Anti-Sporotrichotic Activity, Lambert-W Inhibition Kinetics and 3D Structural Characterization of Sporothrix schenckii Catalase as Target of Glucosinolates from Moringa oleifera
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Extract
2.2. Yeast Cells Culture and MIC
2.3. Catalase Activity
2.4. Protein Determination
2.5. Kinetics Data Processing
2.6. Preparation of Enzyme Structure
2.7. Model Validation
2.8. Catalytic Active Site Prediction
2.9. Preparation of Ligands
2.10. Molecular Docking Simulation
3. Results
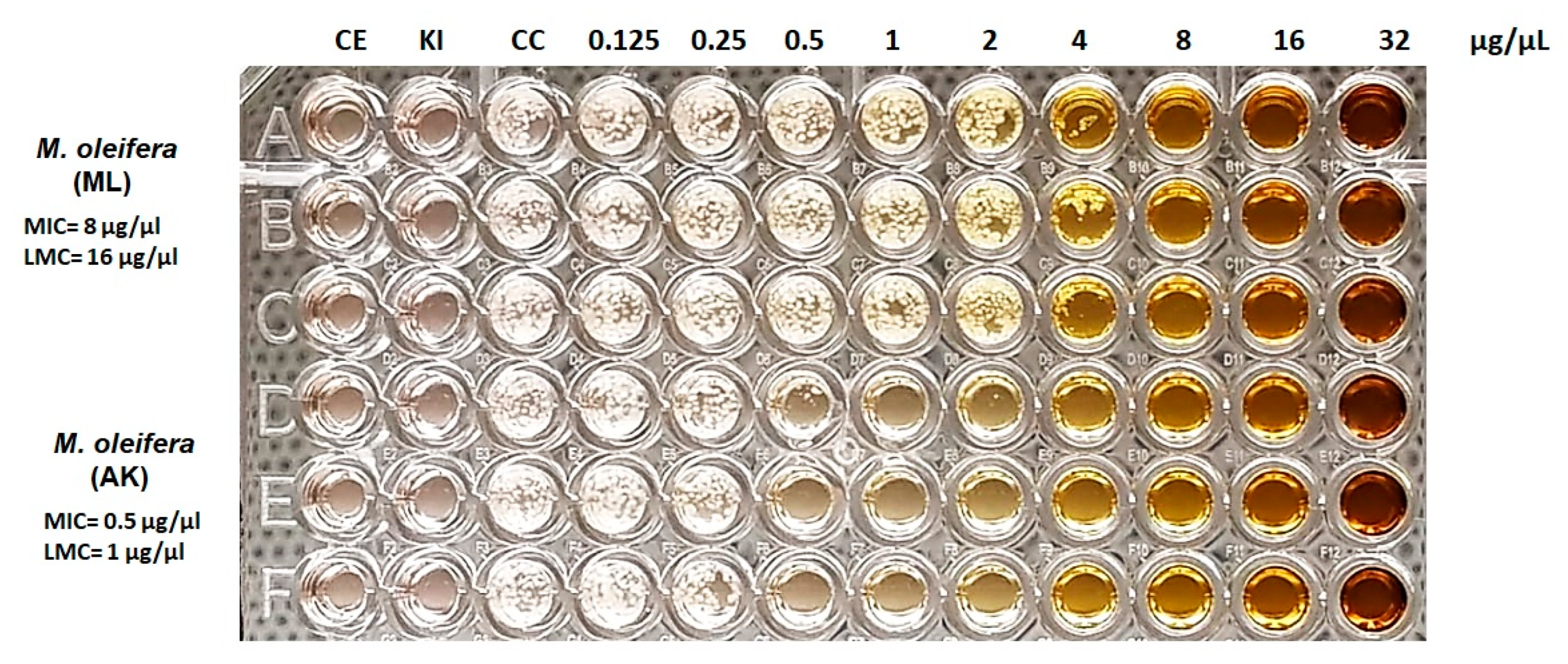
3.1. Antifungal Effect of M. oleifera Extract
3.2. Effect of M. oleifera Leaves Extract on SsCAT Kinetic Parameters
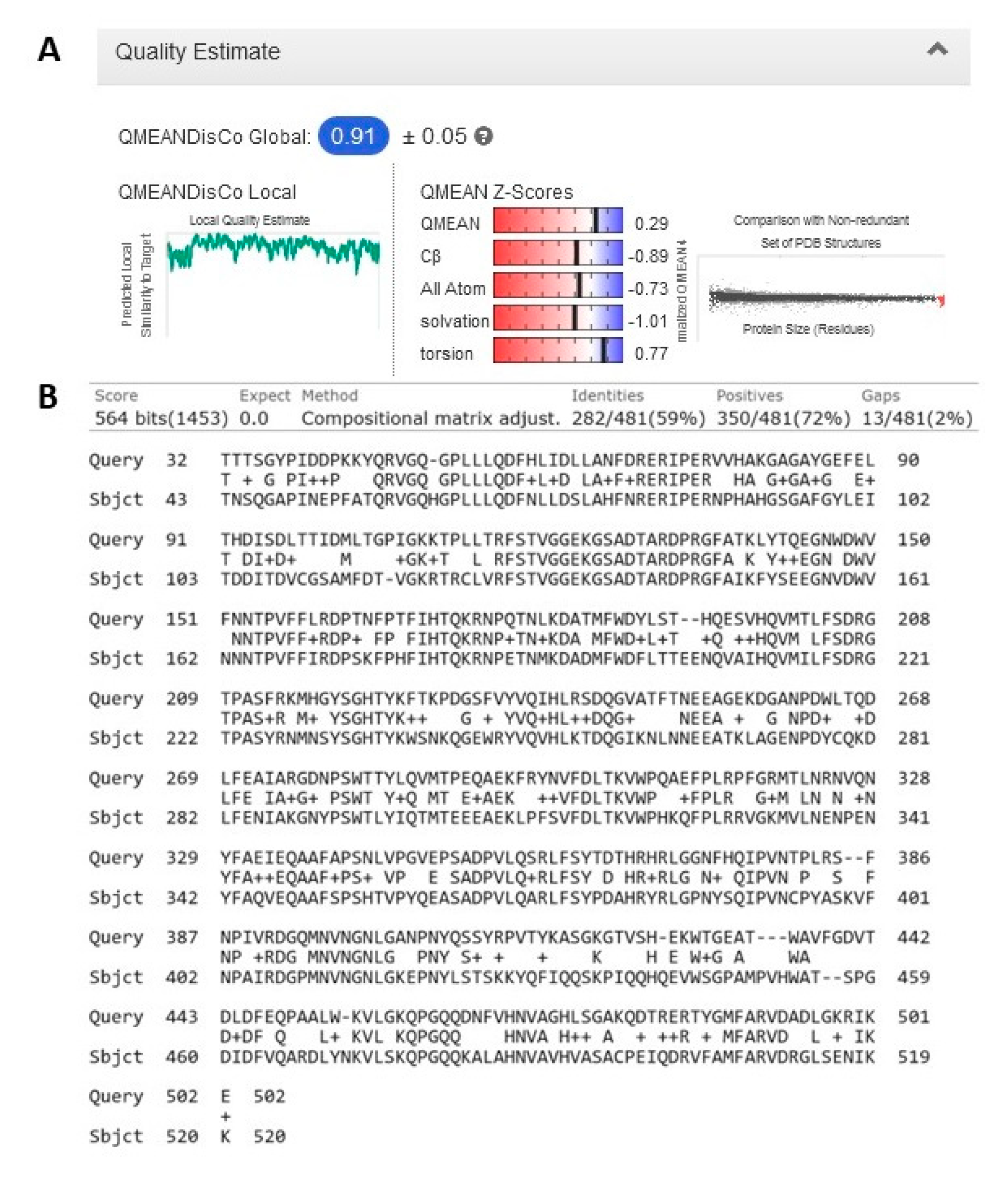
3.3. Homology Modeling
3.4. S. schenckii Catalase Features
3.5. Top Two Drug Candidates from Moringa oleifera
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliveira, M.M.E.; Almeida-Paes, R.; Gutierrez-Galhardo, M.C.; Zancope-Oliveira, R.M. Molecular Identification of the Sporothrix Schenckii Complex. Rev. Iberoam. Micol. 2014, 31, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Sizar, O.; Talati, R. Sporotrichosis (Sporothrix Schenckii). In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2018. [Google Scholar]
- Marek, C.L.; Timmons, S.R. Antimicrobials in Pediatric Dentistry. In Pediatric Dentistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 128–141.e1. [Google Scholar]
- Girois, S.B.; Chapuis, F.; Decullier, E.; Revol, B.G.P. Erratum: Adverse Effects of Antifungal Therapies in Invasive Fungal Infections: Review and Meta-Analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 138–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, N.; Bains, A.; Kaushik, R.; Dhull, S.B.; Melinda, F.; Chawla, P. A Review on Antifungal Efficiency of Plant Extracts Entrenched Polysaccharide-Based Nanohydrogels. Nutrients 2021, 13, 2055. [Google Scholar] [CrossRef] [PubMed]
- Remmele, C.W.; Luther, C.H.; Balkenhol, J.; Dandekar, T.; Müller, T.; Dittrich, M.T. Integrated Inference and Evaluation of Host–Fungi Interaction Networks. Front. Microbiol. 2015, 6, 764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dantas, A.d.S.; Day, A.; Ikeh, M.; Kos, I.; Achan, B.; Quinn, J. Oxidative Stress Responses in the Human Fungal Pathogen, Candida Albicans. Biomolecules 2015, 5, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajiwara, H.; Saito, M.; Ohga, S.; Uenotsuchi, T.; Yoshida, S. Impaired Host Defense against Sporothrix Schenckii in Mice with Chronic Granulomatous Disease. Infect. Immun. 2004, 72, 5073–5079. [Google Scholar] [CrossRef] [Green Version]
- Romero-Martinez, R.; Wheeler, M.; Guerrero-Plata, A.; Rico, G.; Torres-Guerrero, H. Biosynthesis and Functions of Melanin in Sporothrix Schenckii. Rev. Iberoam. Micol. 2000, 68, 3696–3703. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Baca, E.; Leyva-Sánchez, H.; Calderón-Barraza, B.; Esquivel-Naranjo, U.; López-Romero, E.; López-Rodríguez, A.; Cuéllar-Cruz, M. Identification of Proteins in Sporothrixschenckiisensu Stricto in Response to Oxidative Stress Induced by Hydrogen Peroxide. Rev. Iberoam. Micol. 2019, 36, 17–23. [Google Scholar] [CrossRef]
- Zaffer, M.; Ganie, S.A.; Gulia, S.S.; Yadav, S.S.; Singh, R.; Ganguly, S. Antifungal Efficacy of Moringa Oleifera Lam. AJPCT 2015, 3, 28–33. [Google Scholar]
- Chuang, P.; Lee, C.; Chou, J.; Murugan, M.; Shieh, B.; Chen, H. Anti-Fungal Activity of Crude Extracts and Essential Oil of Moringa Oleifera Lam. Bioresour. Technol. 2007, 98, 232–236. [Google Scholar] [CrossRef]
- Sierra-Campos, E.; Valdez-Solana, M.A.; Avitia-Domínguez, C.I.; Téllez-Valencia, A.; Meza-Velázquez, J.A.; Aguilera-Ortiz, M.; Escobar-Ramírez, A. Standarization Based on Chemical Markers of Moringa Oleifera Herbal Products Using Bioautography Assay, Thin Layer Chromatography and High Performance Liquid Chromatography-Diode Array Detector. Malays. J. Anal. Sci. 2020, 24, 449–463. [Google Scholar]
- Aebi, H. Catalase in Vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. ISBN 0076-6879. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Goličnik, M.; Bavec, A. Evaluation of the Paraoxonase-1 Kinetic Parameters of the Lactonase Activity by Nonlinear Fit of Progress Curves. J. Enzym. Inhib. Med. Chem. 2020, 35, 261–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margoliash, E.; Novogrodsky, A.; Schejter, A. Irreversible Reaction of 3-Amino-1:2:4-Triazole and Related Inhibitors with the Protein of Catalase. Biochem. J. 1960, 74, 339–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneidman-Duhovny, D.; Inbar, Y.; Nussinov, R.; Wolfson, H.J. PatchDock and SymmDock: Servers for Rigid and Symmetric Docking. Nucleic Acids Res. 2005, 33, W363-7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Tapia, E.; Nana, R.K.; Querol, A.; Pérez-Torrado, R. Ethanol Cellular Defense Induce Unfolded Protein Response in Yeast. Front. Microbiol. 2016, 7, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadu, T.; Ahmad, K.; Ismail, S.I.; Rashed, O.; Asib, N.; Omar, D. Antifungal Efficacy of Moringa Oleifera Leaf and Seed Extracts against Botrytis Cinerea Causing Gray Mold Disease of Tomato (Solanum lycopersicum L.). Braz. J. Biol. 2021, 81, 1007–1022. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling Protein Tertiary and Quaternary Structure Using Evolutionary Information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.; Lee, K.; Kim, D. Using Reverse Docking for Target Identification and Its Applications for Drug Discovery. Expert Opin. Drug Discov. 2016, 11, 707–715. [Google Scholar] [CrossRef]
- Kochnev, Y.; Hellemann, E.; Cassidy, K.C.; Durrant, J.D. Webina: An Open-Source Library and Web App That Runs AutoDock Vina Entirely in the Web Browser. Bioinformatics 2020, 36, 4513–4515. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Grimm, M.; Dai, W.; Hou, M.; Xiao, Z.-X.; Cao, Y. CB-Dock: A Web Server for Cavity Detection-Guided Protein–Ligand Blind Docking. Acta Pharmacol. Sin. 2020, 41, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Firacative, C. Invasive Fungal Disease in Humans: Are We Aware of the Real Impact? Mem. Inst. Oswaldo Cruz 2020, 115, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mellado, E.; Cuenca-Estrella, M.; Rodríguez-Tudela, J.L. Clinical Relevance of Mechanisms of Antifungal Drug Resistance in Filamentous Fungi. Enferm. Infecc. Microbiol. Clin. 2002, 20, 523–530. [Google Scholar] [CrossRef]
- Orta-Zavalza, E.; Guerrero-Serrano, G.; Gutiérrez-Escobedo, G.; Cañas-Villamar, I.; Juárez-Cepeda, J.; Castaño, I.; De Las Peñas, A. Local Silencing Controls the Oxidative Stress Response and the Multidrug Resistance in Candida Glabrata. Mol. Microbiol. 2013, 88, 1135–1148. [Google Scholar] [CrossRef]
- De Nollin, S.; Van Belle, H.; Goossens, F.; Thone, F.; Borgers, M. Cytochemical and Biochemical Studies of Yeasts After In Vitro Exposure to Miconazole. Antimicrob. Agents Chemother. 1977, 11, 500–513. [Google Scholar] [CrossRef] [Green Version]
- Mesa-Arango, A.C.; del Rocío Reyes-Montes, M.; Pérez-Mejía, A.; Navarro-Barranco, H.; Souza, V.; Zúñiga, G.; Toriello, C. Phenotyping and Genotyping of Sporothrix Schenckii Isolates According to Geographic Origin and Clinical Form of Sporotrichosis. J. Clin. Microbiol. 2002, 40, 3004–3011. [Google Scholar] [CrossRef] [Green Version]
- Minami, P.S.; Oliveira, F. de Atividade Antifúngica de Bidens Pilosa L. Rev. Bras. Farmacogn. 1986, 1, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Waller, S.B.; Madrid, I.M.; de Faria, R.O.; Cleff, M.B.; de Mello, J.R.B.; Meireles, M.C.A. Anti-Sporothrix Spp. Activity of Medicinal Plants. Braz. J. Pharm. Sci. 2016, 52, 221–237. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela-Cota, D.F.; Buitimea-Cantúa, G.V.; Plascencia-Jatomea, M.; Cinco-Moroyoqui, F.J.; Martínez-Higuera, A.A.; Rosas-Burgos, E.C. Inhibition of the Antioxidant Activity of Catalase and Superoxide Dismutase from Fusarium Verticillioides Exposed to a Jacquinia Macrocarpa Antifungal Fraction. J. Environ. Sci. Heal. Part B 2019, 54, 647–654. [Google Scholar] [CrossRef]
- Fouad, E.A.; Abu Elnaga, A.S.M.; Kandil, M.M. Antibacterial Efficacy of Moringa Oleifera Leaf Extract against Pyogenic Bacteria Isolated from a Dromedary Camel (Camelus Dromedarius) Abscess. Vet. World 2019, 12, 802–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, P.; Patel, N.; Patel, D.; Desai, S.; Meshram, D. Phytochemical Analysis and Antifungal Activity of Moringa Oleifera. Int. J. Pharm. Pharm. Sci. 2014, 6, 144–147. [Google Scholar]
- Suraka, B.; Ibrahim, D.; Suleman, K.; Usman, U.; Usman, S.I. Antifungal Activity of Moringa Oleifera Leaves Crude Extract against Aspergillus Flavus and Rhizopus Stolonifer. Dutse J. Pure Appl. Sci. 2022, 7. [Google Scholar] [CrossRef]
- Bennett, R.N.; Mellon, F.A.; Foidl, N.; Pratt, J.H.; Dupont, M.S.; Perkins, L.; Kroon, P.A. Profiling Glucosinolates and Phenolics in Vegetative and Reproductive Tissues of the Multi-Purpose Trees Moringa oleifera L. (Horseradish Tree) and Moringa stenopetala L. J. Agric. Food Chem. 2003, 51, 3546–3553. [Google Scholar] [CrossRef] [PubMed]
- Galuppo, M.; Nicola, G.; Iori, R.; Dell’Utri, P.; Bramanti, P.; Mazzon, E. Antibacterial Activity of Glucomoringin Bioactivated with Myrosinase against Two Important Pathogens Affecting the Health of Long-Term Patients in Hospitals. Molecules 2013, 18, 14340–14348. [Google Scholar] [CrossRef]
- Román-Casiano, K.M.; Martínez-Rocha, A.L.; Romo-Lozano, Y.; López-Rodríguez, A.; Cervantes-García, D.; Sierra-Campos, E.; Cuéllar-Cruz, M.; Ruiz-Baca, E. Enzyme Activity and Expression of Catalases in Response to Oxidative Stress in Sporothrix Schenckii. Microb. Pathog. 2021, 161, 105270. [Google Scholar] [CrossRef]
- Gómez, S.; Navas-Yuste, S.; Payne, A.M.; Rivera, W.; López-Estepa, M.; Brangbour, C.; Fullà, D.; Juanhuix, J.; Fernández, F.J.; Vega, M.C. Peroxisomal Catalases from the Yeasts Pichia Pastoris and Kluyveromyces Lactis as Models for Oxidative Damage in Higher Eukaryotes. Free Radic. Biol. Med. 2019, 141, 279–290. [Google Scholar] [CrossRef]
- Maina, S.; Misinzo, G.; Bakari, G.; Kim, H.-Y. Human, Animal and Plant Health Benefits of Glucosinolates and Strategies for Enhanced Bioactivity: A Systematic Review. Molecules 2020, 25, 3682. [Google Scholar] [CrossRef]






| All-Atom Contacts | Clashscore, All Atoms: | 1 | 99th Percentile * (N = 1784, All Resolutions) | |
|---|---|---|---|---|
| Clashscore is the Number of Serious Steric Overlaps (>0.4 Å) per 1000 Atoms. | ||||
| Protein geometry | Poor rotamers | 11 | 0.63% | Goal: <0.3% |
| Favored rotamers | 1669 | 95.81% | Goal: >98% | |
| Ramachandran outliers | 0 | 0.00% | Goal: <0.05% | |
| Ramachandran favored | 1932 | 96.75% | Goal: >98% | |
| Rama distribution Z-core | 0.08 ± 0.19 | Goal: abs (Z core) < 2 | ||
| MolProbity score∧ | 1 | 100th percentile * (N = 27,675, 0–99 Å) | ||
| Cb deviations > 0.25 Å | 9 | 0.48% | Goal: 0 | |
| Bad bonds: | 0/16,525 | 0.00% | Goal: 0% | |
| Bad angles | 126/22,448 | 0.56% | Goal: <0.1% | |
| Peptide omegas | Cis prolines | 4/140 | 2.86% | Expected: ≤1 per chain, or ≤5% |
| Twisted peptides | 3/2001 | 0.15% | Goal: 0 | |
| Low-resolution criteria | CaBLAM outliers | 41 | 2.1% | Goal: <1.0% |
| CA geometry outliers | 14 | 0.70 | Goal: <0.5% | |
| Additional validations | Chiral volume outliers | 0/2358 | ||
| Waters with clashes | 0/0 | 0.00% | See UnDowser table for details | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra-Campos, E.; Valdez-Solana, M.A.; Ruiz-Baca, E.; Ventura-García, E.K.; Avitia-Domínguez, C.I.; Aguilera-Ortiz, M.; Téllez-Valencia, A. Anti-Sporotrichotic Activity, Lambert-W Inhibition Kinetics and 3D Structural Characterization of Sporothrix schenckii Catalase as Target of Glucosinolates from Moringa oleifera. Sci. Pharm. 2022, 90, 70. https://doi.org/10.3390/scipharm90040070
Sierra-Campos E, Valdez-Solana MA, Ruiz-Baca E, Ventura-García EK, Avitia-Domínguez CI, Aguilera-Ortiz M, Téllez-Valencia A. Anti-Sporotrichotic Activity, Lambert-W Inhibition Kinetics and 3D Structural Characterization of Sporothrix schenckii Catalase as Target of Glucosinolates from Moringa oleifera. Scientia Pharmaceutica. 2022; 90(4):70. https://doi.org/10.3390/scipharm90040070
Chicago/Turabian StyleSierra-Campos, Erick, Mónica A. Valdez-Solana, Estela Ruiz-Baca, Erica K. Ventura-García, Claudia I. Avitia-Domínguez, Miguel Aguilera-Ortiz, and Alfredo Téllez-Valencia. 2022. "Anti-Sporotrichotic Activity, Lambert-W Inhibition Kinetics and 3D Structural Characterization of Sporothrix schenckii Catalase as Target of Glucosinolates from Moringa oleifera" Scientia Pharmaceutica 90, no. 4: 70. https://doi.org/10.3390/scipharm90040070
APA StyleSierra-Campos, E., Valdez-Solana, M. A., Ruiz-Baca, E., Ventura-García, E. K., Avitia-Domínguez, C. I., Aguilera-Ortiz, M., & Téllez-Valencia, A. (2022). Anti-Sporotrichotic Activity, Lambert-W Inhibition Kinetics and 3D Structural Characterization of Sporothrix schenckii Catalase as Target of Glucosinolates from Moringa oleifera. Scientia Pharmaceutica, 90(4), 70. https://doi.org/10.3390/scipharm90040070






