Medicinal Chemistry of Quinazolines as Anticancer Agents Targeting Tyrosine Kinases
Abstract
:1. Introduction
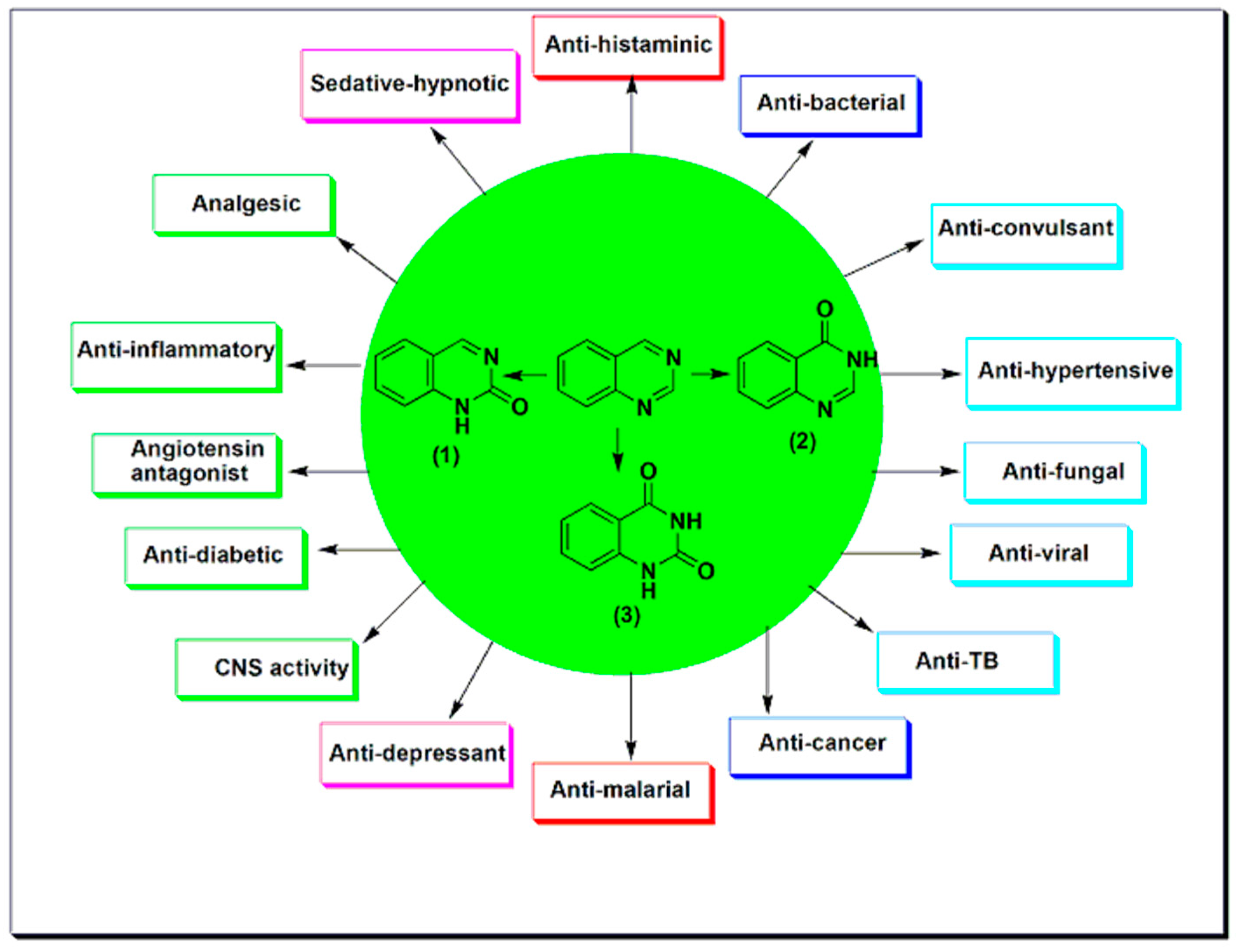
2. Therapeutic Importance of Quinazolines
3. Physicochemical Characters of the Core Structural Feature of Anticancer Quinazolines
4. Methods of Preparation of Quinazolines
- Transition metals-catalyzed method. The nitrobenzamide derivative is reduced by palladium chloride (PdCl2) and iron pentacarbonyl Fe(CO)5 in presence of iodobenzene to give 2-phenyl-4(3H)-quinazolinone. This reaction is performed by a microwave-assisted reaction at 110 °C for 0.5 h with 85% yield (Figure 9) [36].
5. Mode of Action of Quinazolines as Anticancer Agents
5.1. Crystallographic Studies of Quinazolines
5.2. Protein Kinases Inhibitors
- Tyrosine kinases responsible for phosphorylation of phenolic hydroxyl (OH) group.
- Serine-threonine kinases responsible for phosphorylation of serine and threonine amino acids.
- Histidine-kinases responsible for phosphorylation of nitrogen in histidine residues.
- Epidermal growth factor receptor (EGFR);
- Platelet derived growth factor receptor (PDGFR);
- Vascular endothelial growth factor receptor (VEGFR);
- Fibroblast growth factor receptor (FGFR).
5.2.1. Epidermal Growth Factor Receptor (EGFR) Inhibitors
- Tyrosine kinase inhibitors molecules which act as a competitive inhibitor on EGFR;
- Monoclonal antibodies which interfere with the binding of EGF and TGF-α.
6,7-Substituted-4-anilinoquiazolines as Anticancer Agents
6-Substituted-4-anilinoquinazoline Derivatives
Structural activity relationship studies (SAR)
- The 4-anilinoquinazoline with substitution at the C-6 and/or the C-7 positions is the general pharmacophoric group required for the EGFR inhibitory activity. These structural requirements are shown by the common tyrosine-kinase inhibitors such as gefitinib, erlotinib, and other anticancer pharmaceutically marketed products.
- The electron-withdrawing groups such as fluoro, bromo, chloro, and ethylene at the aniline ring is advantageous for the antiproliferative activity.
- The 3-bromo substituted quinazoline molecules displayed potent activity.
- The 3-chloro-4-fluoro-aniline substituted quinazoline molecules showed strong activity.
- Changing the aniline moiety at the 4-position with other groups decreased the activity.
- The electron donating groups at the 6 and/or the 7-positions improved the binding activity of N1 and N3 of quinazoline system with the binding pocket.
- The propoxy linker at the C-6 and/or the C-7 of quinazoline moiety showed stronger activity than the methoxy group.
- Dioxygenated groups at the 6 and the 7 positions of quinazoline moiety improved the cytotoxic activity.
- The Michael addition group at 6-position of quinazoline leads to irreversible binding with the receptor-site.
5.2.2. Vascular Endothelial Growth Factor Receptors (VEGFR) Inhibitors
SAR of VEGFR Inhibitors
- Quinazolin-4-aniloino or quinazoline-4-oxyaryl scaffold is required for VEGFR inhibitory activity.
- Substitution at the 6-position of the quinazoline moiety with an electron-releasing group enhances the activity.
- Substitution at the 7-position of the quinazoline with an aminoalkoxy group increases the activity.
- Aromatic spacer between urea or thiourea and N or O at the 4-position of quinazoline is necessary for the activity.
5.2.3. PDGFR Inhibitors
5.2.4. Serine-Threonine Kinase Inhibitors
- Serine-threonine receptor type kinase (TGFBR).
- Serine-threonine non-receptor type kinas (aurora kinases, CDK, and PI3K).
Aurora Kinase Inhibitors
SAR of Aurora Kinase Inhibitors
- Quinazoline with an aminoalkyl or an aminoaryl moiety at the 4-position of the quinazoline is required for the anticancer activity.
- Substitution at the 5 and the 6-positions of the quinazoline with an electron releasing group increases the activity.
- A lipophilic aromatic group attached to the 4-aminoaryl group increases the activity.
Cyclin-Dependent Kinase (CDK) Inhibitors
Phosphoinositid-3-Kinase (PI3K) Inhibitors
6. Pharmaceutical Marketed Anticancer Quinazolines
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zayed, M.F.; Ahmed, H.E.A.; Ihmaid, S.K.; Omar, A.M.; Abdelrahim, A.S. Synthesis and screening of some new fluorinated quinazolinone–sulphonamide hybrids as anticancer agents. J. Taibah Univ. Sci. 2015, 10, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Bansal, R.; Malhotra, A. Therapeutic progression of quinazolines as targeted chemotherapeutic agents. Eur. J. Med. Chem. 2021, 211, 113016. [Google Scholar] [CrossRef]
- Zayed, M.F.; Rateb, H.; Ahmed, S.; Khaled, O.; Ibrahim, S. Quinazolinone-amino acid hybrids as Dual Inhibitors of EGFR Kinase and Tubulin Polymerization. Molecules 2018, 23, 1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Ali, S.A.; Arshia; Ishtiaq, M.; Khan, K.M. Quinazoline and quinazolinone as important medicinal scaffolds: A comparative patent review (2011–2016). Expert Opin. Ther. Pat. 2018, 28, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.F.; Ahmed, S.; Ihmaid, S.; Ahmed, H.E.; Rateb, H.; Ibrahim, S. Design, Synthesis, Cytotoxic Evaluation and Molecular Docking of New Fluoroquinazolinones as Potent Anticancer Agents with Dual EGFR Kinase and Tubulin Polymerization Inhibitory Effects. Int. J. Mol. Sci. 2018, 19, 1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zayed, M.F.; Ahmed, H.E.A.; Ihmaid, S.; El-Adl, K.; Asiri, A.; Omar, A.M. Modelling and Anticonvulsant Studies of New Quinazolines Showing Three Highly Active Compounds with Low Toxicity and High Affinity to the GABA-A Receptor. Molecules 2017, 22, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zayed, M.F.; Hassan, M.H. Design, Synthesis and Biological Evaluation Studies of Novel Quinazoline Derivatives as Cytotoxic Agents. Drug Res. 2013, 63, 210–215. [Google Scholar] [CrossRef]
- Barreca, M.; Spanò, V.; Rocca, R.; Bivacqua, R.; Abel, A.; Maruca, A.; Montalbano, A.; Raimondi, V.M.; Tarantelli, C.; Gaudio, E.; et al. Development of [1,2]oxazoloisoindoles tubulin polymerization inhibitors: Further chemical modifications and potential therapeutic effects against lymphomas. Eur. J. Med. Chem. 2022, 243, 114744. [Google Scholar] [CrossRef]
- Barreca, M.; Ingarra, M.A.; Raimondi, V.M.; Spanò, V.; Piccionello, P.A.; Franco, D.M.; Menilli, L.; Gandin, V.; Miolo, G.; Barraja, P.; et al. New tricyclic systems as photosensitizers towards triple negative breast cancer cells. Arch. Pharm. Res. 2022, 45, 806–821. [Google Scholar] [CrossRef]
- Duró, C.; Jernei, T.; Szekeres, K.J.; Láng, G.G.; Oláh-Szabó, R.; Bősze, S.; Szabó, I.; Hudecz, F.; Csámpai, A. Synthesis and SAR Analysis of Novel 4-Hydroxytamoxifen Analogues Based on Their Cytotoxic Activity and Electron-Donor Character. Molecules 2022, 27, 6758. [Google Scholar] [CrossRef]
- Fan, C.; Zhong, T.; Yang, H.; Yang, Y.; Wang, D.; Yang, X.; Xu, Y.; Fan, Y. Design, synthesis, biological evaluation of 6-(2-amino-1H-benzo[d]imidazole-6-yl)quinazolin-4(3H)-one derivatives as novel anticancer agents with Aurora kinase inhibition. Eur. J. Med. Chem. 2020, 190, 112108. [Google Scholar] [CrossRef]
- Shagufta; Ahmad, I. An insight into the therapeutic potential of quinazoline derivatives as anticancer agents. Med. Chem. Commun. 2017, 8, 871–885. [Google Scholar] [CrossRef]
- Haider, K.; Das, S.; Joseph, A.; Yar, M.S. An appraisal of anticancer activity with structure-activity relationship of quinazoline and quinazolinone analogues through EGFR and VEGFR inhibition: A review. Drug Dev. Res. 2022, 83, 859–890. [Google Scholar] [CrossRef] [PubMed]
- Auti, P.S.; George, G.; Paul, A.T. Recent advances in the pharmacological diversification of quinazoline/quinazolinone hybrids. RSC Adv. 2020, 10, 41353–41392. [Google Scholar] [CrossRef]
- Sunil Kumar, A.; Kudva, J.; Lahtinen, M.; Peuronen, A.; Sadashiva, R.; Naral, D. Synthesis, characterization, crystal structures and biological screening of 4-amino quinazoline sulfonamide derivatives. J. Mol. Struct. 2019, 1190, 29–36. [Google Scholar] [CrossRef]
- El-Zahabia, M.A.; Bamanie, H.F.; Ghareeb, S.; Alshaeri, K.H.; Alasmari, M.M.; Muostafa, M.; Al-Marzoki, Z.; Zayed, M.F. Design, Synthesis, Molecular Modeling andAnti-Hyperglycemic Evaluation of Quinazoline-Sulfonylurea Hybrids as Peroxisome Proliferator-Activated Receptor Gamma (PPAR) and Sulfonylurea Receptor (SUR) Agonists. Int. J. Mol. Sci. 2022, 23, 9605. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.F.; Ibrahim, S.; Habib, E.E.; Hassan, M.H.; Ahmed, S.; Rateb, H.S. Design, synthesis, antimicrobial and anti-biofilm evaluation, and molecular docking of new substituted fluoroquinazolinones. J. Med. Chem. 2019, 15, 657–673. [Google Scholar]
- Zhang, J.; Liu, J.; Ma, Y.; Ren, D.; Cheng, P.; Zhao, J.; Zhang, F.; Yao, Y. One-pot synthesis and antifungal activity against plant pathogens of quinazolinone derivatives containing an amide moiety. Bioorg. Med. Chem. Lett. 2016, 26, 2273–2277. [Google Scholar] [CrossRef] [PubMed]
- Santos-Ballardo, L.; García-Páez, F.; Picos-Corrales, L.A.; Ochoa-Terán, A.; Bastidas, P.; Calderón-Zamora, L.; Rendón-Maldonado, G.; Osuna-Martínez, U.; Sarmiento-Sánchez, J.I. Synthesis, biological evaluation and molecular docking of 3-substituted quinazoline-2,4(1H, 3H)-diones. J. Chem. Sci. 2020, 132, 100. [Google Scholar] [CrossRef]
- Jain, R.K.; Kashaw, V. Design, synthesis and evaluation of novel 2,3-disubstituted-4-(3H) quinazolinone derivatives. Asian J. Pharm. Pharmacol. 2018, 4, 644–656. [Google Scholar] [CrossRef]
- Hricoviniova, J.; Hricoviniova, Z.; Kozica, K. Antioxidant, Cytotoxic, Genotoxic, and DNA Protective Potential of 2,3-Substituted Quinazolinones: Structure—Activity Relationship Study. Int. J. Mol. Sci. 2021, 22, 610. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.F. New fluorinated quinazolinone derivatives as anticonvulsant agents. J. Taibah Univ. Sci. 2014, 9, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Ghorab, M.M.; Abdel-Kader, M.S.; Alqahtani, A.S.; Soliman, A.M. Synthesis of some quinazolinones inspired from the natural alkaloid L-norephedrine as EGFR inhibitors and radiosensitizers. J. Enzym. Inhib. Med. Chem. 2021, 36, 218–237. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE) Chemical Computing Group. Available online: http://www.chemcomp.com (accessed on 10 May 2019).
- Zayed, M.F.; Hassan, M.H. Synthesis and biological evaluation studies of novel quinazolinone derivatives as antibacterial and anti-inflammatory agents. Saudi Pharm. J. 2014, 22, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Swiss Institute of Bioinformatics (SwissADME). Available online: http://www.swissADME.ch (accessed on 10 November 2022).
- Zayed, M.F.; Ahmed, E.A.; Omar, A.M.; Abdelrahim, A.S.; El-Adl, K. Design, synthesis, and biological evaluation studies of novel quinazolinone derivatives as anticonvulsant agents. Med. Chem. Res. 2013, 22, 5823–5831. [Google Scholar] [CrossRef]
- Wang, D.; Gao, F. Quinazoline derivatives: Synthesis and bioactivities. Chem. Cent. J. 2013, 7, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connolly, D.J.; Cusack, D.; O’Sullivan, T.P.; Guiry, P.J. Synthesis of quinazolinones and quinazolines. Tetrahedron 2005, 61, 10153–10202. [Google Scholar] [CrossRef]
- Meyer, J.F.; Wagner, E.C. The Niementowski reaction. The use of methyl anthranilate or isatoic anhydride with substituted amides or amidines in the formation of 3-substituted-4-keto-3,4-dihydroquinazolines. The course of the reaction. J. Org. Chem. 1943, 8, 239–252. [Google Scholar] [CrossRef]
- Asif, M. Chemical Characteristics, Synthetic Methods, and Biological Potential of Quinazoline and Quinazolinone Derivatives. Int. J. Med. Chem. 2014, 2014, 395637. [Google Scholar] [CrossRef]
- Gupta, T.; Rohilla, A.; Pathak, A.; Akhtar, M.J.; Haider, M.R.; Yar, M.S. Current perspectives on quinazolines with potent biological activities: A review. Synth. Commun. 2018, 48, 1099–1127. [Google Scholar] [CrossRef]
- Bisht, A.S.; Negi, J.S.; Sharma, D.K. Chemistry and activity of quinazoline moiety: A systematic review study. Int. J. Pharm. Chem. Anal. 2020, 7, 61–65. [Google Scholar] [CrossRef]
- Thakur, A.; Tawa, G.J.; Henderson, M.J.; Danchik, C.; Liu, S.; Shah, P.; Wang, A.Q.; Dunn, G.; Kabir, M.; Padilha, E.C.; et al. Design, Synthesis, and Biological Evaluation of Quinazolin-4-one-Based Hydroxamic Acids as Dual PI3K/HDAC Inhibitors. J. Med. Chem. 2020, 63, 4256–4292. [Google Scholar] [CrossRef] [PubMed]
- Arachchige, K.T.P.; Yi, S.C. Synthesis of Quinazoline and Quinazolinone Derivatives via Ligand-Promoted Ruthenium-Catalyzed Dehydrogenative and Deaminative Coupling Reaction of 2-Aminophenyl Ketones and 2-Aminobenzamides with Amines. Org. Lett. 2019, 21, 3337–3341. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.M.; Sivaramakrishna, A. Remarkably flexible quinazolinones—Synthesis and biological applications. J. Heterocycl. Chem. 2019, 57, 942–954. [Google Scholar] [CrossRef]
- Zayed, M.F. Medicinal Chemistry of Quinazolines as Analgesic and Anti-Inflammatory Agents. ChemEngineering 2022, 6, 94. [Google Scholar] [CrossRef]
- Chilin, A.; Conconi, M.T.; Marzaro, G.; Guiotto, A.; Urbani, L.; Tonus, F.; Parnigotto, P. Exploring epidermal growth factor receptor (EGFR) inhibitor features: The role of fused dioxygenated rings on the quinazoline scaffold. J. Med. Chem. 2010, 53, 1862–1866. [Google Scholar] [CrossRef]
- Conconi, M.T.; Marzaro, G.; Urbani, L.; Zanusso, I.; Di, L.R.; Castagliuolo, I.; Brun, P.; Tonus, F.; Ferrarese, A.; Guiotto, A.; et al. Quinazoline-based multi-tyrosine kinase inhibitors: Synthesis, modeling, antitumor and antiangiogenic properties. Eur. J. Med. Chem. 2013, 67, 373–383. [Google Scholar] [CrossRef]
- Kumar, D.; Mariappan, G.; Husain, A.; Monga, J.; Kumar, S. Design, synthesis and cytotoxic evaluation of novel imidazolone fused quinazolinone derivatives. Arab. J. Chem. 2017, 10, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, E.C.; Patel, B.S.; Becker, W.J.; Cameron, M.P.; Zaller, D.; Pikounis, B.V.; O’Keefe, J.S.; Scapin, G. Structural basis for p38α MAP kinase quinazolinone and pyridol-pyrimidine inhibitor specificity. Nat. Struct. Mol. Biol. 2003, 10, 764–769. [Google Scholar] [CrossRef]
- Abouzid, K.; Shouman, S. Design, synthesis and in vitro antitumor activity of 4-aminoquinoline and 4-aminoquinazoline derivatives targeting EGFR tyrosine kinase. Bioorg. Med. Chem. 2008, 16, 7543–7551. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zheng, W.; Ji, L.; Luo, Q.; Hao, X.; Li, X.; Wang, F. Synthesis, characterization, screening and docking analysis of 4-anilinoquinazoline derivatives as tyrosine kinase inhibitors. Eur. J. Med. Chem. 2013, 61, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Peng, T.; Ji, X.; Li, J.; Tong, L.; Li, Z.; Yang, W.; Xu, Y.; Li, M.; Ding, J. Design, synthesis and biological evaluation of novel 4-anilinoquinazolines with C-6 urea-linked side chains as inhibitors of the epidermal growth factor receptor. Bioorg. Med. Chem. 2013, 21, 7988–7998. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Yuan, Y.; Qiu, N.; Peng, P.; Sheng, R.; Hu, Y. Identification of novel 4-anilinoquinazoline derivatives as potent EGFR inhibitors both under normoxia and hypoxia. Bioorg. Med. Chem. 2014, 22, 6796–6805. [Google Scholar] [CrossRef]
- Yu, H.; Li, Y.; Ge, Y.; Song, Z.; Wang, C.; Huang, S.; Jin, Y.; Han, X.; Zhen, Y.; Liu, K.; et al. Novel 4-anilinoquinazoline derivatives featuring an 1-adamantyl moiety as potent EGFR inhibitors with enhanced activity against NSCLC cell lines. Eur. J. Med. Chem. 2016, 110, 195–203. [Google Scholar] [CrossRef]
- Wu, X.; Li, M.; Qu, Y.; Tang, W.; Zheng, Y.; Lian, J.; Ji, M.; Xu, L. Design and synthesis of novel gefitinib analogues with improved anti-tumor activity. Bioorg. Med. Chem. 2010, 18, 3812–3822. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Lin, Z.; Wang, F.; Zhao, W.; Dong, X. Four-membered heterocycles-containing 4-anilino-quinazoline derivative s as epidermal growth factor receptor (EGFR) kinase inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 5385–5388. [Google Scholar] [CrossRef]
- Chang, J.; Ren, H.; Zho, M.; Chong, Y.; Zhao, Y.; He, Y.; Zhao, Y.; Zhang, H.; Qi, C. Development of a se ies of novel 4-anlinoquinazoline derivatives possessing quinazoline skeleton: Design, synthesis, EGFR kinase inhibitory efficacy, and evaluation f anticancer activities in vitro. Eur. J. Med. Chem. 2017, 138, 669–688. [Google Scholar] [CrossRef]
- Cai, J.; Sun, M.; Wu, X.; Chen, P.; Wang, X.; Zong, M.J. Design and synthesis of novel 4-benzothiazole amino quinazolines dasatinib derivatives as potential anti-tumor agents. Eur. J. Med. Chem. 2013, 63, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, Y.; Zhou, H.; Zheng, Q.; Li, Y.; Zheng, S.; Zhao, S.; Chen, D.; Fan, C. Structure-activity study of quinazoline derivatives leading to the discovery of potent EGFR-T790M inhibitors. Eur. J. Med. Chem. 2015, 102, 445–463. [Google Scholar] [CrossRef]
- Hou, W.; Ren, Y.; Zhang, Z.; Sun, H.; Ma, Y.; Yan, B. Novel quinazoline derivatives bearing various 6-benzamide moieties as highly selective and potent EGFR inhibitors. Bioorg. Med. Chem. 2018, 26, 1740–1750. [Google Scholar] [CrossRef]
- Zhang, Y.; Tortorella, M.D.; Liao, J.; Qin, X.; Chen, T.; Luo, J.; Guan, J.; Talley, J.J.; Tu, Z. Synthesis and evaluation of novel erlotinib-NSAID conjugates as more comprehensive anticancer agents. ACS Med. Chem. Lett. 2015, 6, 1086–1090. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Lv, Y.; Liu, P.; Li, Z.; Hu, L.; Zeng, C.; Yang, L. Novel morpholin-3-one fused quinazoline derivatives as EGFR tyrosine kinase inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 1571–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Zhang, Y.; Liu, J.; Wang, W.; Li, X.; Zhao, L.; Wang, W.; Li, B. Novel 4-arylaminoquinazoline derivatives with (E)-propen-1-yl moiety as potent EGFR inhibitors with enhanced antiproliferative activities against tumor cells. Eur. J. Med. Chem. 2017, 138, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, L.; Xu, H.; Li, X.; Zhao, L.; Wang, W.; Li, B.; Zhang, X. 6,7-Dimorpholinoalkoxy quinazoline derivatives as potent EGFR inhibitors with enhanced antiproliferative activities against tumor cells. Eur. J. Med. Chem. 2017, 147, 77–89. [Google Scholar] [CrossRef]
- Liu, L.T.; Yuan, T.T.; Liu, H.H.; Chen, S.F.; Wu, Y.T. Synthesis and biological evaluationJournalofsubstituted6-kynyl -4-anilinoquinazoline derivatives as potent EGFR inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 6373–6377. [Google Scholar] [CrossRef]
- Li, D.D.; Fang, F.; Li, R.; Du, Q.R.; Sun, J.; Gong, H.B.; Zhu, H.L. Discovery of 6-substituted 4-anilin qinazolines with dioxygenated rings as novel EGFR tyrosine kinase inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 5870–5875. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Q.; Li, D.D.; Lu, X.; Xu, Y.Y.; Zhu, H.L. Design and synthesis of 4,6-substituted-(diphenylamino)quinazolines as potent EGFR inhibitors with antitumor activity. Bioorg. Med. Chem. 2012, 20, 317–323. [Google Scholar] [CrossRef]
- Li, D.D.; Lv, P.C.; Zhang, H.; Zhang, H.J.; Hou, Y.P.; Liu, K.; Ye, Y.H.; Zhu, H.L. The combination of 4-anilinoquinazoline and cinnamic acid: A novel mode of binding to the epidermal growth factor receptor tyrosine kinase. Bioorg. Med. Chem. 2011, 19, 5012–5022. [Google Scholar] [CrossRef]
- Mowafy, S.; Farag, N.A.; Abouzid, K.A.M. Design, synthesis and in vitro anti-proliferative activity of 4,6-quinazolinediamines as potent EGFR-TK inhibitors. Eur. J. Med. Chem. 2013, 61, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Chen, L.; Zhao, L.; Li, B.; Wang, W. Synthesis and in vitro biological evaluation of novel quinazoline derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 1584–1587. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Hong, J. Recent advancements of 4-aminoquinazoline derivatives as kinase inhibitors and their applications in medicinal chemistry. Eur. J. Med. Chem. 2019, 170, 55–72. [Google Scholar] [CrossRef]
- Musumeci, F.; Radi, M.; Brullo, C.; Schenone, S. Vascular endothelial growth factor (VEGF) receptors: Drugs and new inhibitors. J. Med. Chem. 2012, 55, 10797–10822. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P. The role of hypoxia-induced factorsproofintumorprogression. Oncologist 2004, 9, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Manley, P.W.; Bold, G.; Bruggen, J.; Fendrich, G.; Fuet, P.; Mestan, J.; Schnell, C.; Stolz, B.; Meyer, T.B.; Meyhack, B.; et al. Advances in the structural biology, design and clinical d v lopmentVEGF-R kinase inhibitors for the treatment of angiogenesis. Biochim. Biophys. Acta 2004, 1697, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Castellana, M.; Virili, C.; Giorgino, F.; Giovanella, L. Efficacy of vandetanib in treating locally advanced or metastatic medullary thyroid carcinoma according to RECIST criteri: A systematic review and meta-analysis. Front. Endocrinol. 2018, 9, 224. [Google Scholar] [CrossRef]
- Batchelr, T.T.; Lholland, P.M.; Neyns, B.; Nabors, L.B.; Campone, M.; Wick, A.; Mason, W.; Mikkelsen, T.; Phuphanich, S.; Ashby, L.S.; et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J. Clin. Oncol. 2013, 31, 3212–3218. [Google Scholar] [CrossRef] [Green Version]
- Garofalo, A.; Goossens, L.; Six, P.; Lemoine, A.; Ravez, S.; Farce, A.; Depreux, P. Impact of aryloxy-linked quinazolines: A novel series of selective VEGFR-2 receptor tyrosine kinase inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 2106–2112. [Google Scholar] [CrossRef]
- Garofalo, A.; Farce, A.; Ravez, S.; Lemoine, A.; Six, P.; Chavatte, P.; Goossens, L.; Depreux, P. Synthesis and structure-activity relationships of (aryloxy)quinazoline ureas as novel, potent, and selective vascular endothelial growth factor receptor-2 inhibitors. J. Med. Chem. 2012, 55, 1189–1204. [Google Scholar] [CrossRef]
- Ravez, S.; Barczyk, A.; Six, P.; Cagnon, A.; Garofalo, A.; Goossens, L.; Depreux, P. Inhibition of tumor cell growth and angiogenesis by 7-aminoalkoxy-4-aryloxy-quinazoline ureas, a novel series of multi-tyrosine kinase inhibitors. Eur. J. Med. Chem. 2014, 79, 369–381. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, J.; Wang, N.; Kong, X.; Fu, F.; Wang, H.; Yao, J. Design and discovery of quinazoline- and thiourea-containing sorafenib analogs as EGFR and VEGFR-2 dual TK inhibitors. Molecules 2018, 23, 24. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, M.L.; Lima, L.M.; Tesch, R.; Sant’Anna, C.M.; Totzke, F.; Kubbutat, M.H.; Schächtele, C.; Laufer, S.A.; Barreiro, E.J. Novel 2-chloro-4-anilino-quinazoline derivatives as EGFR and VEGFR-2 dual inhibitors. Eur. J. Med. Chem. 2014, 71, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Gong, F.H.; Li, C.G.; Zhang, C.; Wang, Y.J.; Xu, Y.G.; Sun, L.P. Design and discovery of 4-anilinoquinazoline-acylamino derivatives as EGFR and VEGFR-2 dual TK inhibitors. Eur. J. Med. Chem. 2016, 109, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Gong, F.H.; Ye, J.Q.; Zhang, C.; Yue, X.H.; Li, C.G.; Xu, Y.G.; Sun, L.P. Design an discovery of 4-anilinoquinazoline-urea derivatives as dual TK inhibitors of EGFR and VEGFR-2. Eur. Med. Chem. 2017, 125, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Zhang, J.Q.; Liu, Z.C.; Zhang, J.H.; Yan, J.F.; Jin, Y.; Lin, J. Novel 5-anilinoquinaz line-8-nitro derivatives as inhibitors of VEGFR-2 tyrosine kinase: Synthesis, biological evaluation and molecular docking. Org. Biomol. Chem. 2013, 11, 4367–4378. [Google Scholar] [CrossRef]
- Elsayed, N.M.Y.; Serya, R.A.T.; Tolba, M.F.; Ahmed, M.; Barakat, K.; Abou El Ella, D.A.; Abouzid, K.A.M. Design, synthesis, biological evaluation and dynamics simulation of indazole derivatives with antiangiogenic and antiproliferative anticancer activity. Bioorg. Chem. 2019, 82, 340–359. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 11, 1276–1312. [Google Scholar] [CrossRef] [Green Version]
- Shepard, D.R.; Cooney, M.M.; Elson, P.; Bukowski, R.M.; Dreicer, R.; Rini, B.J.; Garcia, J.A. A phase II study of tandutinib (MLN518), a selective inhibitor of type III tyrosine receptor kinases, in patients with metastaticrenal cell carcinoma. Invest. New Drugs 2012, 30, 364–367. [Google Scholar] [CrossRef]
- Blume-Jensen, P.; Hunter, T. Oncogenic kinase signaling. Nature 2001, 411, 355–365. [Google Scholar] [CrossRef]
- Wu, P.; Nielsen, T.E.; Clausen, M.H. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol. Sci. 2015, 36, 422–439. [Google Scholar] [CrossRef] [Green Version]
- Andrews, P.D. Aurora kinases: Shining lights on the therapeutic horizon. Oncogene 2005, 24, 5005–5015. [Google Scholar] [CrossRef] [Green Version]
- Borisa, A.C.; Bhatt, H.G. A comprehensive review on Aurora kinase: Small molecule inhibitors and clinical trial studies. Eur. J. Med. Chem. 2017, 140, 1–19. [Google Scholar] [CrossRef]
- Gadea, B.B.; Ruderman, J.V. Aurora kinase inhibit ZM447439 blocks chromosome-induced spindle assembly, the completion of ch m s me condensation, and the establishment of the spindle integrity check oint in Xenopus egg extracts. Mol. Biol. Cell. 2005, 16, 1305–1318. [Google Scholar] [CrossRef] [Green Version]
- Heron, N.M.; Anderson, M.; Blows, D.P.; Bred, J.; Eden, J.M.; Green, S.; Hill, G.B.; Johnson, T.; Jung, F.H.; McMiken, H.H.J.; et al. SAR and inhibitor complex structure determination of a novel class of potent specific Aurora kinase inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 1320–1323. [Google Scholar] [CrossRef]
- Jung, F.H.; Pasqet, G.; Brempt, C.L.; Lohmann, J.J.M.; Warin, N.; Renaud, F.; Germain, H.; De Savi, C.; Roberts, N.; Johnston, T.; et al. Discovery of novel and potent thiazoloquinazolines as selective aurora A and B kinase inhibitors. J. Med. Chem. 2006, 49, 955–970. [Google Scholar] [CrossRef] [PubMed]
- Grundy, M.; Seedhouse, C.; Shang, S.; Richardson, J.; Russell, N.; Pallis, M. The FLT3 internal tandem duplication mutation is a secondary target of the aurora B kinase inhibitor AZD1152-HQPA in acute myelogenous leukemia cells. Mol. Cancer Ther. 2010, 9, 661–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortlock, A.A.; Foote, K.M.; Heron, N.M.; Jung, F.H.; Pasquet, G.; Lohmann, J.J.M.; Warin, N.; Renaud, F.; De Savi, C.; Roberts, N.J.; et al. Discovery, synthesis and in vivo activity of a new class of pyrazoloquinazolines as selective inhibitors of aurora B kinase. J. Med. Chem. 2007, 50, 2213–2324. [Google Scholar] [CrossRef] [PubMed]
- Foote, K.M.; Mortlock, A.A.; Heron, N.M.; Jung, F.H.; Hill, G.B.; Pasquet, G.; Brady, M.C.; Green, S.; Heaton, S.P.; Kearney, S.; et al. Synthesis and SAR of 1-acetanilide-4-aminopyrazole-substituted quinazolines: Selective inhibitors of aurora B kinase with potent anti-tumor activity. Bioorg. Med. Chem. Lett. 2008, 18, 1904–1909. [Google Scholar] [CrossRef]
- Long, L.; Wang, Y.H.; Zhuo, J.X.; Tu, Z.C.; Wu, R.; Yan, M.; Liu, Q.; Lu, G. Structure-based drug design: Synthesis and biological evaluation of quinazolin-4-amine derivatives as selective aurora A kinase inhibitors. Eur. J. Med. Chem. 2018, 157, 1361–1375. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Li, L.; Hong, K.H.; Wu, X.; Chen, J.; Wang, P.; Cao, M.; Zong, X.; Ji, M. Discovery of 4-aminoquinazoline-u rea derivatives as aurora kinase inhibitors with antiproliferative activity. Bioorg. Med. Chem. 2014, 22, 5813–5823. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Coumar, M.S.; Wang, W.C.; Shiao, H.Y.; Ke, Y.Y.; Lin, W.H.; Kuo, C.C.; Chang, C.W.; Kuo, F.M.; Chen, P.Y.; et al. Discovery of BPR1K871, a quinazoline based, multi-kinase inhibit f r the treatment of AML and solid tumors: Rational design, synthesis, in vitro and in vivo evaluation. Oncotarget 2016, 7, 86239–86256. [Google Scholar]
- Malumbres, M.; Barbacid, M.; Mammalian, M. Cyclin-dependent kinases. Trends Biochem. Sci. 2005, 30, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Besson, A.; Dowdy, S.F.; Roberts, J.M. CDK inhibitors: Cycle regulators and beyond. Dev. Cell. 2008, 14, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Martínez, C.; Gelbert, L.M.; Lallena, M.J.; Dios, A.D. Cyclin dependent kinase (CDK) inhibitors as anticancer drugs. Bioorg. Med. Chem. Lett. 2015, 25, 3420–3435. [Google Scholar] [CrossRef]
- Shewchuk, L.; Hassell, A.; Wisely, B.; Rocque, W.; Holmes, W.; Veal, J.; Kuyper, L.F. Binding mode of the 4-anilinoquinazoline class of protein kinase inhibitor: X-ray crystallographic studies of 4-anilinoquinazolines bound to cyclin-dependent kinase 2 and p38 kinase. J. Med. Chem. 2000, 43, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Sielecki, T.M.; Johnson, T.L.; Liu, J.; Muckelbauer, J.K.; Grafstrom, R.H.; Cox, S.; Boylan, J.; Burton, C.R.; Chen, H.; Smallwood, A.; et al. Quinazolines as cyclin dependent kinase inhibitors. Bioorg. Med. Chem. Lett. 2001, 11, 1157–1160. [Google Scholar] [CrossRef]
- Bathini, Y.; Singh, I.; Harvey, P.J.; Keller, P.R.; Singh, R.; Micetich, R.G.; Fry, D.W.; Dobrusin, E.M.; Toogood, P.L. 2-Aminoquinazoline inhibitors of cyclin-dependent kinases. Bioorg. Med. Chem. Lett. 2005, 15, 3881–3885. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Wang, S.T.; Sun, P.H.; Song, F.J. Molecular modeling studies of 4,5-dihydro-1H-pyrazolo[4,3-h]quinazoline derivatives as otent CDK2/cyclin A inhibitors using 3D-QSAR and docking. Int. J. Mol. Sci. 2010, 11, 3705–3724. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Li, Y.; Ren, X.; Zhang, Y.; Yang, Z.; Qi, C. A novel quinazoline-based analog induces G2/M cell cycle arrest and apoptosis in human A549 lung cancer cells via a ROS-dependentJournalmechanism. Biochem. Biophys. Res. Commun. 2017, 486, 314–320. [Google Scholar] [CrossRef]
- Marone, R.; Cmiljaovic, V.; Giese, B.; Wymann, M.P. Targeting phosphoinositide 3-kinase: Moving towards therapy. Biochim. Biophys. Acta 2008, 1784, 159–185. [Google Scholar] [CrossRef]
- Markham, A. Idelalisib: First global approval. Drugs 2014, 74, 1701–1707. [Google Scholar] [CrossRef]
- Dreyling, M.; Santoro, A.; Mollica, L.; Leppa, S.; Follows, G.A.; Lenz, G.; Kim, W.S.; Nagler, A.; Panayiotidis, P.; Demeter, P.; et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J. Clin. Oncol. 2017, 35, 3898–3905. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Wang, J.; Chen, J.G.; Wang, X.M.; Li, H.; Li, Y.P.; Li, Y.; Yang, G.D.; Mei, Q.B.; Zhang, S.Q. Discovery of 2-methoxy-3-phenylsulfonamido-5-(quinazolin-6-yl or quinolin-6-yl)benzamides as novel PI3K inhibitors and anticancer agents by bioisostere. Eur. J. Med. Chem. 2014, 75, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Wani, Z.A.; Guru, S.K.; Rao, A.V.; Sharma, S.; Mahajan, G.; Behl, A.; Kumar, A.; Sharma, P.R.; Kamal, A.; Bhushan, S.; et al. A novel quinazolinone chalcone derivative induces mitochondrial dependent apoptosis and inhibits PI3K/Akt/mTOR signaling pathway in human colon cancer HCT-116 cells. Food Chem. Toxicol. 2016, 87, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dai, W.; Chen, X.; Geng, A.; Chen, Y.; Lu, T.; Zhu, Y. Design, synthesis and biological evaluation of 2,3-dihydroimidazo[1,2-c]quinazoline derivatives as novel phosphatidylinositol 3-kinase and histone deacetylase dual inhibitors. RSC Adv. 2017, 7, 52180–52186. [Google Scholar] [CrossRef] [Green Version]
- Xin, M.; Hei, Y.Y.; Zhang, H.; Xie, X.X.; Mao, S.; Zu, S.J.; Zhang, S.Q. Discovery of 6-benzamide containing 4-phenylquinazoline de ivatives as novel PI3Kδ inhibitors. Lett. Drug Des. Dis. 2017, 14, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Xin, M.; Hei, Y.Y.; Zhang, H.; Shen, Y.; Zhang, S.Q. Design and synthesis of novel 6-aryl substituted 4-anilinequinazoline de ivatives as potential PI3Kδ inhibitors. Bioorg. Med. Chem. Lett. 2017, 14, 1972–1977. [Google Scholar] [CrossRef]
- Xin, M.; Duan, W.; Feng, Y.; Hei, Y.; Zhang, H.; Shen, Y.; Zhao, H.Y.; Mao, S.; Zhang, S.Q. Novel 6- yl substituted 4-pyrrolidineaminoquinazoline derivatives as potent phosphoinositide 3-kinase delta (PI3Kδ) inhibitors. Bioorg. Med. Chem. 2018, 26, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 25 January 2023).



































| Character | 4-Aminoquinazoline |
|---|---|
| Molecular formula | C8H9N3 |
| Molecular weight | 147.18 g/mol |
| Number of heavy atoms | 11 |
| Number of aromatic heavy atoms | 6 |
| Fraction Csp3 | 0.12 |
| Number of rotatable bonds | 0 |
| Number of H-bond acceptors | 2 |
| Number of H-bond donors | 2 |
| Molar refractivity | 51.25 |
| Tropological polar surface area | 50.41 A2 |
| Lipophilicity | 0.66 |
| Water solubility | Soluble |
| GI absorption | High |
| BBB permeation | No |
| Bioavailability score | 0.55 |
| Lipinski | Yes |
| Synthetic accessibility | Easy |
| Molecular Structure | Generic Name | Chemical Name | Biological Target |
|---|---|---|---|
 | Gefitinib Iressa Irressat NSC 759856 UNII-S65743JHBS ZD 1839 CCRIS 9011 | 4-(3’-Chloro-4’-fluoroanilino)-7-methoxy-6-(3-morpholinopropoxy)quinazoline | Tyrosine kinase (EGFR) IC50 = 33 nM |
 | Erlotinib HSDB 8082 UNII-J4T82NDH7E | 4-Quinazolinamine, N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)- | Tyrosine kinase (EGFR) IC50 = 2 nM |
 | Vandetanib Caprelsa HSDB 8198 UNII-YO460OQ37K Zactima ZD 6474 | 4-Quninazolinamine, N-(4-bromo-2-fluorophenyl)-6-methoxy-7-((1-methyl-4-piperidinyl)methoxy)- | Tyrosine kinase (VEGFR2) IC50 = 40 nM |
 | Dacomitinib Vizimpro UNII-5092U85G58 PF-00299804 | 2-Butenamide, N-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxy-6-quinazolinyl)-4-(1-piperidinyl)-, hydrate (1:1), (2E)- | Tyrosine kinase (EGFR) IC50 = 50 nM |
 | Afatinib BIBW 2992 Tomtovok Tovok UNII-41UD74L59M | 2-Butenamide, N-(4-((3-chloro-4-fluorophenyl)amino)-7-(((3S)-tetrahydro-3-furanyl)oxy)-6-quinazolinyl)-4-(dimethylamino)-, (2E)- | Tyrosine kinase (EGFR) IC50 = 0.5 nM |
 | Canertinib UNII-C78W1K5ASF | N-(4-((3-Chloro-4-fluorophenyl)amino)-7-(3-(morpholin-4-yl)propoxy)quinazolin-6-yl)prop-2-enamide | Tyrosine kinase (EGFR) IC50 = 0.8 nM |
 | Trimetrexate Trimetrexatum HSDB 6545 JB 11 NSC 249008 TMQ UNII-UPN4ITI8T4 | 2,4-Quinazolinediamine, 5-methyl-6-(((3,4,5-trimethoxyphenyl)amino)methyl) | Dihydrofolate reductase (DHFR) IC50 = 0.04 nM |
 | Lapatinib GSK 572016 HSDB 8209 Tykerb UNII-0VUA21238F | 4-Quinazolinamine, N-(3-chloro-4-((3-fluorophenyl)methoxy)phenyl)-6-(5-(((2-(methylsulfonyl)ethyl)amino)methyl)-2-furanyl) | Tyrosine Kinase (EGFR) IC50 = 10.8 nM; (HER) IC50 = 29.2 nM |
 | Raltitrexed D 1694 UNII-FCB9EGG971 ZD1694 | L-Glutamic acid, N-((5-(((1,4-dihydro-2-methyl-4-oxo-6-quinazolinyl)methyl)methylamino)-2-thienyl)carbonyl) | Thymidylate synthase inhibitor IC50 = 9 nM |
 | Cediranib USAN AZD-2171 Recentin UNII-NQU9IPY4K9 | Quinazoline, 4-((4-fluoro-2-methyl-1H-indol-5-yl)oxy)-6-methoxy-7-(3-(1-pyrrolidinyl)propoxy) | Tyrosine Kinase (VEGFR) IC50 = 0.4 nM |
 | Tandutinib CT 53518 MLN 518 NSC 759851 NII-E1IO3ICJ9A | 1-Piperazinecarboxamide, 4-(6-methoxy-7-(3-(1-piperidinyl)propoxy)-4-quinazolinyl)-N-(4-(1-methylethoxy)phenyl) | Tyrosine Kinase (PDGFR) IC50 = 0.20 μM |
 | Barasertib AZD 1152 UNII-16XC2U7W8N | 1H-Pyrazole-3-acetamide, 5-((7-(3-(ethyl(2- (phosphonooxy)ethyl)amino)propoxy)-4-quinazolinyl)amino)-N-(3-fluorophenyl) | Tyrosine Kinase (Aurora B) IC50 = 0.37 nM |
 | Idelalisib GS 1101 UNII-YG57I8T5M0 Zydelig HSDB 8408 CAL-101 | 5-Fluoro-3-phenyl-2-((S)-1-(9H-purin-6-ylamino)-propyl)-3H-quinazolin-4-one | Tyrosine Kinase (PI3Kδ) IC50 = 2.5 nM |
 | Copanlisib BAY 80-6946 UNII-WI6V529FZ9 Aliqopa | 2-amino-N-(7-methoxy-8-(3-morpholinopropoxy)-2,3-dihydroimidazo(1,2-c)quinazolin-4-yl)pyrimidine-5-carboxamide | Tyrosine Kinase (PI3Kα) (PI3Kϒ) IC50 = 19 nM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayed, M.F. Medicinal Chemistry of Quinazolines as Anticancer Agents Targeting Tyrosine Kinases. Sci. Pharm. 2023, 91, 18. https://doi.org/10.3390/scipharm91020018
Zayed MF. Medicinal Chemistry of Quinazolines as Anticancer Agents Targeting Tyrosine Kinases. Scientia Pharmaceutica. 2023; 91(2):18. https://doi.org/10.3390/scipharm91020018
Chicago/Turabian StyleZayed, Mohamed F. 2023. "Medicinal Chemistry of Quinazolines as Anticancer Agents Targeting Tyrosine Kinases" Scientia Pharmaceutica 91, no. 2: 18. https://doi.org/10.3390/scipharm91020018
APA StyleZayed, M. F. (2023). Medicinal Chemistry of Quinazolines as Anticancer Agents Targeting Tyrosine Kinases. Scientia Pharmaceutica, 91(2), 18. https://doi.org/10.3390/scipharm91020018






