Old Dogs with New Tricks: Antiparasitic Potential of Structurally Diverse 5-Nitrofuran and 5-Nitrothiophene Imines and Acyl Hydrazones
Abstract
:1. Introduction
2. Results
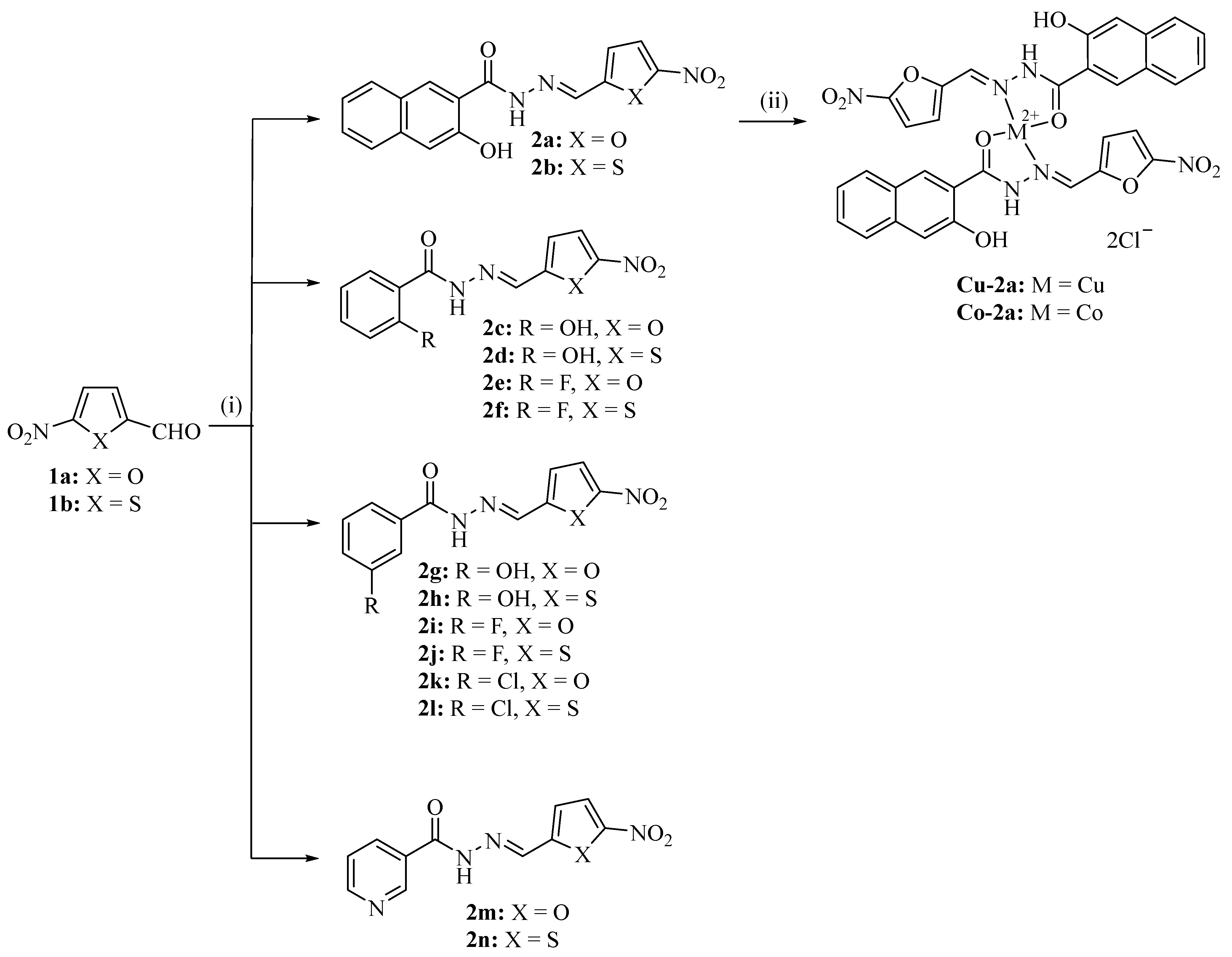
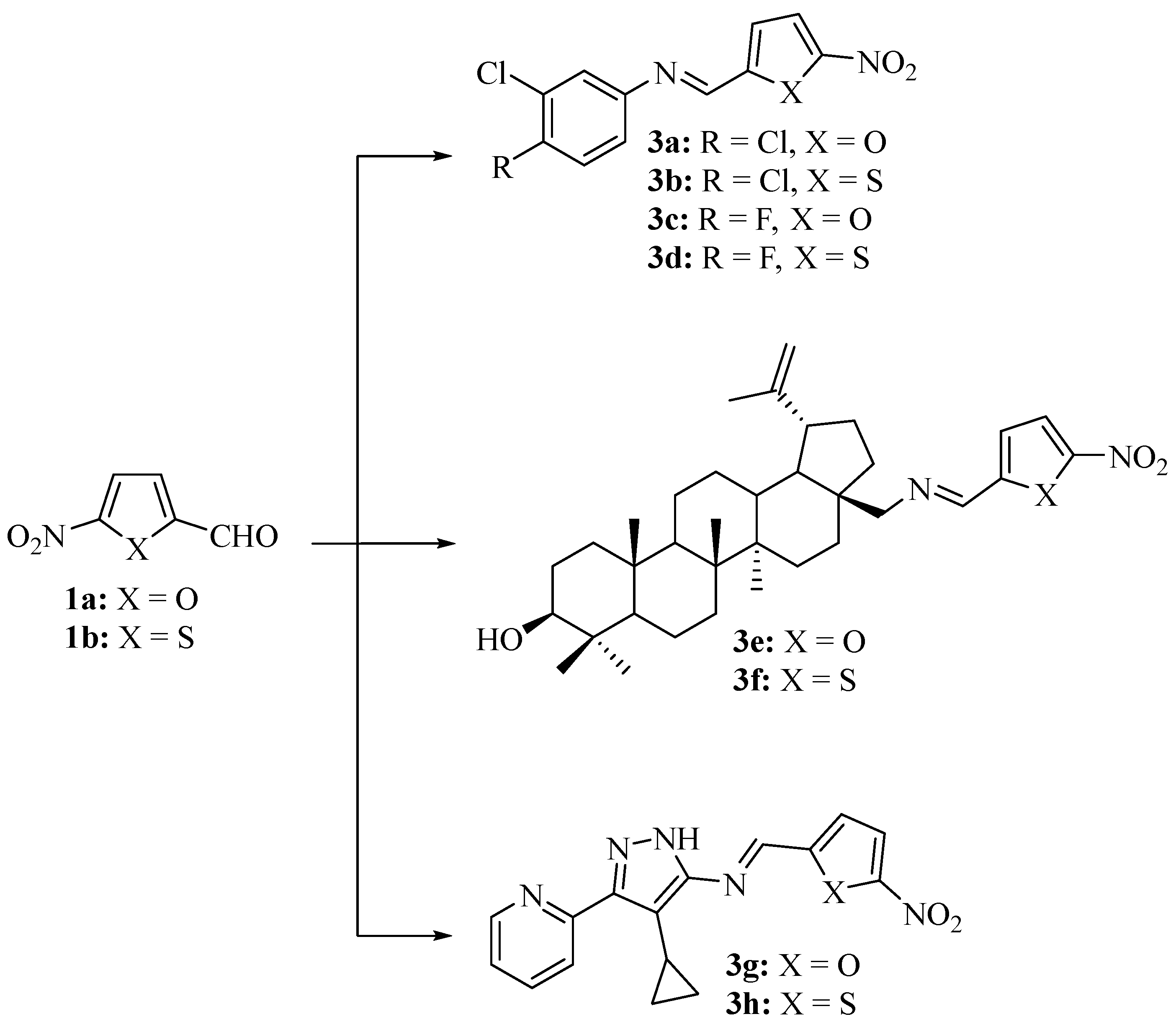
2.1. Chemistry
2.2. Antiparasitic Activity
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. Toxoplasma gondii Cell Line, Culture Conditions, and Assay
4.3. Leishmania Major Cell Isolation, Culture Conditions, and Assays
4.4. In Vitro Cytotoxicity Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meymandi, S.; Hernandez, S.; Park, S.; Sanchez, D.R.; Forsyth, C. Treatment of Chagas Disease in the USA. Curr. Treat. Options Infect. Dis. 2018, 10, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Priotto, G.; Kasparian, S.; Mutombo, W.; Ngouama, D.; Ghorashian, S.; Arnold, U.; Ghabri, S.; Baudin, E.; Buard, V.; Kazadi-Kyanza, S.; et al. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: A multicentre, randomised, phase III, non-inferiority trial. Lancet 2009, 374, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Kuemmerle, A.; Schmid, C.; Bernhard, S.; Kande, W.; Mutombo, W.; Ilunga, M.; Lumpungu, I.; Mutanda, S.; Nganzobo, P.; Tete, D.N.; et al. Effectiveness of Nifurtimox Eflornithine Combination Therapy (NECT) in T. b. gambiense second stage sleeping sickness patients in the Democratic Republic of Congo: Report from a field study. PLoS Negl. Trop. Dis. 2021, 15, e0009903. [Google Scholar] [CrossRef]
- Thota, S.; Rodrigues, D.A.; de Sena Murteira Pinheiro, P.; Lima, L.M.; Fraga, C.A.M.; Barreiro, E.J. N-Acylhydrazones as drugs. Bioorg. Med. Chem. Lett. 2018, 28, 2797–2806. [Google Scholar] [CrossRef] [PubMed]
- Socea, L.-I.; Barbuceanu, S.-F.; Pahontu, E.M.; Dumitru, A.-C.; Nitulescu, G.M.; Sfetea, R.C.; Apostol, T.-V. Acylhydrazones and their biological activity: A review. Molecules 2022, 27, 8719. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Toward a repositioning of the antibacterial drug nifuroxazide for cancer treatment. Drug Discov. Today 2019, 24, 1930–1936. [Google Scholar] [CrossRef]
- Rando, D.G.; Avery, M.A.; Tekwani, B.L.; Khan, S.I.; Ferreira, E.I. Antileishmanial activity screening of 5-nitro-2-heterocyclic benzylidene hydrazides. Bioorg. Med. Chem. 2008, 16, 6724–6731. [Google Scholar] [CrossRef]
- Caldas, L.A.; Attias, M.; de Souza, W. Dynamin inhibitor impairs Toxoplasma gondii invasion. FEMS Microbiol. Lett. 2009, 301, 103–108. [Google Scholar] [CrossRef]
- Paruch, K.; Biernasiuk, A.; Khylyuk, D.; Paduch, R.; Wujec, M.; Popiołek, Ł. Synthesis, biological activity and molecular docking studies of novel nicotinic acid derivatives. Int. J. Mol. Sci. 2022, 23, 2823. [Google Scholar] [CrossRef]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef] [PubMed]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of toxoplasmosis: Historical perspective, animal models, and current clinical practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, R.; Tawk, L.; Itani, S.; Hamie, M.; Ezzeddine, J.; El Sabban, M.; El Hajj, H. Toxoplasmosis: Current and emerging parasite druggable targets. Microorganisms 2021, 9, 2531. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; Quereda, C.; Pascual, J.; Rivera, M.; Clemente, L.; Cano, T. Patterns of sulfadiazine acute nephrotoxicity. Clin. Nephrol. 2000, 54, 68–72. [Google Scholar] [PubMed]
- WHO-Factsheets. Available online: http://www.who.int/mediacentre/factsheets/fs375/en/ (accessed on 12 November 2020).
- van Bocxlaer, K.; Caridha, D.; Black, C.; Vesely, B.; Leed, S.; Sciotti, R.J.; Wijnant, G.-J.; Yardley, V.; Braillard, S.; Mowbray, C.E.; et al. Novel benzoxaborole, nitroimidazole and aminopyrazoles with activity against experimental cutaneous leishmaniasis. Int. J. Parasitol. Drugs Drug Resist. 2019, 11, 129–138. [Google Scholar] [CrossRef]
- Alves, F.; Bilbe, G.; Blesson, S.; Goyal, V.; Monnerat, S.; Mowbray, C.; Ouattara, G.M.; Pécoul, B.; Rijal, S.; Rode, J.; et al. Recent development of visceral leishmaniasis treatments: Successes, pitfalls, and perspectives. Clin. Microbiol. Rev. 2018, 31, e00048-18. [Google Scholar] [CrossRef]
- Koko, W.S.; Al Nasr, I.S.; Khan, T.A.; Schobert, R.; Biersack, B. An update on natural antileishmanial treatment options from plants, fungi and algae. Chem. Biodivers. 2021, 19, e202100542. [Google Scholar] [CrossRef]
- Mioc, M.; Prodea, A.; Racoviceanu, R.; Mioc, A.; Ghiulai, R.; Milan, A.; Voicu, M.; Mardale, G.; Soica, C. Recent advances regarding the molecular mechanisms of triterpenic acids: A review (part II). Int. J. Mol. Sci. 2022, 23, 8896. [Google Scholar] [CrossRef]
- Rocha, V.; Quadros, H.; Meira, C.; Silva, L.; Carvalho, D.; Hodel, K.; Moreira, D.; Soares, M. Potential of triterpenic natural compound betulinic acid for neglected tropical diseases new treatments. Biomedicines 2022, 10, 831. [Google Scholar] [CrossRef]
- Vana den Kerkhof, M.; Mabille, D.; Hendrickx, S.; Leprohon, P.; Mowbray, C.E.; Braillard, S.; Ouellette, M.; Maes, L.; Caljon, G. Antileishmanial aminopyrazoles: Studies into mechanisms and stability of experimental drug resistance. Antimicrob. Agents Chemother. 2020, 64, e00152-20. [Google Scholar] [CrossRef] [PubMed]
- Mowbray, C.E.; Braillard, S.; Speed, W.; Glossop, P.A.; Whitlock, G.A.; Gibson, K.R.; Mills, J.E.J.; Brown, A.D.; Gardner, J.M.F.; Cao, Y.; et al. Novel amino-pyrazole ureas with potent in vitro and in vivo antileishmanial activity. J. Med. Chem. 2015, 58, 9615–9624. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Shafiq, Z.; Nawaz, M.H.; Shad, M.A.; Nawaz, H.; Yaqub, M.; Ahmad, H.B. Synthesis, characterization and biological evaluation of some novel hydrazide Schiff’s bases and their metal complexes. Asian J. Chem. 2013, 25, 2668. [Google Scholar] [CrossRef]
- Cunha, A.B.; Batista, R.; Castro, M.Á.; David, J.M. Chemical strategies towards the synthesis of betulinic acid and its more potent antiprotozoal analogues. Molecules 2021, 26, 1081. [Google Scholar] [CrossRef]
- DNDi–Open Synthesis Network. Available online: https://dndi.org/research-development/portfolio/open-synthesis-network/ (accessed on 31 July 2023).
- Al Nasr, I.S.; Hanachi, R.; Said, R.B.; Rahali, S.; Tangour, B.; Abdelwahab, S.I.; Farasani, A.; Taha, M.M.E.; Bidwai, A.; Koko, W.S.; et al. p-Trifluoromethyl- and p-pentafluorothio-substituted curcuminoids of the 2,6-di[(E)-benzylidene)]cycloalkanone type: Syntheses and activities against Leishmania major and Toxoplasma gondii parasites. Bioorg. Chem. 2021, 114, 105099. [Google Scholar] [CrossRef]
- Wilkinson, S.R.; Kelly, J.M. Trypanocidal drugs: Mechanisms, resistance and new targets. Expert Rev. Mol. Med. 2009, 11, e31. [Google Scholar] [CrossRef]
- Yeo, S.-J.; Jin, C.M.; Kim, S.Y.; Park, H. In vitro and in vivo effects of nitrofurantoin on experimental toxoplasmosis. Korean J. Parasitol. 2016, 54, 155–161. [Google Scholar] [CrossRef]
- Feitosa, I.B.; Mori, B.; de Azevedo dos Santos, A.P.; Villanova, J.C.O.; Teles, C.B.G.; Costa, A.G. What are the immunopharmacological effects of furazolidone? A systematic review. Immunopharmacol. Immunotoxicol. 2021, 43, 674–679. [Google Scholar] [CrossRef]
- Da Silva Santos-Júnior, P.F.; Silva, L.R.; Quintans-Júnior, L.J.; da Silva-Júnior, E.F. Nitro compounds against trypanosomatidae parasites: Heroes or villains? Bioorg. Med. Chem. Lett. 2022, 75, 128930. [Google Scholar] [CrossRef]
- Miyata, N.; Tang, Z.; Conti, M.A.; Johnson, M.E.; Douglas, C.J.; Hasson, S.A.; Damoiseaux, R.; Chang, C.-E.A.; Koehler, C.M. Adaptation of a genetic screen reveals an inhibitor for mitochondrial protein import component Tim44. J. Biol. Chem. 2017, 292, 5429–5442. [Google Scholar] [CrossRef]
- Preta, G.; Cronin, J.G.; Sheldon, I.M. Dynasore–not just a dynamin inhibitor. Cell Commun. Signal. 2015, 13, 24. [Google Scholar] [CrossRef]
- Asthana, A.; Corona, A.; Shin, W.-J.; Kwak, M.-J.; Gaughan, C.; Tramontano, E.; Jung, J.U.; Schobert, R.; Jha, B.K.; Silverman, R.H.; et al. Analogs of the catechol derivative dynasore inhibit HIV-1 ribonuclease H, SARS-CoV-2 nsp14 exoribonuclease, and virus replication. Viruses 2023, 15, 1539. [Google Scholar] [CrossRef]
- Verhoef, J.M.J.; Meissner, M.; Kooji, T.W.A. Organelle dynamics in apicomplexan parasites. mBio 2021, 12, e01409-21. [Google Scholar] [CrossRef]
- Webster, W.A.J.; McFadden, G.I. Organelle division: Dynamin-related proteins in apicomplexans. Curr. Biol. 2009, 19, R336. [Google Scholar] [CrossRef]
- Caldas, L.A.; Soares, L.L.; Seabra, S.H.; Attias, M.; de Souza, W. Monitoring of dynamin during the Toxoplasma gondii cell cycle. Pathog. Dis. 2016, 74, ftw108. [Google Scholar] [CrossRef]
- Ramírez-Flores, C.J.; Cruz-Mirón, R.; Lagunas-Cortés, N.; Mondragón-Castelán, M.; Mondragon-Gonzalez, R.; González-Pozos, S.; Mondragón-Flores, R. Toxoplasma gondii excreted/secreted proteases disrupt intercellular junction proteins in epithelial cell monolayers to facilitate tachyzoites paracellular migration. Cell. Microbiol. 2021, 23, e13283. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Azevedo, R.; Mendonca-Natividade, F.C.; Santana, A.C.; Diniz, J.A.; Roque-Barreira, M.C. Microneme proteins 1 and 4 from Toxoplasma gondii induce IL-10 production by macrophages through TLR4 endocytosis. Front. Immunol. 2021, 12, 655371. [Google Scholar] [CrossRef] [PubMed]
- Canfield, C.J.; Pudney, M.; Gutteridge, W.E. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp. Parasitol. 1995, 80, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Gajurel, K.; Gomez, C.A.; Dhakal, R.; Vogel, H.; Montoya, J.G. Failure of primary atovaquone prophylaxis for prevention of toxoplasmosis in hematopoietic cell transplant recipients. Transpl. Infect. Dis. 2016, 18, 446–452. [Google Scholar] [CrossRef]
- Lucaciu, R.L.; Hangan, A.C.; Sevastre, B.; Oprean, L.S. Metallo-drugs in cancer therapy: Past, present and future. Molecules 2022, 27, 6485. [Google Scholar] [CrossRef]
- Da Silva, D.A.; De Luca, A.; Squitti, R.; Rongioletti, M.; Rossi, L.; Machado, C.M.L.; Cerchiaro, G. Copper in tumors and the use of copper-based compounds in cancer treatment. J. Inorg. Biochem. 2022, 226, 111634. [Google Scholar] [CrossRef] [PubMed]
- Portes, J.A.; Motta, C.S.; Azeredo, N.F.; Fernandes, C.; Horn, A., Jr.; de Souza, W.; DaMatta, R.A.; Seabra, S.H. In vitro treatment of Toxoplasma gondii with copper(II) complexes induces apoptosis-like and cellular division alterations. Vet. Parasitol. 2017, 245, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.M.; Horn, A., Jr.; Terra, A.R.d.M.L.; Rezende, L.M.; Moreira, F.F.; DaMatta, R.A.; Xavier, F.R.; Cervo, R.; Cargnelutti, R.; Moorkkannur, S.N.; et al. In vitro anti-Leishmania activity of new isomeric cobalt(II)complexes and in silico insights: Mitochondria impairment and apoptosis-like cell death of the parasite. J. Inorg. Biochem. 2023, 240, 112088. [Google Scholar] [CrossRef] [PubMed]
- Tsiliou, S.; Kefala, L.-A.; Perdih, F.; Turel, I.; Kessissoglou, D.P.; Psomas, G. Cobalt(II) complexes with non-steroidal anti-inflammatory drug tolfenamic acid: Structure and biological evaluation. Eur. J. Med. Chem. 2012, 48, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, L.; Talaie, F.; Chiniforoshan, H. Copper(II), cobalt(II) and nickel(II) complexes of lapachol: Synthesis, DNA interaction, and cytotoxicity. J. Biomol. Struct. Dyn. 2017, 35, 3330–3341. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.S.; Eseola, A.O.; Plass, W.; Kato, K.; Otuchere, C.A.; Awakan, O.J.; Atolani, O.; Otohinoy, D.A.; Elebiyo, T.C.; Evbuomwan, I.O. The anti-parasitic action of imidazole derivatives likely involves oxidative stress but not HIF-1α signaling. Chem. Biol. Interact. 2021, 349, 109676. [Google Scholar] [CrossRef]
- Jelali, H.; Al Nasr, I.; Koko, W.; Khan, T.; Deniau, E.; Sauthier, M.; Alresheedi, F.; Hamdi, N. Synthesis, characterization and in vitro bioactivity studies of isoindolin-1-3-phosophonate compounds. J. Heterocycl. Chem. 2022, 59, 493–506. [Google Scholar] [CrossRef]
- Osorio, E.; Arango, G.; Jiménez, N.; Alzate, F.; Ruiz, G.; Gutiérrez, D.; Paco, M.A.; Giménez, A.; Robledo, S. Antiprotozoal and cytotoxic activities in vitro of Colombian Annonaceae. J. Ethnopharmacol. 2007, 111, 630–635. [Google Scholar] [CrossRef]
- Koko, W.S.; Mesaik, M.A.; Yousaf, S.; Galal, M.; Choudhary, M.I. In vitro immunomodulating properties of selected Sudanese medicinal plants. J. Ethnopharmacol. 2008, 118, 26–34. [Google Scholar] [CrossRef]

| Compd. | IC50 (T. gondii) | CC50 (Vero) | IC50 (Macrophages) | SI (Vero/T. gondii) 2 | SI (Macrophages/T. gondii) 2 |
|---|---|---|---|---|---|
| 2a | 0.12 | 2.77 | 14.8 | 23.1 | 123 |
| 2b | 0.35 | 3.28 | 20.3 | 9.4 | 58.0 |
| 2c | 1.16 | 20.5 | 28.8 | 17.7 | 24.8 |
| 2d | 12.7 | 11.2 | 24.7 | 0.88 | 2.0 |
| 2e | 20.2 | 40.0 | 24.5 | 2.0 | 1.2 |
| 2f | 3.07 | 23.2 | 16.4 | 7.6 | 5.3 |
| 2g | 22.5 | 47.6 | 18.9 | 2.1 | 0.84 |
| 2h | 3.78 | 30.6 | 9.27 | 8.1 | 2.5 |
| 2i | 0.90 | 2.71 | 7.72 | 3.0 | 8.6 |
| 2j | 1.30 | 10.1 | 7.23 | 7.8 | 5.6 |
| 2k | 19.8 | 31.7 | 15.0 | 1.6 | 0.76 |
| 2l | 1.61 | 14.2 | 9.36 | 8.8 | 5.8 |
| 2m | 12.9 | 14.2 | 28.4 | 1.1 | 2.2 |
| 2n | 1.27 | 4.53 | 10.5 | 3.6 | 8.3 |
| Cu-2a | 0.28 | 1.78 | 11.2 | 6.4 | 40.0 |
| Co-2a | 0.15 | 2.70 | 11.0 | 17.9 | 73.3 |
| 3a | 13.7 | 12.1 | 9.96 | 0.88 | 0.73 |
| 3b | 4.15 | 12.6 | 12.3 | 3.0 | 3.0 |
| 3c | 4.77 | 13.2 | 15.8 | 2.8 | 3.3 |
| 3d | 4.92 | 11.2 | 15.8 | 2.3 | 3.2 |
| 3e | 7.97 | 5.67 | 13.5 | 0.71 | 1.7 |
| 3f | 15.8 | 17.7 | 7.51 | 1.1 | 0.48 |
| 3g | 9.90 | 24.4 | 17.9 | 2.5 | 1.8 |
| 3h | 14.1 | 15.6 | 26.2 | 1.1 | 1.9 |
| 28-AB | 0.16 | 0.34 | 10.5 | 2.1 | 65.6 |
| ATO 3 | 0.07 | 9.5 | - | 136 | - |
| AmB 3 | - | - | 8.1 | - | - |
| Compd. | IC50 Promastigotes | IC50 Amastigotes | SI (Macrophages/ Promastigotes) 2 | SI (Macrophages/ Amastigotes) 2 |
|---|---|---|---|---|
| 2a | 5.84 | 5.53 | 2.53 | 2.68 |
| 2b | 0.88 | 24.0 | 23.1 | 0.85 |
| 2c | 4.83 | 32.0 | 5.96 | 0.90 |
| 2d | 3.78 | 28.8 | 6.53 | 0.86 |
| 2e | 6.49 | 22.7 | 3.78 | 1.08 |
| 2f | 5.11 | 17.7 | 3.21 | 0.93 |
| 2g | 8.36 | 21.1 | 2.26 | 0.90 |
| 2h | 17.9 | 2.75 | 0.52 | 3.37 |
| 2i | 0.087 | 9.01 | 88.7 | 0.86 |
| 2j | 1.36 | 3.75 | 5.32 | 1.93 |
| 2k | 1.02 | 16.4 | 14.7 | 0.92 |
| 2l | 4.84 | 2.91 | 1.93 | 3.22 |
| 2m | 5.23 | 32.7 | 5.43 | 0.87 |
| 2n | 3.26 | 11.2 | 3.22 | 0.94 |
| Cu-2a | 7.52 | 8.15 | 1.49 | 1.37 |
| Co-2a | 4.74 | 6.66 | 2.32 | 1.65 |
| 3a | 4.63 | 11.6 | 2.15 | 0.86 |
| 3b | 4.25 | 9.30 | 2.89 | 1.32 |
| 3c | 15.7 | 11.5 | 1.01 | 1.37 |
| 3d | 4.92 | 9.48 | 3.21 | 1.67 |
| 3e | 3.01 | 5.49 | 4.49 | 2.46 |
| 3f | 5.51 | 14.1 | 1.36 | 0.53 |
| 3g | 1.11 | 14.2 | 16.1 | 1.26 |
| 3h | 1.00 | 29.8 | 26.2 | 0.88 |
| 28-AB | 2.94 | 1.59 | 3.57 | 6.60 |
| AmB 3 | 0.83 | 0.47 | 9.76 | 17.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Nasr, I.S.; Koko, W.S.; Khan, T.A.; Schobert, R.; Biersack, B. Old Dogs with New Tricks: Antiparasitic Potential of Structurally Diverse 5-Nitrofuran and 5-Nitrothiophene Imines and Acyl Hydrazones. Sci. Pharm. 2023, 91, 44. https://doi.org/10.3390/scipharm91030044
Al Nasr IS, Koko WS, Khan TA, Schobert R, Biersack B. Old Dogs with New Tricks: Antiparasitic Potential of Structurally Diverse 5-Nitrofuran and 5-Nitrothiophene Imines and Acyl Hydrazones. Scientia Pharmaceutica. 2023; 91(3):44. https://doi.org/10.3390/scipharm91030044
Chicago/Turabian StyleAl Nasr, Ibrahim S., Waleed S. Koko, Tariq A. Khan, Rainer Schobert, and Bernhard Biersack. 2023. "Old Dogs with New Tricks: Antiparasitic Potential of Structurally Diverse 5-Nitrofuran and 5-Nitrothiophene Imines and Acyl Hydrazones" Scientia Pharmaceutica 91, no. 3: 44. https://doi.org/10.3390/scipharm91030044
APA StyleAl Nasr, I. S., Koko, W. S., Khan, T. A., Schobert, R., & Biersack, B. (2023). Old Dogs with New Tricks: Antiparasitic Potential of Structurally Diverse 5-Nitrofuran and 5-Nitrothiophene Imines and Acyl Hydrazones. Scientia Pharmaceutica, 91(3), 44. https://doi.org/10.3390/scipharm91030044







