Topical Application of Cha-Koji, Green Tea Leaves Fermented with Aspergillus Luchuensis var Kawachii Kitahara, Promotes Acute Cutaneous Wound Healing in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Mice
2.3. Preparation of Cha-Koji
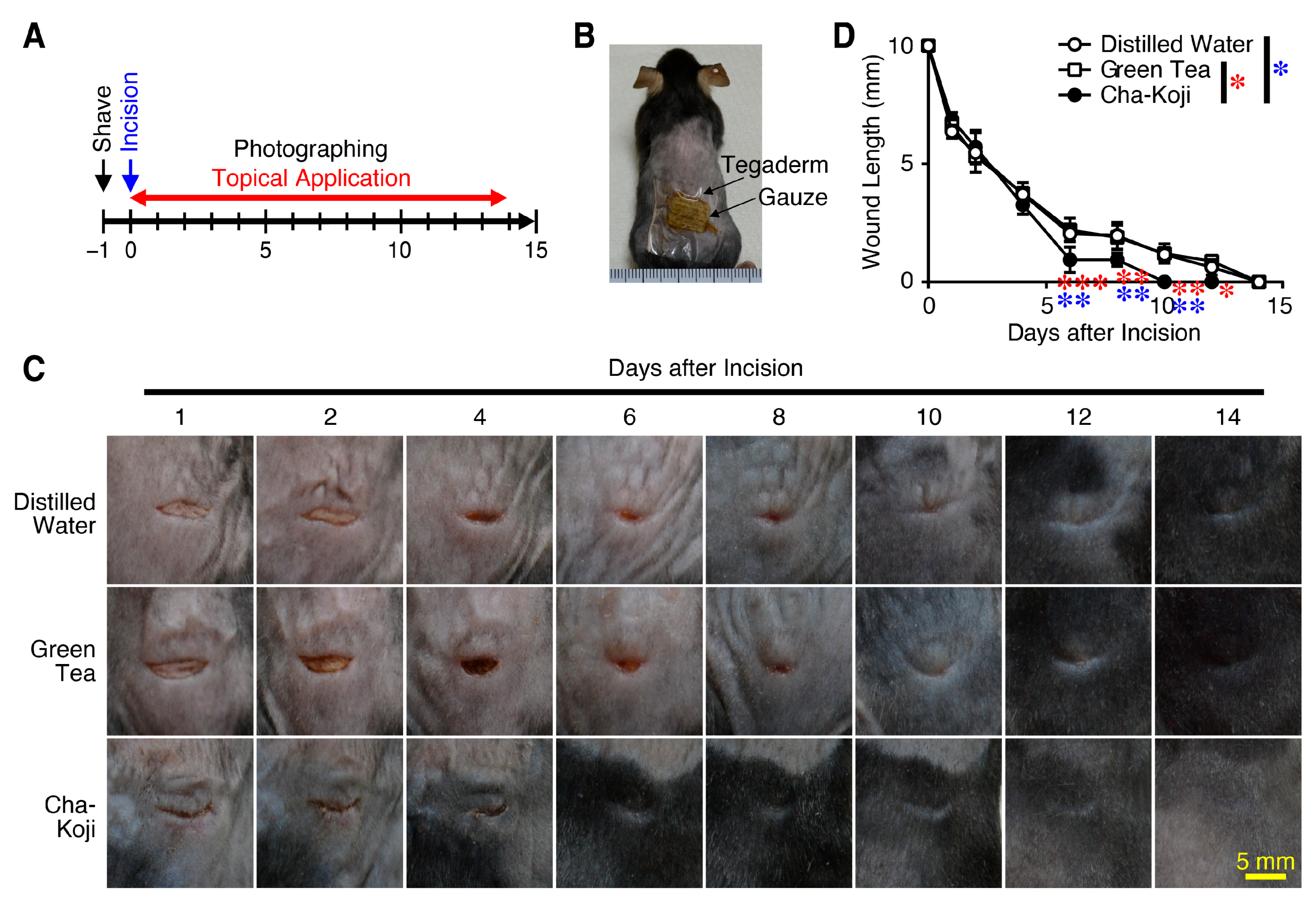
2.4. Evaluation of Wound Healing in a Linear Incision Wound Mouse Model
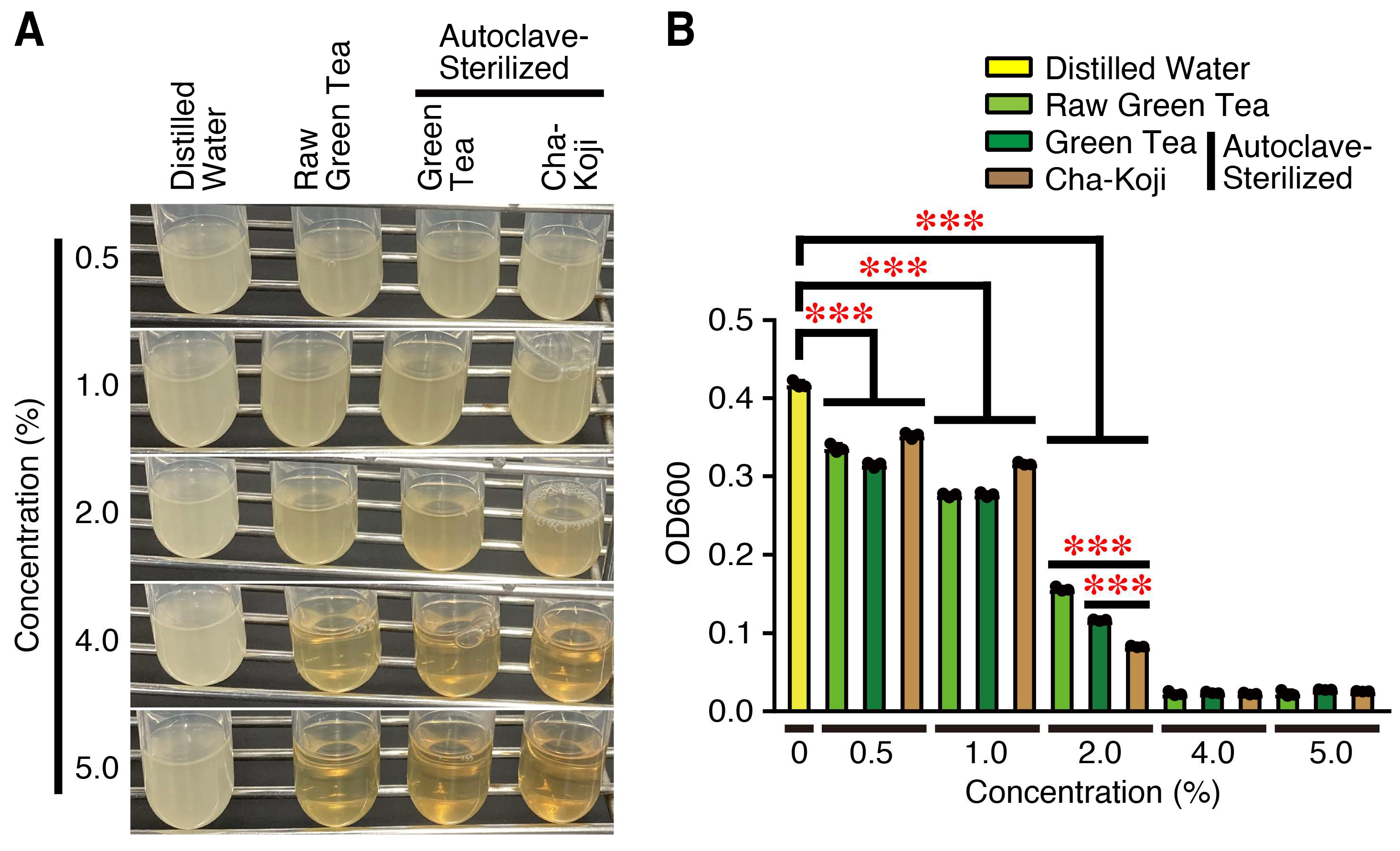
2.5. Anti-Bacterial Activity Test
2.6. Cell Proliferation Assay
2.7. Scratch Assay
2.8. RT-qPCR
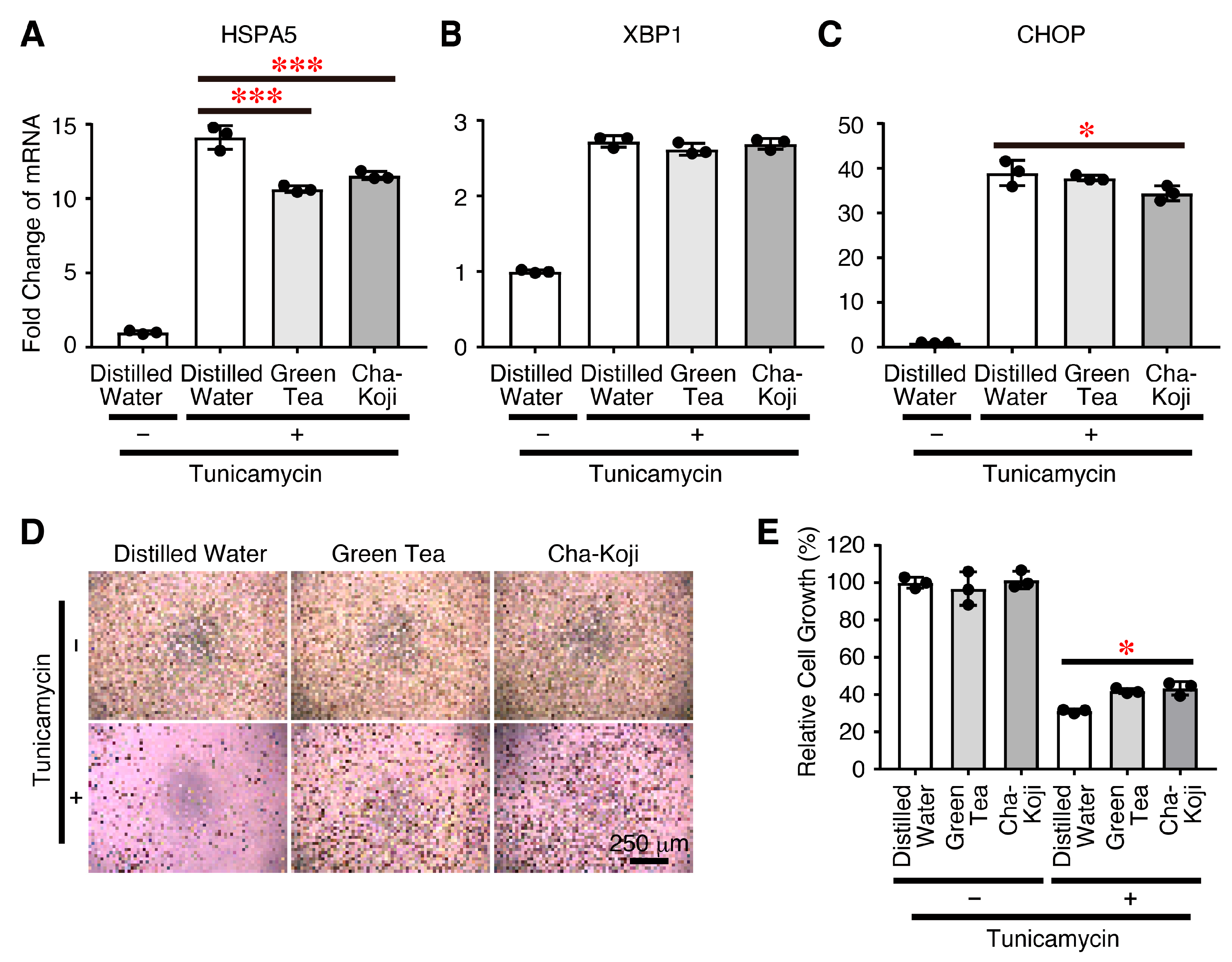
2.9. ER Stress
2.10. h-CLAT Assay
2.11. Statistical Analyses
3. Results
3.1. Autoclave-Sterilized Cha-Koji Suspension Promotes Cutaneous Wound Healing
3.2. Autoclave-Sterilized Cha-Koji Suspension Shows Anti-Bacterial Activity
3.3. Autoclave-Sterilized Cha-Koji Suspension Promotes Skin Epidermal Cell Proliferation and Migration
3.4. Autoclave-Sterilized Cha-Koji Suspension Promotes the Expression of Anti-Inflammatory Cytokine Transforming Growth Factor (TGF)-β1
3.5. Autoclave-Sterilized Cha-Koji Suspension Suppresses ER Stress
3.6. Autoclave-Sterilized Cha-Koji Suspension Poses No Potential Skin Sensitizing Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gravitz, L. Skin. Nature 2018, 563, S83. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, A.L.; Rendon, J.L.; Ramirez, L.; Choudhry, M.A.; Kovacs, E.J. Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. J. Immunol. 2013, 190, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Cai, Y.; Zhang, G.; Jing, Z.; Liang, J.; Zhang, R.; Dang, X.; Zhang, C. Exosomes derived from M2 macrophages induce angiogenesis to promote wound healing. Front. Mol. Biosci. 2022, 9, 1008802. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Ratsep, M.; Kilk, K.; Zilmer, M.; Kuus, L.; Songisepp, E. A Novel Bifidobacterium longum ssp. longum Strain with Pleiotropic Effects. Microorganisms 2024, 12, 174. [Google Scholar] [CrossRef]
- Liu, K.; Cai, Y.; Song, K.; Yuan, R.; Zou, J. Clarifying the effect of gut microbiota on allergic conjunctivitis risk is instrumental for predictive, preventive, and personalized medicine: A Mendelian randomization analysis. EPMA J. 2023, 14, 235–248. [Google Scholar] [CrossRef]
- Piriyaprasath, K.; Kakihara, Y.; Kurahashi, A.; Taiyoji, M.; Kodaira, K.; Aihara, K.; Hasegawa, M.; Yamamura, K.; Okamoto, K. Preventive Roles of Rice-koji Extracts and Ergothioneine on Anxiety- and Pain-like Responses under Psychophysical Stress Conditions in Male Mice. Nutrients 2023, 15, 3989. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Escalera, S.; Lund, M.L.; Hermes, G.D.A.; Choi, B.S.; Sakamoto, K.; Wellejus, A. In Vitro Screening for Probiotic Properties of Lactobacillus and Bifidobacterium Strains in Assays Relevant for Non-Alcoholic Fatty Liver Disease Prevention. Nutrients 2023, 15, 2361. [Google Scholar] [CrossRef]
- Summer, M.; Ali, S.; Fiaz, U.; Tahir, H.M.; Ijaz, M.; Mumtaz, S.; Mushtaq, R.; Khan, R.; Shahzad, H.; Fiaz, H. Therapeutic and immunomodulatory role of probiotics in breast cancer: A mechanistic review. Arch. Microbiol. 2023, 205, 296. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, B.; Suzuki, Y.; Yonezu, T.; Mizushima, N.; Watanabe, N.; Sato, T.; Inoue, S.; Inokuchi, S. Cha-Koji, comprising green tea leaves fermented with Aspergillus luchuensis var kawachii kitahara, increases regulatory T cell production in mice and humans. Biosci. Biotechnol. Biochem. 2018, 82, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Ozaki, A.; Hakariya, H.; Takahashi, K.; Tanimoto, T. The Beni-Koji scandal and Japan’s unique health food system. Lancet 2024, 403, 2287–2288. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Masumoto, N.; Makino, T.; Matsushima, Y.; Morikawa, T.; Ito, M. Novel compounds isolated from health food products containing beni-koji (red yeast rice) with adverse event reports. J. Nat. Med. 2024, 1–4. [Google Scholar] [CrossRef]
- Kadooka, C.; Izumitsu, K.; Onoue, M.; Okutsu, K.; Yoshizaki, Y.; Takamine, K.; Goto, M.; Tamaki, H.; Futagami, T. Mitochondrial Citrate Transporters CtpA and YhmA Are Required for Extracellular Citric Acid Accumulation and Contribute to Cytosolic Acetyl Coenzyme A Generation in Aspergillus luchuensis mut. kawachii. Appl. Environ. Microbiol. 2019, 85, e03136. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.Y.; Parsaei, P.; Karimi, M.; Ezzati, S.; Zamiri, A.; Mohammadizadeh, F.; Rafieian-Kopaei, M. Effect of green tea (Camellia sinensis) extract on healing process of surgical wounds in rat. Int. J. Surg. 2013, 11, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lu, Z.; Lu, J.; Wu, D. Evaluation of the antioxidant characteristics of craft beer with green tea. J. Food Sci. 2023, 88, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Jaiganesh, R.; Rajeshkumar, S. Green Synthesis of Zinc Oxide Nanoparticles Using Chamomile and Green Tea Extracts and Evaluation of Their Anti-inflammatory and Antioxidant Activity: An In Vitro Study. Cureus 2023, 15, e46088. [Google Scholar] [CrossRef]
- Ansell, D.M.; Campbell, L.; Thomason, H.A.; Brass, A.; Hardman, M.J. A statistical analysis of murine incisional and excisional acute wound models. Wound Repair Regen. 2014, 22, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Guideline for the Testing of Chemicals No. 442E: In Vitro Skin Sensitisation: Human Cell Line Activation Test (h-CLAT); Organisation for Economic Cooperation and Development: Paris, France, 2016. [Google Scholar]
- Efaq, A.N.; Rahman, N.N.N.A.; Nagao, H.; Ai-Gheethi, A.A.; Kadir, M.O.A. Inactivation of Aspergillus Spores in Clinical Wastes by Supercritical Carbon Dioxide. Arab. J. Sci. Eng. 2017, 42, 39–51. [Google Scholar] [CrossRef]
- Colwell, A.S.; Krummel, T.M.; Kong, W.; Longaker, M.T.; Lorenz, H.P. Skin wounds in the MRL/MPJ mouse heal with scar. Wound Repair Regen. 2006, 14, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Bobadilla, A.V.P.; Arevalo, J.; Sarro, E.; Byrne, H.M.; Maini, P.K.; Carraro, T.; Balocco, S.; Meseguer, A.; Alarcon, T. In vitro cell migration quantification method for scratch assays. J. R. Soc. Interface 2019, 16, 20180709. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. TGF-beta induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef] [PubMed]
- Pena, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Hiratsuka, T.; Taniguchi, M.; Shingaki, K.; Kubo, T.; Kiya, K.; Fujiwara, T.; Kanazawa, S.; Kanematsu, R.; Maeda, T.; et al. Physiological ER Stress Mediates the Differentiation of Fibroblasts. PLoS ONE 2015, 10, e0123578. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Ishise, H.; Kubo, T.; Larson, B.; Fujiwara, T.; Nishimoto, S.; Kakibuchi, M. Stretching Promotes Wound Contraction Through Enhanced Expression of Endothelin Receptor B and TRPC3 in Fibroblasts. Plast. Reconstr. Surg. Glob. Open 2023, 11, e4954. [Google Scholar] [CrossRef] [PubMed]
- Ishise, H.; Larson, B.; Hirata, Y.; Fujiwara, T.; Nishimoto, S.; Kubo, T.; Matsuda, K.; Kanazawa, S.; Sotsuka, Y.; Fujita, K.; et al. Hypertrophic scar contracture is mediated by the TRPC3 mechanical force transducer via NFkB activation. Sci. Rep. 2015, 5, 11620. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Ansari, A.A.; Upadhyay, S.P.; Mettman, D.J.; Hibdon, J.R.; Quadir, M.; Ghosh, P.; Kambhampati, A.; Banerjee, S.K. Benefits and Pitfalls of a Glycosylation Inhibitor Tunicamycin in the Therapeutic Implication of Cancers. Cells 2024, 13, 395. [Google Scholar] [CrossRef]
- Katahira, Y.; Sakamoto, E.; Watanabe, A.; Furusaka, Y.; Inoue, S.; Hasegawa, H.; Mizoguchi, I.; Yo, K.; Yamaji, F.; Toyoda, A.; et al. Upregulation of CD86 and IL-12 by rhododendrol in THP-1 cells cocultured with melanocytes through ROS and ATP. J. Dermatol. Sci. 2022, 108, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, E.; Katahira, Y.; Mizoguchi, I.; Watanabe, A.; Furusaka, Y.; Sekine, A.; Yamagishi, M.; Sonoda, J.; Miyakawa, S.; Inoue, S.; et al. Chemical- and Drug-Induced Allergic, Inflammatory, and Autoimmune Diseases Via Haptenation. Biology 2023, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Heydari Nasrabadi, H.; Tajabadi Ebrahimi, M.; Dehghan Banadaki, S.; Torabi Kajousangi, M.; Zahedi, F. Study of cutaneous wound healing in rats treated with Lactobacillus plantarum on days 1, 3, 7, 14 and 21. Afr. J. Pharm. Pharmacol. 2011, 5, 2395–2401. [Google Scholar] [CrossRef]
- Zheng, Z.; Sun, N.; Lu, Z.; Zheng, J.; Zhang, S.; Lin, S. The potential mechanisms of skin wound healing mediated by tetrapeptides from sea cucumber. Food Sci. 2023, 53, 102742. [Google Scholar] [CrossRef]
- Kapoor, M.; Howard, R.; Hall, I.; Appleton, I. Effects of epicatechin gallate on wound healing and scar formation in a full thickness incisional wound healing model in rats. Am. J. Pathol. 2004, 165, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, F.; Esposito, D.; Grace, M.H.; Pirovani, M.E.; Lila, M.A. Anti-inflammatory and wound healing properties of polyphenolic extracts from strawberry and blackberry fruits. Food Res. Int. 2019, 121, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M. Green Tea Catechins: Nature’s Way of Preventing and Treating Cancer. Int. J. Mol. Sci. 2022, 23, 10713. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhu, Q.Y.; Tsang, D.; Huang, Y. Degradation of green tea catechins in tea drinks. J. Agric. Food Chem. 2001, 49, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Yousef-Nezhad, H.; Hejazi, N. Anti-Bacterial Properties of Herbs against Helicobacter Pylori Infection: A Review. Int. J. Nutr. Sci. 2017, 2, 126–133. [Google Scholar]
- Nishitani, A.; Hiramatsu, K.; Kadooka, C.; Mori, K.; Okutsu, K.; Yoshizaki, Y.; Takamine, K.; Tashiro, K.; Goto, M.; Tamaki, H.; et al. Expression of heterochromatin protein 1 affects citric acid production in Aspergillus luchuensis mut. kawachii. J. Biosci. Bioeng. 2023, 136, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Burel, C.; Kala, A.; Purevdorj-Gage, L. Impact of pH on citric acid antimicrobial activity against Gram-negative bacteria. Lett. Appl. Microbiol. 2021, 72, 332–340. [Google Scholar] [CrossRef]
- Todokoro, T.; Fukuda, K.; Matsumura, K.; Irie, M.; Hata, Y. Production of the natural iron chelator deferriferrichrysin from Aspergillus oryzae and evaluation as a novel food-grade antioxidant. J. Sci. Food Agric. 2016, 96, 2998–3006. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, N.; Gessho, M.; Torii, T.; Ashida, Y.; Akamatsu, M.; Guo, A.K.; Lee, S.; Katsuno, T.; Nakajima, W.; Budirahardja, Y.; et al. The iron chelator deferriferrichrysin induces paraptosis via extracellular signal-related kinase activation in cancer cells. Genes Cells 2023, 28, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Hahm, K.M.; Park, S.H.; Oh, S.W.; Kim, J.H.; Yeom, H.S.; Lee, H.J.; Yang, S.; Cho, J.Y.; Park, J.O.; Lee, J. Aspergillus oryzae-Fermented Wheat Peptone Enhances the Potential of Proliferation and Hydration of Human Keratinocytes through Activation of p44/42 MAPK. Molecules 2021, 26, 6074. [Google Scholar] [CrossRef]
- Yu, H.; Li, Y.; Pan, Y.; Wang, H.; Wang, W.; Ren, X.; Yuan, H.; Lv, Z.; Zuo, Y.; Liu, Z.; et al. Multifunctional porous poly (L-lactic acid) nanofiber membranes with enhanced anti-inflammation, angiogenesis and antibacterial properties for diabetic wound healing. J. Nanobiotechnology 2023, 21, 110. [Google Scholar] [CrossRef] [PubMed]
- Kotwal, G.J.; Chien, S. Macrophage Differentiation in Normal and Accelerated Wound Healing. In Results and Problems in Cell Differentiation; Springer: Berlin/Heidelberg, Germany, 2017; Volume 62, pp. 353–364. [Google Scholar] [CrossRef]
- Darby, I.A.; Laverdet, B.; Bonte, F.; Desmouliere, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katahira, Y.; Sonoda, J.; Yamagishi, M.; Horio, E.; Yamaguchi, N.; Hasegawa, H.; Mizoguchi, I.; Yoshimoto, T. Topical Application of Cha-Koji, Green Tea Leaves Fermented with Aspergillus Luchuensis var Kawachii Kitahara, Promotes Acute Cutaneous Wound Healing in Mice. Sci. Pharm. 2024, 92, 44. https://doi.org/10.3390/scipharm92030044
Katahira Y, Sonoda J, Yamagishi M, Horio E, Yamaguchi N, Hasegawa H, Mizoguchi I, Yoshimoto T. Topical Application of Cha-Koji, Green Tea Leaves Fermented with Aspergillus Luchuensis var Kawachii Kitahara, Promotes Acute Cutaneous Wound Healing in Mice. Scientia Pharmaceutica. 2024; 92(3):44. https://doi.org/10.3390/scipharm92030044
Chicago/Turabian StyleKatahira, Yasuhiro, Jukito Sonoda, Miu Yamagishi, Eri Horio, Natsuki Yamaguchi, Hideaki Hasegawa, Izuru Mizoguchi, and Takayuki Yoshimoto. 2024. "Topical Application of Cha-Koji, Green Tea Leaves Fermented with Aspergillus Luchuensis var Kawachii Kitahara, Promotes Acute Cutaneous Wound Healing in Mice" Scientia Pharmaceutica 92, no. 3: 44. https://doi.org/10.3390/scipharm92030044
APA StyleKatahira, Y., Sonoda, J., Yamagishi, M., Horio, E., Yamaguchi, N., Hasegawa, H., Mizoguchi, I., & Yoshimoto, T. (2024). Topical Application of Cha-Koji, Green Tea Leaves Fermented with Aspergillus Luchuensis var Kawachii Kitahara, Promotes Acute Cutaneous Wound Healing in Mice. Scientia Pharmaceutica, 92(3), 44. https://doi.org/10.3390/scipharm92030044







