Targeted Gene Delivery to Muscle Cells In Vitro and In Vivo Using Electrostatically Stabilized DNA—Peptide Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Peptide and Peptide-Based Polymer Preparation
2.3. Plasmids Preparation
2.4. Preparation of Ternary Polyplexes
2.5. Relaxation of Ternary Polyplexes by Dextran Sulfate
2.6. Gene Transfection and Cytotoxicity Assays
2.7. Size and Zeta-Potential of Ternary Polyplexes
2.8. Cellular Uptake of Ternary Polyplexes
2.9. In Vivo Gene Transfer to Skeletal Muscle Tissue
2.10. Statistical Analysis
3. Results
3.1. Design of Carriers
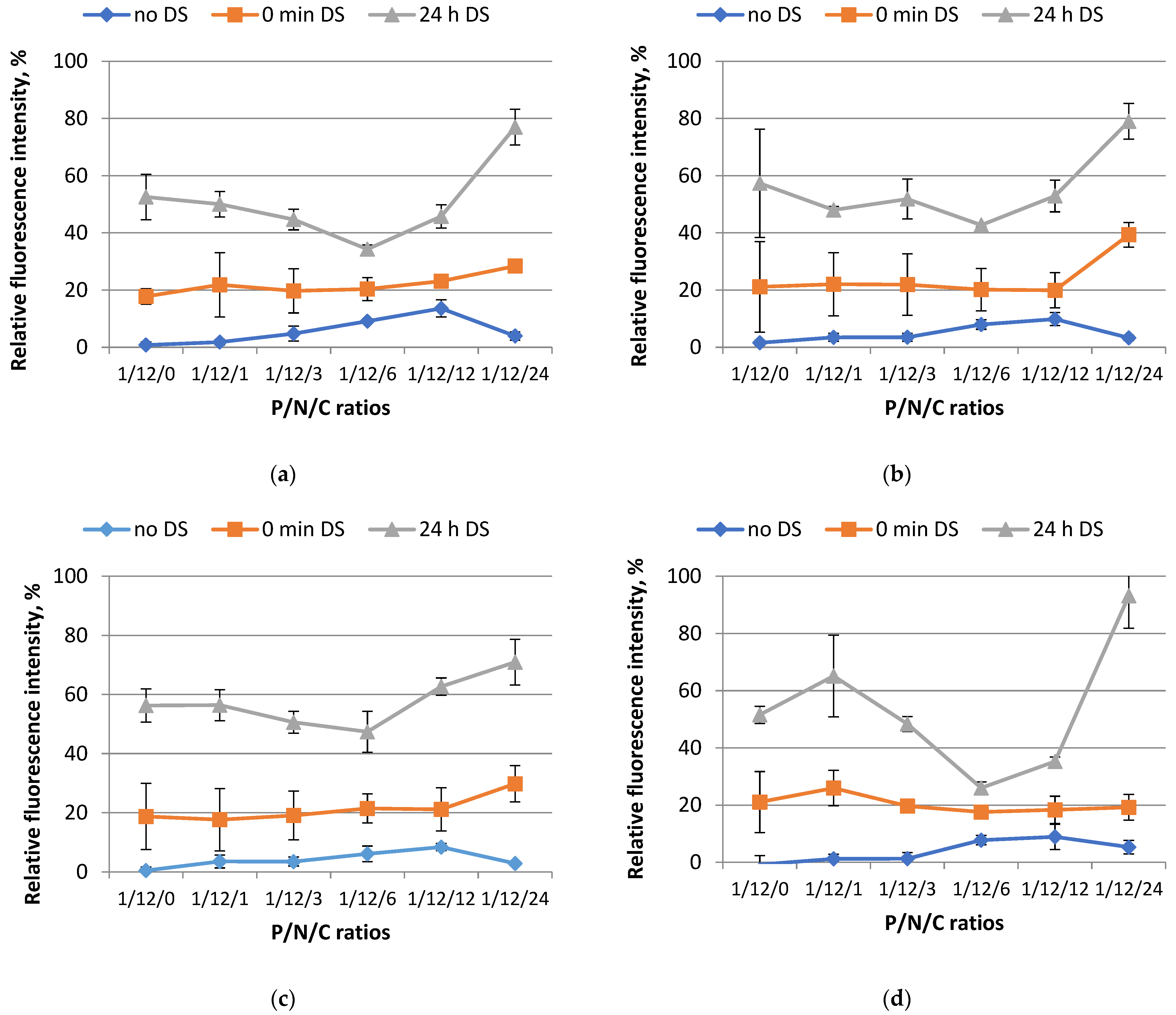
3.2. Study of Polyplex Relaxation by Polyanions
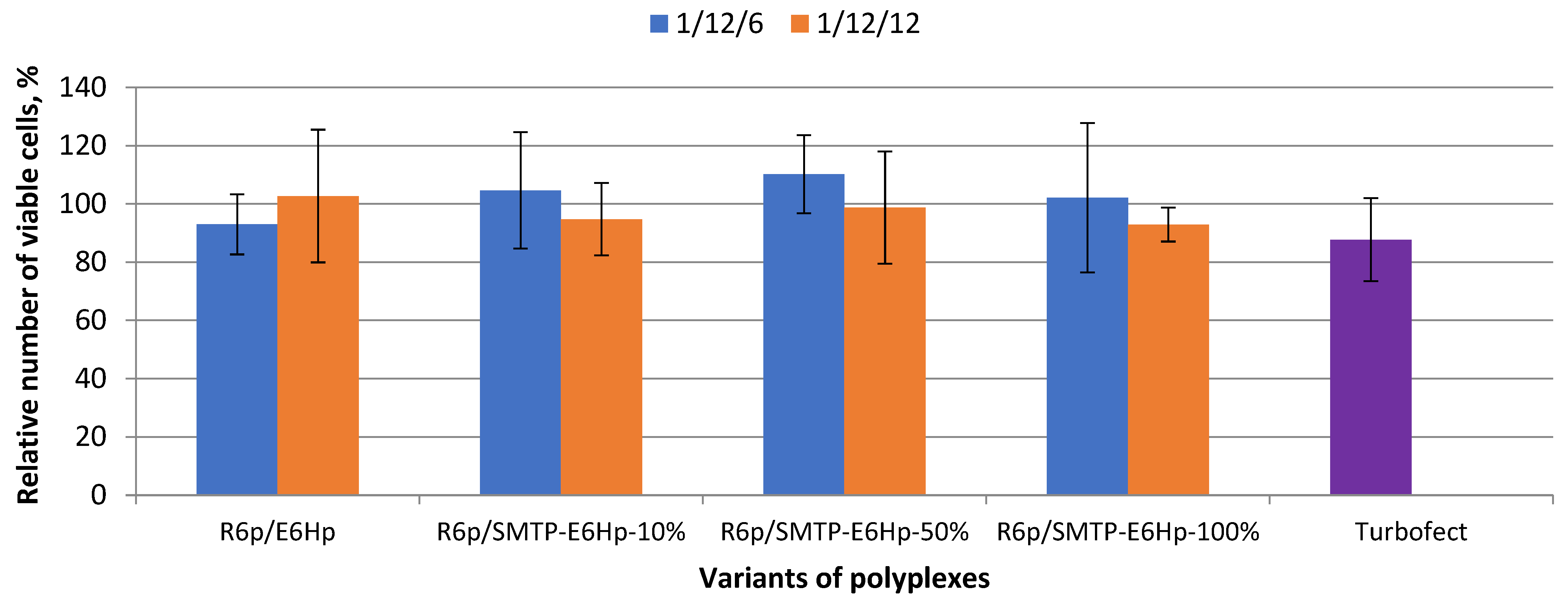
3.3. Evaluation of Ternary Polyplex Cytotoxicity
3.4. Evaluation of Transfectional Properties of Ternary Polyplexes In Vitro
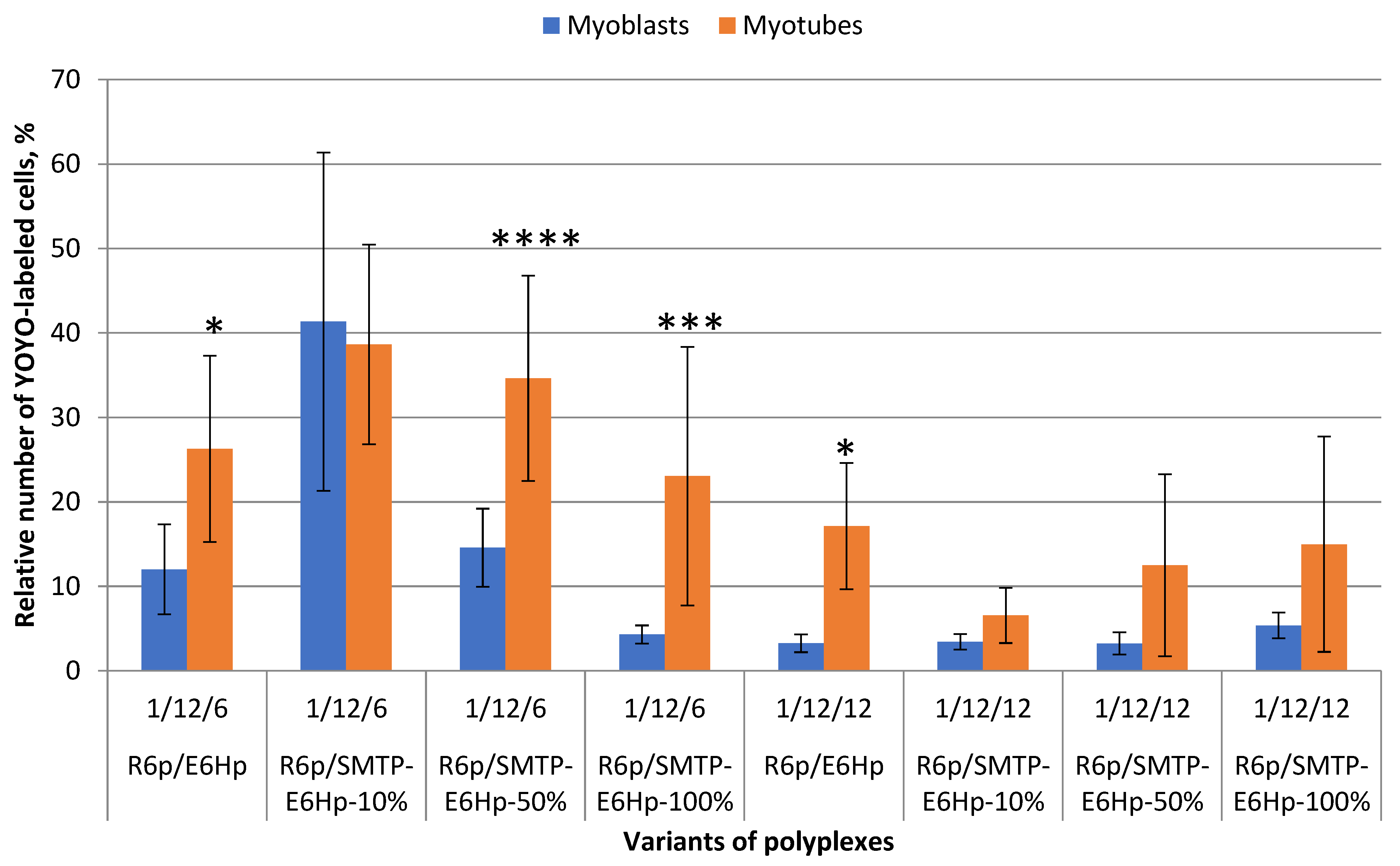
3.5. Evaluation of Cellular Uptake of Ternary Polyplexes
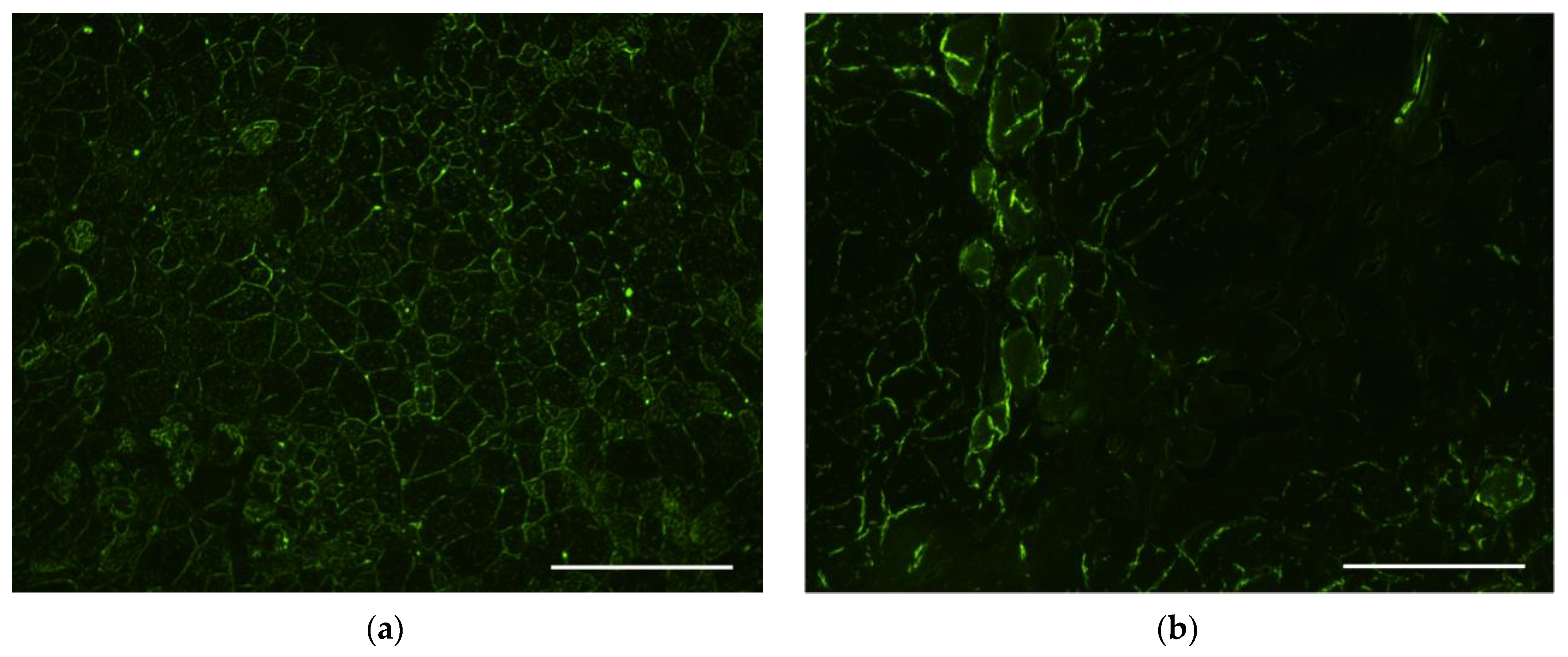
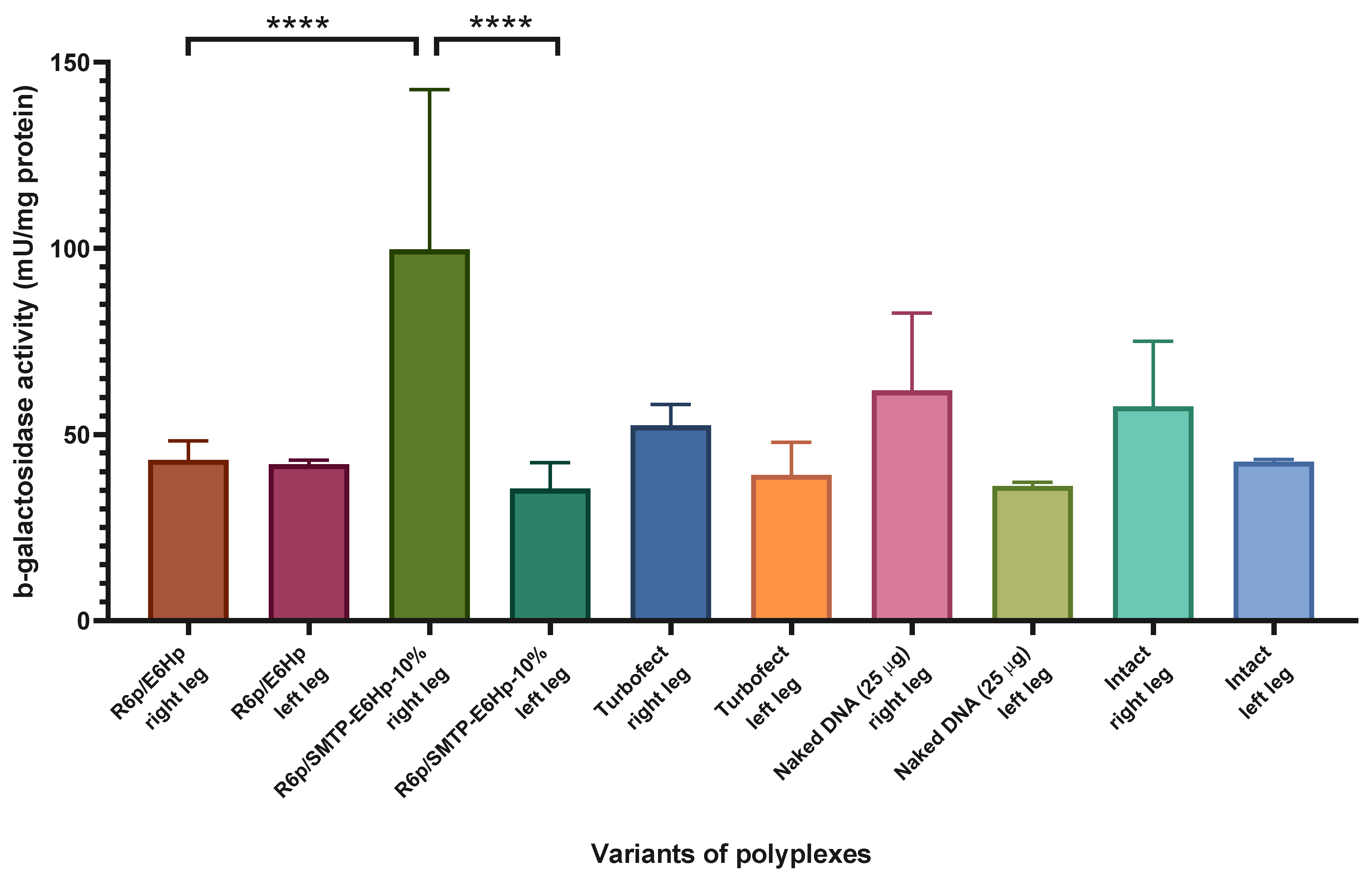
3.6. Evaluation of Transfectional Properties of Ternary Polyplexes In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butt, M.; Zaman, M.; Ahmad, A.; Khan, R.; Mallhi, T.; Hasan, M.; Khan, Y.; Hafeez, S.; Massoud, E.; Rahman, M.; et al. Appraisal for the Potential of Viral and Nonviral Vectors in Gene Therapy: A Review. Genes 2022, 13, 1370. [Google Scholar] [CrossRef] [PubMed]
- Shtykalova, S.; Deviatkin, D.; Freund, S.; Egorova, A.; Kiselev, A. Non-Viral Carriers for Nucleic Acids Delivery: Fundamentals and Current Applications. Life 2023, 13, 903. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Xia, Z.L.; Ze, L.J.; Jing, H.; Xin, B.; Fu, S. Research Progress of nucleic acid delivery vectors for gene therapy. Biomed. Microdevices 2020, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Mastrobattista, E.; van der Aa, M.A.E.M.; Hennink, W.E.; Crommelin, D.J.A. Artificial viruses: A nanotechnological approach to gene delivery. Nat. Rev. Drug Discov. 2006, 5, 115–121. [Google Scholar] [CrossRef]
- A Egorova, A.; V Kiselev, A. Peptide modules for overcoming barriers of nucleic acids transport to cells. Curr. Top. Med. Chem. 2015, 16, 330–342. [Google Scholar] [CrossRef]
- McErlean, E.M.; McCrudden, C.M.; McCarthy, H.O. Delivery of Nucleic Acids for Cancer Gene therapy: Overcoming extra- and intra-cellular Barriers. Ther. Deliv. 2016, 7, 619–637. [Google Scholar] [CrossRef]
- Lu, Q.L.; Bou-Gharios, G.; Partridge, T.A. Non-viral gene delivery in skeletal muscle: A protein factory. Gene Ther. 2003, 10, 131–142. [Google Scholar] [CrossRef]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef]
- Ke, H.; Guay, K.P.; Flotte, T.R.; Gierasch, L.M.; Gershenson, A.; Hebert, D.N. Secretion of functional α1-antitrypsin is cell type dependent: Implications for intramuscular delivery for gene therapy. Proc. Natl. Acad. Sci. USA 2022, 119, 1–12. [Google Scholar] [CrossRef]
- Duan, D.; Mendell, J.R. (Eds.) Muscle Gene Therapy, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–811. [Google Scholar] [CrossRef]
- Nirasawa, K.; Hamada, K.; Naraki, Y.; Kikkawa, Y.; Sasaki, E.; Endo-Takahashi, Y.; Hamano, N.; Katagiri, F.; Nomizu, M.; Negishi, Y. Development of A2G80 peptide-gene complex for targeted delivery to muscle cells. J. Control. Release 2021, 329, 988–996. [Google Scholar] [CrossRef]
- Samoylova, T.I.; Smith, B.F. Elucidation of muscle-binding peptides by phage display screening. Muscle Nerve 1999, 22, 460–466. [Google Scholar] [CrossRef]
- Seow, Y.; Yin, H.; Wood, M.J.A. Identification of a novel muscle targeting peptide in mdx mice. Peptides 2010, 31, 1873–1877. [Google Scholar] [CrossRef] [PubMed]
- Jativa, S.D.; Thapar, N.; Broyles, D.; Dikici, E.; Daftarian, P.; Jiménez, J.J.; Daunert, S.; Deo, S.K. Enhanced Delivery of Plasmid DNA to Skeletal Muscle Cells using a DLC8-Binding Peptide and ASSLNIA-Modified PAMAM Dendrimer. Mol. Pharm. 2019, 16, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Hersh, J.; Condor Capcha, J.M.; Iansen Irion, C.; Lambert, G.; Noguera, M.; Singh, M.; Kaur, A.; Dikici, E.; Jiménez, J.J.; Shehadeh, L.A.; et al. Peptide-Functionalized Dendrimer Nanocarriers for Targeted Microdystrophin Gene Delivery. Pharmaceutics 2021, 13, 2159. [Google Scholar] [CrossRef]
- Harris, T.J.; Green, J.J.; Fung, P.W.; Langer, R.; Anderson, D.G.; Bhatia, S.N. Tissue-specific gene delivery via nanoparticle coating. Biomaterials 2010, 31, 998–1006. [Google Scholar] [CrossRef]
- Tang, G.P.; Zeng, J.M.; Gao, S.J.; Ma, Y.X.; Shi, L.; Li, Y.; Too, H.-P.; Wang, S. Polyethylene glycol modified polyethylenimine for improved CNS gene transfer: Effects of PEGylation extent. Biomaterials 2003, 24, 2351–2362. [Google Scholar] [CrossRef]
- Shimizu, T.; Lila, A.S.A.; Kitayama, Y.; Abe, R.; Takata, H.; Ando, H.; Ishima, Y.; Ishida, T. Peritoneal B Cells Play a Role in the Production of Anti-polyethylene Glycol (PEG) IgM against Intravenously Injected siRNA-PEGylated Liposome Complexes. Biol. Pharm. Bull. 2024, 47, b23-00733. [Google Scholar] [CrossRef]
- Iwanaga, M.; Kodama, Y.; Muro, T.; Nakagawa, H.; Kurosaki, T.; Sato, K.; Nakamura, T.; Kitahara, T.; Sasaki, H. Biocompatible complex coated with glycosaminoglycan for gene delivery. J. Drug Target. 2017, 25, 370–378. [Google Scholar] [CrossRef]
- Kodama, Y.; Tokunaga, A.; Hashizume, J.; Nakagawa, H.; Harasawa, H.; Kurosaki, T.; Nakamura, T.; Nishida, K.; Nakashima, M.; Hashida, M.; et al. Evaluation of transgene expression characteristics and DNA vaccination against melanoma metastasis of an intravenously injected ternary complex with biodegradable dendrigraft poly-L-lysine in mice. Drug Deliv. 2021, 28, 542–549. [Google Scholar] [CrossRef]
- Egorova, A.; Shtykalova, S.; Maretina, M.; Freund, S.; Selutin, A.; Shved, N.; Selkov, S.; Kiselev, A. Serum-Resistant Ternary DNA Polyplexes for Suicide Gene Therapy of Uterine Leiomyoma. Int. J. Mol. Sci. 2023, 25, 34. [Google Scholar] [CrossRef]
- Islam, M.S.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Extracellular matrix in uterine leiomyoma pathogenesis: A potential target for future therapeutics. Hum. Reprod. Update 2018, 24, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Aitken, A.; Learmonth, M. Estimation of Disulfide Bonds Using Ellman’s Reagent. In The Protein Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 1996; pp. 1053–1055. ISBN 978-1-60327-259-9. [Google Scholar]
- Kiselev, A.; Egorova, A.; Laukkanen, A.; Baranov, V.; Urtti, A. Characterization of reducible peptide oligomers as carriers for gene delivery. Int. J. Pharm. 2013, 441, 736–747. [Google Scholar] [CrossRef]
- Egorova, A.; Kiselev, A.; Hakli, M.; Ruponen, M.; Baranov, V.; Urtti, A. Chemokine-derived peptides as carriers for gene delivery to CXCR4 expressing cells. J. Gene Med. 2009, 11, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Egorova, A.; Selutin, A.; Maretina, M.; Selkov, S.; Kiselev, A. Peptide-Based Nanoparticles for αvβ3 Integrin-Targeted DNA Delivery to Cancer and Uterine Leiomyoma Cells. Molecules 2022, 27, 8363. [Google Scholar] [CrossRef]
- Midoux, P.; Monsigny, M. Efficient Gene Transfer by Histidylated Polylysine/pDNA Complexes. Bioconjug. Chem. 1999, 10, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Anchordoquy, T. Drug Delivery Trends in Clinical Trials and Translational Medicine: Challenges and Opportunities in the Delivery of Nucleic Acid-Based Therapeutics. J. Pharm. Sci. 2011, 100, 38–52. [Google Scholar] [CrossRef]
- Wang, T.; Upponi, J.R.; Torchilin, V.P. Design of multifunctional non-viral gene vectors to overcome physiological barriers: Dilemmas and strategies. Int. J. Pharm. 2012, 427, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, T.R.; Raj, A.; Tseng, T.H.; Xiao, H.; Nguyen, R.; Mohammed, F.S.; Halder, S.; Xu, M.; Wu, M.J.; Bao, S.; et al. Biocompatibility of nanomaterials and their immunological properties. Biomed. Mater. 2021, 16, 042005. [Google Scholar] [CrossRef]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In Vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Zhu, L.; Weller, H.; Mews, A.; Parak, W.J.; Barz, M.; Feliu, N. Ligand density on nanoparticles: A parameter with critical impact on nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 22–36. [Google Scholar] [CrossRef]
- Wang, J.; Tian, S.; Petros, R.A.; Napier, M.E.; DeSimone, J.M. The Complex Role of Multivalency in Nanoparticles Targeting the Transferrin Receptor for Cancer Therapies. J. Am. Chem. Soc. 2010, 132, 11306–11313. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, J.; García, I.; Liz-Marzán, L.M. Cellular Uptake of Nanoparticles versus Small Molecules: A Matter of Size. Acc. Chem. Res. 2018, 51, 2305–2313. [Google Scholar] [CrossRef]
- Egorova, A.; Shtykalova, S.; Maretina, M.; Selutin, A.; Shved, N.; Deviatkin, D.; Selkov, S.; Baranov, V.; Kiselev, A. Polycondensed Peptide Carriers Modified with Cyclic RGD Ligand for Targeted Suicide Gene Delivery to Uterine Fibroid Cells. Int. J. Mol. Sci. 2022, 23, 1164. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Yuan, Z.; Cao, Z.; Wang, B.; Qiao, C.; Li, J.; Xiao, X. A muscle-targeting peptide displayed on AAV2 improves muscle tropism on systemic delivery. Gene Ther. 2009, 16, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Sicinski, P.; Geng, Y.; Ryder-Cook, A.S.; Barnard, E.A.; Darlison, M.G.; Barnard, P.J. The Molecular Basis of Muscular Dystrophy in the mdx Mouse: A Point Mutation. Science 1989, 244, 1578–1580. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct Gene Transfer into Mouse Muscle In Vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Plank, C.; Mechtler, K.; Szoka, F.C.; Wagner, E. Activation of the complement system by synthetic DNA complexes: A potential barrier for intravenous gene delivery. Hum. Gene Ther. 1996, 7, 1437–1446. [Google Scholar] [CrossRef]
- Ibaraki, H.; Kanazawa, T.; Owada, M.; Iwaya, K.; Takashima, Y.; Seta, Y. Anti-Metastatic Effects on Melanoma via Intravenous Administration of Anti-NF-κB siRNA Complexed with Functional Peptide-Modified Nano-Micelles. Pharmaceutics 2020, 12, 64. [Google Scholar] [CrossRef]
- Kodama, Y.; Nakamura, T.; Kurosaki, T.; Egashira, K.; Mine, T.; Nakagawa, H.; Muro, T.; Kitahara, T.; Higuchi, N.; Sasaki, H. Biodegradable nanoparticles composed of dendrigraft poly-l-lysine for gene delivery. Eur. J. Pharm. Biopharm. 2014, 87, 472–479. [Google Scholar] [CrossRef]
- Shtykalova, S.; Egorova, A.; Maretina, M.; Baranov, V.; Kiselev, A. Magnetic Nanoparticles as a Component of Peptide-Based DNA Delivery System for Suicide Gene Therapy of Uterine Leiomyoma. Bioengineering 2022, 9, 112. [Google Scholar] [CrossRef]
- Cui, Z.; Jiao, Y.; Pu, L.; Tang, J.Z.; Wang, G. The Progress of Non-Viral Materials and Methods for Gene Delivery to Skeletal Muscle. Pharmaceutics 2022, 14, 2428. [Google Scholar] [CrossRef] [PubMed]



| Name | Composition | |
|---|---|---|
| Monomers | R6 | CHRRRRRRHC |
| E6H | CHHEEEEEEHHC | |
| Ligand monomer | SMTP-E6H | ASSLNIAXXCHHEEEEEEHHC |
| Polycondensed carriers | R6p | (CHRRRRRRHC)n |
| E6Hp | (CHHEEEEEEHHC)n | |
| SMTP-E6Hp-100% | (ASSLNIAXXCHHEEEEEEHHC)n | |
| SMTP-E6Hp-50% | 50 mol % (ASSLNIAXXCHHEEEEEEHHC)n + 50 mol% (CHHEEEEEEHHC)n | |
| SMTP-E6Hp-10% | 10 mol % (ASSLNIAXXCHHEEEEEEHHC)n + 90 mol% (CHHEEEEEEHHC)n |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorova, A.; Chepanov, S.; Selkov, S.; Kogan, I.; Kiselev, A. Targeted Gene Delivery to Muscle Cells In Vitro and In Vivo Using Electrostatically Stabilized DNA—Peptide Complexes. Sci. Pharm. 2024, 92, 51. https://doi.org/10.3390/scipharm92030051
Egorova A, Chepanov S, Selkov S, Kogan I, Kiselev A. Targeted Gene Delivery to Muscle Cells In Vitro and In Vivo Using Electrostatically Stabilized DNA—Peptide Complexes. Scientia Pharmaceutica. 2024; 92(3):51. https://doi.org/10.3390/scipharm92030051
Chicago/Turabian StyleEgorova, Anna, Sergei Chepanov, Sergei Selkov, Igor Kogan, and Anton Kiselev. 2024. "Targeted Gene Delivery to Muscle Cells In Vitro and In Vivo Using Electrostatically Stabilized DNA—Peptide Complexes" Scientia Pharmaceutica 92, no. 3: 51. https://doi.org/10.3390/scipharm92030051







