2380 mmol).
TLC: (Et2O, 100%), Rf = 0,43 (UV or p-ASD
1H NMR (400MHz, CDCl3) δ 8.03 - 7.97 (m, 1 H), 7.71 - 7.58 (m, 3 H), 5.82 - 5.68 (m, 2 H), 5.48 - 5.37 (m, 1 H), 5.36 - 5.24 (m, 1 H), 4.32 (br s, 1 H), 4.14 - 3.93 (m, 2 H), 3.04 - 2.89 (m, 1 H), 2.45 - 2.27 (m, 4 H), 2.12 (s, 3 H), 1.99 - 1.85 (m, 2 H), 1.61 - 1.45 (m, 2 H), 1.34 - 1.28 (m, 1 H), 1.25 (br s, 7 H)
13C NMR (101MHz, CDCl3) δ 210.0, 147.8, 134.7, 134.0, 133.6, 131.9, 130.6, 130.2, 128.9, 124.6, 124.3, 62.7, 54.2, 45.2, 43.8, 37.7, 32.4, 29.9, 29.1(×2), 29.0, 23.8, 23.8 (×2)
IR (cm−1, thin film, ATR) 3427, 2928, 2855, 1706, 1543, 1371, 1165, 970, 851, 745
HRMS (ESI) calculated for C23H32N2O6SNa [M+Na]+: 487.1879; found 487.1858
14-(5-hydroxy-1-((2-nitrophenyl)sulfonyl)-1,2,5,6-tetrahydropyridin-2-yl)tetradecadec-12-en-2-one (E/Z mixture) (9c): the title compound was prepared according to general procedure C using 8a (97 mg, 0.3 mmol, 1.0 eq), methyl ketone C (297 mg, 1.5 mmol, 5.0 eq) and Hoveyda–Grubbs II catalyst (9.700 mg, 0.015 mmol, 0.075 eq). Yield was 82% (121.0 mg, 0.246 mmol).
TLC: (Et2O, 100%), Rf = 0,43 (UV or p-ASD
1H NMR (400MHz, CDCl3) δ 8.03 - 7.97 (m, 1 H), 7.71 - 7.58 (m, 3 H), 5.82 - 5.68 (m, 2 H), 5.48 - 5.37 (m, 1 H), 5.36 - 5.24 (m, 1 H), 4.32 (br s, 1 H), 4.14 - 3.93 (m, 2 H), 3.04 - 2.89 (m, 1 H), 2.45 - 2.27 (m, 4 H), 2.12 (s, 3 H), 1.99 - 1.85 (m, 2 H), 1.61 - 1.45 (m, 2 H), 1.34 - 1.28 (m, 1 H), 1.25 (Br. s., 7 H)
13C NMR (101MHz, CDCl3) δ 210.1, 147.6, 134.6, 133.8, 133.5, 131.8, 130.4, 130.1, 128.6, 124.4, 124.1, 62.5, 54.1, 45.0, 43.6, 37.5, 32.3, 29.7 (×2), 29.2, 29.1, 29,0, 28.9 (×2), 23.7
IR (cm−1, thin film, ATR) 3417, 2923, 1706, 1544, 1370, 1165, 970, 744
HRMS (ESI) calculated for C25H37N2O6SK [M+H]+: 493.2372; found 493.2291
12-(5-hydroxy-6-methyl-1-((2-nitrophenyl)sulfonyl)-1,2,5,6-tetrahydropyridin-2-yl)dodec-10-en-2-one (E/Z mixture) (9d): the title compound was prepared according to general procedure C using 8b (102 mg, 0.30 mmol, 1.00 eq), methyl ketone B (255 mg, 1.50 mmol, 5.0 eq) and Hoveyda–Grubbs II catalyst (14.00 mg, 0.023 mmol, 0.075 eq). Brown oil, 57% yield (115.0 mg, 0.227 mmol).
TLC: (hexanes: EtOAc = 1:1), Rf = 0,23 (UV or p-ASD)
1H NMR (250 MHz, CDCl3) δ 8.09 - 8.00 (m, 1 H), 7.75 - 7.57 (m, 3 H), 5.87 - 5.76 (m, 1 H), 5.63 - 5.35 (m, 3 H), 4.33 - 4.20 (m, 1 H), 4.20 - 4.12 (m, 1 H), 4.09 (d, J = 4.9 Hz, 1 H), 2.68 - 2.55 (m, 1 H), 2.42 (t, J = 7.4 Hz, 2 H), 2.13 (s, 3 H), 2.08 - 1.92 (m, 2 H), 1.69 - 1.48 (m, 4 H), 1.42 - 1.17 (m, 11 H)
13C NMR (63 MHz, CDCl3) δ 209.9, 148.0, 134.7, 133.8, 133.7, 131.9, 131.1, 127.0, 126.8, 125.6, 124.4, 65.5, 54.0, 50.6, 43.9, 41.1, 32.6, 30.0, 29.3, 29.3, 29.2, 29.0, 23.9, 14.8
IR (cm−1, thin film, ATR) 2926, 2853, 1705, 1543, 1370, 1170, 1138, 757
HRMS (ESI) calculated for C24H35N2O6S [M+H]+: 479.2210; found 479.2207
14-(5-hydroxy-6-methyl-1-((2-nitrophenyl)sulfonyl)-1,2,5,6-tetrahydropyridin-2-yl)tetradec-12-en-2-one (E/Z mixture) (9e): the title compound was prepared according to general procedure C using 8b (117 mg, 0.350 mmol, 1.00 eq), methyl ketone C (343 mg, 1.73 mmol, 5.00 eq) and Hoveyda–Grubbs II catalyst (17.0 mg, 0.026 mmol, 0.075 eq). Yield was 66% (115 mg, 0.227 mmol).
TLC: (hexanes: EtOAc = 7:3), Rf = 0,33 (UV or p-ASD)
1H NMR (400 MHz, CDCl3) δ 8.04 - 8.00 (m, 1 H), 7.70 - 7.65 (m, 2 H), 7.62 - 7.58 (m, 1 H), 5.80 (td, J = 2.8, 10.6 Hz, 1 H), 5.60 - 5.36 (m, 3 H), 4.24 (m, 1 H), 4.15 (m, 1 H), 4.08 (br s, 1 H), 2.63 - 2.54 (m, 1 H), 2.40 (t, J = 7.5 Hz, 2 H), 2.32 (ddd, J = 7.9, 10.2, 13.2 Hz, 1 H), 2.12 (s, 3 H), 2.08 - 1.94 (m, 3 H), 1.55 (t, J = 6.8 Hz, 2 H), 1.39 - 1.18 (m, 15 H)
13C NMR (101 MHz, CDCl3) δ 209.8, 148.0, 134.8, 133.8, 133.7, 131.9, 131.1, 127.0, 126.8, 125.6, 124.4, 65.6, 54.0, 50.6, 43.9, 41.1, 32.6, 30.0, 29.5, 29.5, 29.3, 29.3, 29.2, 29.2, 24.0, 14.8
IR (cm−1, thin film, ATR) 3463, 2926, 2853, 1708, 1544, 1370, 1170, 1138, 1020, 778, 757
HRMS (ESI) calculated for C26H38N2O6SNa [M+Na]+: 529.2343; found 529.2339
General Procedure D (N-deprotection)
To a solution of N-nosylpiperidine in MeCN (sufficient for 0.05 M) was added K2CO3 (5 eq) and benzenethiol (3 eq). The resulting yellow solution was stirred for 45 min at room temperature or until total consumption of starting material, then filtered. The solvent was removed under reduced pressure and the residue was purified by flash column chromatography (SiO2, hexanes 100% to eliminate yellow compounds, then DCM:MeOH 0% to 10%, with 0,5% Et3N as additive).
7-(5-hydroxy-1,2,5,6-tetrahydropyridin-2-yl)hept-5-en-2-one (10a,E/Z mixture): the title compound was prepared according to general procedure D using 9a (66.00 mg, 0.096 mmol, 1.000 eq), K2CO3 (115 mg, 0.84 mmol, 5.00 eq) and benzenethiol (53 µL, 0.5 mmol, 3.0 eq). Yield was 82% (29.00 mg, 0.138 mmol).
TLC: (DCM:MeOH = 8:2), Rf 0.33= (KMnO4 or Dragendorff)
1H NMR (400 MHz, CDCl3) δ 5.97 −-5.91 (m, 1 H), 5.79 - 5.73 (m, 1 H), 5.57 - 5.46 (m, 1 H), 5.46 - 5.36 (m, 1 H), 3.98 - 3.91 (m, 1 H), 3.36 - 3.25 (m, 1 H), 3.13 (d, J = 12.8 Hz, 1 H), 3.01 (br s, 2 H), 2.92 (dd, J = 2.9, 12.8 Hz, 1 H), 2.51 (q, J = 6.8 Hz, 2 H), 2.29 (q, J = 6.9 Hz, 2 H), 2.25 - 2.15 (m, 2 H), 2.13 (s, 2 H)
13C NMR (101 MHz, CDCl3) δ 208.6, 133.6, 132.3, 128.1, 126.6, 62.2, 54.0, 50.4, 43.0, 38.2, 30.0, 26.7
IR (cm−1, thin film, ATR) 3353, 2917, 1708, 1436, 1362, 1041, 971, 734
HRMS (ESI) calculated for C12H20NO2 [M+H]+: 210. 1494; found 210. 1477
12-(5-hydroxy-1,2,5,6-tetrahydropyridin-2-yl)dodec-10-en-2-one (10b,E/Z mixture): the title compound was prepared according to general procedure D using 9b (110 mg, 0.24 mmol, 1.00 eq), K2CO3 (164 mg, 1.19 mmol, 5.00 eq) and benzenethiol (75 µL, 0.7 mmol, 3.0 eq). Yield was 84% (56.0 mg, 0.20 mmol).
TLC: (DCM: MeOH = 9:1), Rf = 0,33 (KMnO4 or Dragendorff)
1H NMR (500 MHz, CDCl3) δ 6.01 - 5.90 (m, 1 H), 5.77 (d, J = 10.1 Hz, 1 H), 5.60 - 5.51 (m, 1 H), 5.40 - 5.28 (m, 1 H), 4.69 (br s, 2 H), 4.01 (br s, 1 H), 3.54 (q, J = 7.2 Hz, 1 H), 3.44 - 3.33 (m, 1 H), 3.33 - 3.14 (m, 1 H), 3.05 - 2.91 (m, 1 H), 2.45 - 2.31 (m, 2 H), 2.11 (s, 3 H), 2.06 - 1.90 (m, 2 H), 1.62 - 1.49 (m, 2 H), 1.49 - 1.39 (m, 1 H), 1.37 - 1.14 (m, 7 H)
13C NMR (126 MHz, CDCl3) δ 209.4, 135.2, 131.2, 128.0, 124.5, 61.6, 53.9, 52.8, 50.0, 43.7, 37.4, 32.5, 29.8, 29.2, 29.0, 28.9, 23.7
IR (cm−1, thin film, ATR) 3330, 2925, 2853, 1711, 1438, 1361, 1038, 970, 749
HRMS (ESI) calculated for C17H30NO2 [M+H]+: 280.2277; found 280.2264
14-(5-hydroxy-1,2,5,6-tetrahydropyridin-2-yl)tetradec-12-en-2-one (10c,E/Z mixture): the title compound was prepared according to general procedure D using 9c (202 mg, 0.41 mmol, 1.00 eq), K2CO3 (283 mg, 2.05 mmol, 5.00 eq) and benzenethiol (130 µL, 1.2 mmol, 3.0 eq). Yield was 85% (107 mg, 0.35 mmol).
TLC: (DCM: MeOH = 9:1), Rf = 0,33 (KMnO4 or Dragendorff)
1H NMR (500 MHz, CDCl3) δ 5.94 - 5.84 (m, 1 H), 5.74 (d, J = 10.1 Hz, 1 H), 5.56 - 5.45 (m, 1 H), 5.39 - 5.26 (m, 1 H), 3.93 (br s, 1 H), 3.48 (br s, 2 H), 3.23 (t, J = 6.1 Hz, 1 H), 3.09 (d, J = 12.6 Hz, 1 H), 2.88 (dd, J = 2.8, 12.7 Hz, 1 H), 2.37 (t, J = 7.5 Hz, 2 H), 2.16 (t, J = 6.9 Hz, 1 H), 2.09 (s, 3 H), 2.03 - 1.90 (m, 2 H), 1.52 (t, J = 6.9 Hz, 2 H), 1.36 - 1.26 (m, 2 H), 1.22 (br s, 9 H)
13C NMR (126 MHz, CDCl3) δ 209.3, 134.6, 133.1, 127.8, 125.1, 77.3, 76.8, 62.1, 54.1, 50.2, 43.6, 38.2, 32.5, 29.7, 29.3, 29.2, 29.1, 29.0, 23.7
IR (cm−1, thin film, ATR) 3318, 2923, 2852, 1713, 1436, 1360, 1020, 969, 720
HRMS (ESI) calculated for C19H34NO2 [M+H]+: 308.2589; found 308.2502
12-(5-hydroxy-6-methyl-1,2,5,6-tetrahydropyridin-2-yl)dodec-10-en-2-one (10d,E/Z mixture): the title compound was prepared according to general procedure D using 9d (52.0 mg, 0.10 mmol, 1.00 eq), K2CO3 (70.0 mg, 0.51 mmol, 5.00 eq) and benzenethiol (33.0 µL, 0.31 mmol, 3.00 eq). Light yellow oil, 81% yield (26 mg, 0.09 mmol).
TLC: (DCM:MeOH = 9:1), Rf = 0.5 (p-ASD)
1H NMR (500 MHz, CDCl3) δ 5.96 (ddd, J = 2.3, 5.1, 9.9 Hz, 1 H), 5.74 (d, J = 9.9 Hz, 1 H), 5.55 −-5.46 (m, 1 H), 5.39 - 5.30 (m, 1 H), 3.66 (d, J = 5.0 Hz, 1 H), 3.35 (t, J = 6.6 Hz, 1 H), 2.85 (dq, J = 2.0, 6.5 Hz, 1 H), 2.39 (t, J = 7.5 Hz, 2 H), 2.29 - 2.12 (m, 3 H), 2.11 (s, 3 H), 2.05 - 1.95 (m, 2 H), 1.54 (t, J = 6.9 Hz, 2 H), 1.38 - 1.19 (m, 8 H), 1.19 - 1.11 (m, 3 H)
13C NMR (126 MHz, CDCl3) δ 209.5, 134.5, 133.8, 128.7, 125.7, 65.9, 55.8, 53.4, 43.9, 39.0, 32.7, 30.0, 29.5, 29.3, 29.2, 29.1, 23.9, 17.7
IR (cm−1, thin film, ATR) 337, 2925, 2853, 1713, 1359, 972
HRMS (ESI) calculated for C18H32NO2 [M+H]+: 294.2428; found 294.2426
14-(5-hydroxy-6-methyl-1,2,5,6-tetrahydropyridin-2-yl)tetradec-12-en-2-one (10e,E/Z mixture): the title compound was prepared according to general procedure D using 9e (90.0 mg, 0.18 mmol, 1.00 eq), K2CO3 (123 mg, 0.89 mmol, 5.00 eq) and benzenethiol (57.0 µL, 0.54 mmol, 3.00 eq). Light yellow solid, 92% yield (56 mg, 0.16 mmol).
TLC: (DCM: MeOH: NH4OH (27%) = 88:10:2), Rf = 0,5 (p-ASD)
1H NMR (250 MHz, CDCl3) δ 5.72 (dd, J = 1.4, 10.0 Hz, 1 H), 5.58 - 5.42 (m, 1 H), 5.41 - 5.23 (m, 1 H), 3.67 - 3.61 (m, 1 H), 3.32 (t, J = 6.7 Hz, 1 H), 2.82 (dq, J = 2.1, 6.5 Hz, 1 H), 2.38 (t, J = 7.4 Hz, 2 H), 2.22 - 2.06 (m, 6 H), 1.97 (q, J = 6.7 Hz, 2 H), 1.52 (t, J = 7.0 Hz, 2 H), 1.36 - 1.18 (m, 11 H), 1.14 (d, J = 6.6 Hz, 3 H)
13C NMR (63 MHz, CDCl3) δ 209.4, 134.4, 133.9, 128.7, 125.7, 77.7, 76.7, 65.9, 55.8, 53.4, 43.9, 39.0, 32.7, 29.9, 29.5, 29.4, 29.4, 29.2, 23.9, 17.7
IR (cm−1, thin film, ATR) 3402, 2923, 2852, 1714, 1462, 1359, 971, 718, 640
HRMS (ESI) calculated for C20H36NO2 [M+H]+: 322.2741; found 322.2736
General Procedure E (synthesis of compounds 11a, 11b, 11c)
To a solution of compound 10a–c (1.0 eq) in EtOAc (sufficient for 0.1 M) at 0 °C, was added Boc2O (1.3 eq), and it was allowed to reach room temperature. After total consumption of starting material, according to TLC, Ac2O (2.0 eq), Et3N (4.00 eq) and DMAP (0.05 eq) were added. The reaction mixture was stirred for 2 h, then diluted with EtOAc and washed with a citric acid 5% solution. The aqueous phase was extracted with EtOAc and washed with saturated NaCl solution, dried over Na2SO4, filtered and concentrated under reduced pressure. To the residue was added a HCl 4 M solution in EtOAc. After total consumption of starting material, the reaction was treated with saturated NaHCO3 solution and extracted with EtOAc (3×). The combined organic phases were washed with saturated NaCl solution, dried over Na2SO4, filtered and concentrated under reduced pressure. The material was purified by column chromatography (SiO2, DCM/MeOH 0% to 10%, 2% increases).
6-(6-oxohept-2-en-1-yl)-1,2,3,6-tetrahydropyridin-3-yl acetate (11a, E/Z mixture): the title compound was prepared according to general procedure E using 10a (21 mg, 0.1 mmol, 1.0 eq), Boc2O (30.0 µL, 0.13 mmol, 1.30 eq), Ac2O (19 µL, 0.2 mmol, 2.0 eq), Et3N (56 µL, 0.4 mmol, 4.0 eq) and DMAP (0.600 mg, 0.005 mmol, 0.050 eq). Yield was 48% (12 mg, 0.05 mmol).
1H NMR (400 MHz, CDCl3) δ 6.01 - 5.93 (m, 1 H), 5.93 - 5.86 (m, 1 H), 5.61 - 5.51 (m, 1 H), 5.50 - 5.39 (m, 1 H), 5.09 - 5.01 (m, 1 H), 3.32 (dt, J = 1.7, 6.5 Hz, 1 H), 3.25 - 3.18 (m, 1 H), 3.08 - 2.98 (m, 1 H), 2.90 (br s, 1 H), 2.56 - 2.48 (m, 2 H), 2.39 - 2.20 (m, 4 H), 2.17 - 2.12 (m, 3 H), 2.10 - 2.05 (m, 3 H)
13C NMR (101 MHz, CDCl3) δ 208.2, 170.7, 136.5, 132.6, 126.3, 123.7, 64.7, 53.5, 47.1, 43.1, 37.9, 29.9, 26.7, 21.3
IR (cm−1, thin film, ATR) 2920, 1728, 1715, 1370, 1238, 1024, 971
HRMS (ESI) calculated for C14H22NO3 [M+H]+: 252.1600; found 252.1616
6-(11-oxododec-2-en-1-yl)-1,2,3,6-tetrahydropyridin-3-yl acetate (11b,E/Z mixture): the title compound was prepared according to general procedure E using 10b (56.0 mg, 0.16 mmol, 1.0 eq), Boc2O (51.0 µL, 0.18 mmol, 1.30 eq), Ac2O (39 µL, 0.3 mmol, 2.0 eq), Et3N (113 µL, 0.65 mmol, 4.0 eq) and DMAP (1.200 mg, 0.008 mmol, 0.050 eq), resulting in 29% yield (18 mg, 0.06 mmol).
1H NMR (400 MHz, CDCl3) δ 6.00 (d, J = 10.3 Hz, 1 H), 5.92 - 5.84 (m, 1 H), 5.60 - 5.49 (m, 1 H), 5.45 - 5.31 (m, 1 H), 5.01 (br s, 1 H), 3.33 - 3.23 (m, 1 H), 3.17 (d, J = 13.9 Hz, 1 H), 2.99 (dd, J = 3.4, 14.0 Hz, 1 H), 2.40 (t, J = 7.5 Hz, 2 H), 2.32 −-2.16 (m, 2 H), 2.16 - 2.09 (m, 3 H), 2.09 - 2.03 (m, 4 H), 2.03 - 1.90 (m, 2 H), 1.62 - 1.48 (m, 2 H), 1.38 - 1.20 (m, 8 H)
13C NMR (101 MHz, CDCl3) δ 209.3, 170.7, 137.3, 134.5, 125.3, 123.5, 65.1, 53.7, 47.4, 43.7, 38.2, 32.5, 29.8, 29.3, 29.2, 29.1, 28.9, 23.8, 21.3
IR (cm−1, thin film, ATR) 2926, 2853, 1731, 1715, 1433, 1369, 1237, 1026, 968
HRMS (ESI) calculated for C19H32NO3 [M+H]+: 322.2382; found 322.2375
6-(13-oxotetradec-2-en-1-yl)-1,2,3,6-tetrahydropyridin-3-yl acetate (11c,E/Z mixture): the title compound was prepared according to general procedure E using 10c (60.0 mg, 0.16 mmol, 1.0 eq), Boc2O (50.0 µL, 0.18 mmol, 1.30 eq), Ac2O (38 µL, 0.3 mmol, 2.0 eq), Et3N (110 µL, 0.65 mmol, 4.0 eq) and DMAP (1.200 mg, 0.008 mmol, 0.050 eq), resulting in 34% yield (18 mg, 0.06 mmol).
1H NMR (400 MHz, CDCl3) δ 5.99 (d, J = 10.1 Hz, 1 H), 5.93 - 5.83 (m, 1 H), 5.61 - 5.49 (m, 1 H), 5.43 - 5.31 (m, 1 H), 5.00 (br s, 1 H), 3.32 - 3.21 (m, 1 H), 3.16 (d, J = 14.1 Hz, 1 H), 2.99 (dd, J = 3.3, 14.1 Hz, 1 H), 2.40 (t, J = 7.5 Hz, 2 H), 2.30 - 2.16 (m, 2 H), 2.12 (s, 3 H), 2.09 - 2.02 (m, 3 H), 1.99 (q, J = 7.0 Hz, 2 H), 1.92 - 1.80 (m, 1 H), 1.65 - 1.48 (m, 2 H), 1.39 - 1.29 (m, 2 H), 1.26 (br s, 10 H)
13C NMR (101 MHz, CDCl3) δ 209.3, 170.7, 137.5, 134.5, 125.4, 123.7, 123.5, 65.2, 53.7, 47.5, 43.7, 38.3, 32.6, 29.8, 29.4, 29.3, 29.2, 29.1, 29.1, 23.8, 21.3
IR (cm−1, thin film, ATR) 2918, 2849, 1731, 1716, 1369, 1238, 1025, 968, 719
HRMS (ESI) calculated for C21H36NO3 [M+H]+: 350.2695; found 350.2684
General Procedure F (catalytic hydrogenation)
To a solution of compound 10a–e (1 eq) in AcOEt (sufficient for 0.1 M) under N2 atmosphere was added Pd(OH)2 20%/C (20 mol%). Then, the atmosphere was changed to H2 (1 atm) and the reaction was left stirring overnight. After this period, the mixture was filtered through a pad of Celite and the solvent was removed under reduced pressure. The residue was purified by flash column chromatography (SiO2, isocratic DCM: MeOH: NH4OH, 88:10:2).
7-(5-hydroxypiperidin-2-yl)heptan-2-one (12a): the title compound was prepared according to general procedure F using 10a (31.0 mg, 0.15 mmol, 1.00 eq) and Pd(OH)2 (4.00 mg, 0.03 mmol, 0.20 eq). Isolated in 47% yield (15.0 mg, 0.07 mmol).
TLC: (DCM: MeOH = 8:2), Rf = 0,33 (p-ASD)
1H NMR (500 MHz, CDCl3) δ 3.89 (br s, 1 H), 3.77 (br s, 2 H), 3.12 (d, J = 12.1 Hz, 1 H), 2.81 (d, J = 12.1 Hz, 1 H), 2.55 (Br. s., 1 H), 2.42 (t, J = 7.3 Hz, 2 H), 2.16 - 2.08 (m, 3 H), 1.85 (br s, 1 H), 1.59 - 1.45 (m, 5 H), 1.43 - 1.24 (m, 5 H)
13C NMR (126 MHz, CDCl3) δ 209.2, 63.7, 56.7, 51.9, 43.5, 35.9, 30.6, 29.9, 29.1, 26.0, 25.4, 23.6
IR (cm−1, thin film, ATR) 3353, 2915, 2851, 1704, 1448, 1163, 1074
HRMS (ESI) calculated for C12H24NO2 [M+H]+: 214.1807; found 214.1793
12-(5-hydroxypiperidin-2-yl)dodecan-2-one (12b): the title compound was prepared according to general procedure F using 10b (62.0 mg, 0.22 mmol, 1.00 eq) and Pd(OH)2 (18.0 mg, 0.13 mmol). Isolated in 38% yield (24.0 mg, 0.08 mmol).
TLC: (CHCl3: MeOH = 9:1), Rf = 0,16 (p-ASD)
1H NMR (500 MHz, CDCl3) δ 3.83 (Br. s., 1 H), 3.03 (d, J = 12.1 Hz, 1 H), 2.77 (d, J = 11.9 Hz, 1 H), 2.51 - 2.44 (m, 1 H), 2.41 (t, J = 7.5 Hz, 2 H), 2.24 (d, J = 19.6 Hz, 2 H), 2.13 (s, 3 H), 1.87 - 1.79 (m, 1 H), 1.61 - 1.47 (m, 4 H), 1.47 - 1.38 (m, 1 H), 1.38 - 1.29 (m, 4 H), 1.27 (br s, 11 H)
13C NMR (126 MHz, CDCl3) δ 209.4, 63.9, 56.8, 52.0, 43.8, 36.3, 30.8, 29.8, 29.6, 29.5, 29.5, 29.4, 29.3, 29.1, 26.2, 25.6, 23.8
IR (cm−1, thin film, ATR) 3397, 2915, 2848, 1718, 1445, 1152, 962
HRMS (ESI) calculated for C17H34NO2 [M+H]+: 284.2589; found 284.2581
14-(5-hydroxypiperidin-2-yl)tetradecan-2-one (12c): the title compound was prepared according to general procedure F using 10c (107 mg, 0.35 mmol, 1.00 eq) and Pd(OH)2 (21.0 mg, 0.15 mmol). Isolated in 46% yield (50.0 mg, 0.16 mmol).
TLC: (DCM:MeOH = 8:2), Rf = 0,33 (p-ASD)
1H NMR (400 MHz, CDCl3) δ 3.80 (br s, 1 H), 3.01 (d, J = 12.1 Hz, 1 H), 2.75 (d, J = 11.9 Hz, 1 H), 2.54 (br s, 2 H), 2.49 - 2.42 (m, 1 H), 2.39 (t, J = 7.5 Hz, 2 H), 2.11 (s, 3 H), 1.81 (d, J = 13.3 Hz, 1 H), 1.57 - 1.40 (m, 4 H), 1.39 - 1.27 (m, 5 H), 1.23 (br s, 15 H)
13C NMR (126 MHz, CDCl3) δ 209.4, 64.3, 56.8, 52.4, 43.8, 36.8, 31.0, 29.8, 29.7, 29.5 (×3), 29.4, 29.3, 29.1, 26.8, 25.7, 23.8
IR (cm−1, thin film, ATR) 3329, 2923, 2852, 1715, 1439, 1358, 1163, 753
HRMS (ESI) calculated for C19H38NO2 [M+H]+: 312.2903; found 312.2885
12-(5-hydroxy-6-methylpiperidin-2-yl)dodecan-2-one [1, (±)-cassine]: the title compound was prepared according to general procedure F using 10d (25.0 mg, 0.08 mmol, 1.00 eq) and Pd(OH)2 (5.00 mg, 0.04 mmol). Light yellow solid, 87% yield (23.0 mg, 0.08 mmol).
TLC: (DCM:MeOH = 8:2), Rf = 0,4 (p-ASD)
MP: 66.6–67.9 °C
1H NMR (500 MHz, CDCl3) δ 3.53 (br s, 1 H), 2.75 (q, J = 6.4 Hz, 1 H), 2.57 - 2.49 (m, 1 H), 2.40 (t, J = 7.4 Hz, 2 H), 2.12 (s, 3 H), 1.91 - 1.85 (m, 1 H), 1.59 - 1.49 (m, 2 H), 1.49 - 1.46 (m, 1 H), 1.45 (dd, J = 2.2, 4.4 Hz, 1 H), 1.36 - 1.22 (m, 18 H), 1.09 (d, J = 6.4 Hz, 3 H)
13C NMR (126 MHz, CDCl3) δ 209.5, 68.1, 57.3, 55.9, 43.9, 37.1, 32.2, 30.0, 29.9, 29.7, 29.6, 29.5, 29.5, 29.3, 26.2, 25.9, 24.0, 18.8
IR (cm−1, thin film, ATR) 2919, 2850, 1708, 1472, 1425, 1357, 1161, 993
HRMS (ESI) calculated for C18H36NO2 [M+H]+: 298.2741; found 298.2739
14-(5-hydroxy-6-methylpiperidin-2-yl)tetradecan-2-one [3, (±)-spectaline]: the title compound was prepared according to general procedure F using 10e (53 mg, 0.16 mmol, 1.00 eq) and Pd(OH)2 (10.0 mg, 0.07 mmol). Light yellow solid, 90% yield (48.0 mg, 0.15 mmol).
TLC: (DCM:MeOH = 8:2), Rf = 0,4 (p-ASD)
1H NMR (500 MHz, MeOD(d4)) δ 3.60 - 3.57 (m, 1 H), 2.76 (dq, J = 1.4, 6.7 Hz, 1 H), 2.60 - 2.53 (m, 1 H), 2.47 (t, J = 7.4 Hz, 2 H), 2.13 (s, 1 H), 2.12 - 2.10 (m, 1 H), 1.94 - 1.87 (m, 1 H), 1.67 - 1.59 (m, 1 H), 1.59 −-1.46 (m, 4 H), 1.44 - 1.32 (m, 6 H), 1.30 (br s, 15 H), 1.11 (d, J = 6.6 Hz, 3 H)
13C NMR (126 MHz, MeOD(d4)) δ 212.4, 68.2, 57.9, 56.4, 44.5, 37.6, 32.8, 31.0, 30.9, 30.8, 30.8, 30.8, 30.7, 30.4, 29.9, 27.0, 26.2, 25.0, 18.4
IR (cm−1, thin film, ATR) 2917, 2849, 1712, 1470, 1261, 1090, 993
HRMS (ESI) calculated for C20H40NO2 [M+H]+: 326.3054; found 326.3049
General Procedure G (synthesis of compounds 13a-c)
To a solution of compound 12a–c in EtOAc (sufficient for 0.1 M) was added 0.1 mL HCl 4 M in dioxane. After 18 h the solvent was removed under reduced pressure, the residue was suspended in 1 mL of DCM and acetyl chloride (1.8 eq), freshly distilled, was added. The mixture was kept under reflux for 18 h. After this period was added NaHCO3 saturated solution and extracted with EtOAc. The combined organic phases were washed with saturated NaCl solution, dried over Na2SO4, filtered and concentrated under reduced pressure. The material was purified by column chromatography (SiO2, DCM/MeOH 0% to 10%, 2% increases).
6-(6-oxoheptyl)piperidin-3-yl acetate (13a): the title compound was prepared according to general procedure G using 12a (24.0 mg, 0,06 mmol, 1.00 eq) and AcCl (8.0 µL, 0,1 mmol, 1.8 eq). Yield was 63% (10.0 mg, 0.04 mmol).
TLC: (DCM:MeOH = 9:1), Rf = 0,26 (p-ASD)
1H NMR (500 MHz, CDCl3) δ 4.85 (br s, 1 H), 3.22 - 3.08 (m, 1 H), 2.84 (d, J = 13.8 Hz, 1 H), 2.51 (br s, 1 H), 2.41 (t, J = 7.4 Hz, 2 H), 2.12 (s, 2 H), 2.11 - 2.02 (m, 3 H), 1.96 (d, J = 14.5 Hz, 1 H), 1.69 - 1.51 (m, 4 H), 1.45 - 1.27 (m, 6 H), 1.24 (s, 2 H)
13C NMR (101 MHz, CDCl3) δ 208.8, 170.3, 67.0, 55.6, 48.8, 43.3, 35.9, 29.5, 28.8, 27.9, 26.9, 25.3, 23.3, 21.1
IR (cm−1, thin film, ATR) 2915, 2850, 1738, 1716, 1465, 1376, 1235, 1087, 1022, 668
HRMS (ESI) calculated for C14H26NO3 [M+H]+: 256.1913; found 256.1910
6-(11-oxododecyl)piperidin-3-yl acetate (13b): the title compound was prepared according to general procedure G using 12b (15.0 mg, 0,05 mmol, 1.00 eq) and AcCl (6.0 µL, 0,08 mmol, 1.80 eq). Yield was 58% (15.0 mg, 0.03 mmol).
TLC: (DCM:MeOH = 9:1), Rf = 0,4 (p-ASD)
1H NMR (500 MHz, CDCl3) δ 4.88 (br s, 1 H), 3.20 (d, J = 13.8 Hz, 1 H), 2.87 (d, J = 13.7 Hz, 1 H), 2.55 (br s, 1 H), 2.42 (t, J = 7.5 Hz, 2 H), 2.14 (s, 3 H), 2.11 (s, 3 H), 2.02 - 1.94 (m, 1 H), 1.70 - 1.51 (m, 4 H), 1.51 - 1.31 (m, 5 H), 1.27 (br s, 13 H)
13C NMR (126 MHz, CDCl3) δ 209.4, 170.7, 67.4, 56.1, 49.2, 43.8, 36.5, 29.9, 29.7, 29.5, 29.5, 29.4, 29.4, 29.2, 28.2, 27.2, 25.9, 23.9, 21.5
IR (cm−1, thin film, ATR) 2923, 2850, 1733, 1716, 1372, 1240, 1022, 668
HRMS (ESI) calculated for C19H36NO3 [M+H]+: 326.2695; found 326.2712
6-(13-oxotetradecyl)piperidin-3-yl acetate (13c): the title compound was prepared according to general procedure G using 12c (23.0 mg, 0,07 mmol, 1.00 eq) and AcCl (10.0 µL, 0,14 mmol, 1.80 eq). Yield was 57% (15.0 mg, 0.04 mmol).
TLC: (DCM:MeOH = 9:1), Rf = 0,46 (p-ASD)
1H NMR (400 MHz, CDCl3) δ 4.88 - 4.81 (m, 2 H), 3.15 (td, J = 2.3, 13.8 Hz, 2 H), 2.84 (dd, J = 2.0, 13.8 Hz, 2 H), 2.59 - 2.44 (m, 2 H), 2.40 (t, J = 7.5 Hz, 3 H), 2.12 (s, 5 H), 2.10 - 2.07 (m, 5 H), 2.00 −-1.90 (m, 3 H), 1.73 - 1.61 (m, 2 H), 1.61 - 1.51 (m, 6 H), 1.47 - 1.30 (m, 8 H)
13C NMR (101 MHz, CDCl3) δ 209.5, 170.9, 67.9, 56.2, 49.7, 44.0, 37.0, 30.0, 29.9, 29.7(×3), 29.6 (×2), 29.5, 29.3, 28.6, 27.8, 26.1, 24.0, 21.6
IR (cm−1, thin film, ATR) 2924, 2852, 1734, 1717, 1436, 1373, 1240, 1022, 668
HRMS (ESI) calculated for C21H40NO3 [M+H]+: 354.3008; found 354.3005