Abstract
Cannabigerol (CBG), a phytocannabinoid, has shown promise in pain management. Previous studies by our research group identified an increase in pain sensitivity as a consequence of prenatal hypoxia-ischemia (HI) in an animal model. This study aimed to investigate the efficacy of CBG in acute and chronic hyperalgesia induced by prenatal HI. A pharmacological screening was first conducted using hot plate and open-field tests to evaluate the antinociceptive and locomotor activities of animals administered with a 50 mg/kg oral dose of cannabis extract with a high CBG content. Prenatal HI was induced in pregnant rats, and the offspring were used to evaluate the acute antinociceptive effect of CBG in the formalin-induced peripheral pain model, while chronic antinociceptive effects were observed through spinal nerve ligation (SNL) surgery, a model used to induce neuropathic pain. Our results show that CBG exhibited an antinociceptive effect in the hot plate test without affecting the animals’ motor function in the open-field test. CBG significantly reduced formalin-induced reactivity in HI offspring during both the neurogenic and inflammatory phases. CBG treatment alleviated thermal and mechanical hypernociception induced by SNL. Biomolecular analysis revealed CBG’s ability to modulate expression, particularly reducing TNFα and Nav1.7 in HI male and female rats, respectively. These results highlight CBG as a potential antinociceptive agent in acute and chronic pain models, suggesting it as a promising therapeutic option without inducing motor impairment. Further research is needed to fully elucidate its mechanisms and clinical applications in pain management.
1. Introduction
Pain is a crucial physiological response that serves as an essential warning mechanism for living organisms, signaling potential or actual tissue damage and helping to define the safety limits of certain actions. When the body’s physical integrity is threatened, an alert system is activated, making pain a primary symptom of injury [1,2,3]. The International Association for the Study of Pain (IASP) defines it as an unpleasant sensory and emotional experience related to actual or potential tissue damage, or a condition resembling such damage [4]. Pain is generally categorized into acute and chronic types, with chronic pain further divided into neuropathic, inflammatory, and cancer pain [5,6]. Understanding these categories and their implications is crucial for effective diagnosis and treatment, considering its complex interaction with psychological, social, and environmental factors.
Hypoxic-ischemic (HI) events are closely associated with pain, especially when they impact the central nervous system (CNS) during the peri- and prenatal periods, significantly affecting neurological development. The consequences of HI depend on factors such as timing, severity, and the specific CNS regions involved [7,8,9]. HI involves a reduction in oxygen and blood flow, which can lead to various cognitive and motor impairments, including hyperactivity, attention deficits [10,11], speech and language disorders, growth issues, discomfort, and, in severe cases, cerebral palsy [12]. Our research group recently found that adult rats exposed to prenatal HI exhibited hypernociception in the formalin pain model at 30 and 90 days post-birth (P30 and P90) [13]. Building on these preclinical results, we investigated the clinical prevalence of pain and heightened sensitivity in children who experienced perinatal HI, considering sociodemographic factors. Our findings revealed a higher prevalence of pain, particularly in the head region, among premature infants, with more intense pain reported in girls and children who self-identified as black or brown-skinned [14].
Despite significant technological advancements in recent decades, the development of new therapies for pain management has been largely unsatisfactory. Pain remains a highly individualized experience [15], and difficulties in finding effective treatments may arise from the complex interplay between peripheral and central mechanisms that both generate and sustain pain. This complexity often involves neuroinflammation, driven by the activation of microglia and astrocytes, as well as immune cell infiltration in peripheral and central regions such as the dorsal root ganglia (DRG) and the spinal cord [16,17,18]. Consequently, there is growing interest in exploring new strategies for pain relief, with one promising approach being the therapeutic potential of phytocannabinoids derived from Cannabis sativa [19].
The resurgence in interest regarding the medicinal properties of Cannabis sativa can be attributed to the endocannabinoid system (ECS) and its potential for therapeutic applications of cannabinoids and phytocannabinoids [20,21,22]. The cannabis plant contains approximately 500 distinct compounds, with cannabinoids being the most prominent class. Over 120 cannabinoids have been identified, including delta-8 and delta-9 tetrahydrocannabinol (Δ8-THC and Δ9-THC), cannabidiol (CBD), and cannabinol (CBN) [23]. Research has historically concentrated on Δ9-THC and CBD, leading to a better understanding of their pharmacological effects and sparking the growing interest in the plant’s broader medicinal potential [24]. Recently, the United Nations removed Cannabis sativa from its list of the most dangerous drugs [25], formally acknowledging its therapeutic potential, including for chronic pain relief [26].
In addition to Δ9-THC and CBD, Cannabis sativa also produces cannabigerol (CBG), a non-psychoactive cannabinoid typically found in plants with low THC content. CBG is derived from cannabigerolic acid (CBGA) [27,28] and interacts with CB1 and CB2 receptors similarly to Δ9-THC and CBD, but also uniquely engages with α2-adrenergic receptors, suggesting potential analgesic properties [29]. A PubMed search for studies on CBG, pain, and antinociceptive effects—using keywords such as “cannabigerol”, “pain”, and “antinociceptive effect”—reveals only eight articles as of September 15, 2024, with one being a review. Notably, none of these studies use the spinal nerve ligation model, which is considered one of the most reliable methods for replicating neuropathic pain in animal models. Given CBG’s pharmacological profile, this study aims to investigate its potential to alleviate hypernociception in animals subjected to prenatal HI, utilizing cannabis extracts rich in CBG as the treatment.
2. Materials and Methods
2.1. Laboratory Animals
The experimental protocols used in this study were approved by the Ethics Committee for the Care and Use of Experimental Animals of Rio de Janeiro State University (CEUA UERJ 010/2022). Male and female Swiss mice (Mus musculus), weighing between 25 and 30 g and age-matched (between 4 and 6 weeks), as well as male and female Wistar rats (Rattus norvegicus), weighing 100–200 g, were used. These animals were obtained and housed at the animal facility of the Department of Pharmacology and Psychobiology (DFP) at the Instituto de Biologia Roberto Alcantara Gomes (IBRAG) of the State University of Rio de Janeiro (UERJ). They were maintained under a 12 h light–dark cycle (lights off from 6 p.m. to 6 a.m.), with a temperature of 21 ± 1 °C and relative humidity of 50 ± 2%. The animals were housed in polypropylene cages with sawdust bedding, randomized and separated into groups of 7–10 animals by sex, and had free access to water and a controlled diet consisting of pellet food. Prior to testing, they were acclimated at room temperature for 30 min.
2.2. Substances
The cannabis extracts were kindly provided by the Association for the Support of Research and Medicinal Cannabis Patients (APEPI). These extracts were produced from a standardized genetic strain of cannabis designed to contain less than 0.2% Δ9-THC. The chemical analysis of the cannabis extract was performed by the Center for Information and Toxicological Assistance (CIATox) at Campinas University (UNICAMP), which issues reports on the active ingredient content in cannabis extracts. The analysis was conducted under Technical Cooperation Agreement No. 65065921/2023 (Process No. SEI-260007/026216/2023). The other substances used were the following: Acetylsalicylic acid (Sigma, Saint Louis, MO, USA); Dimethyl sulfoxide (DMSO) (Dinâmica, Indaiatuba, Brazil); Formaldehyde P.A. 36% (Proquimios, Rio de Janeiro, Brazil); Xylazine hydrochloride 2% (Vetecia, Louveira, Brazil); ketamine hydrochloride (Cristália, Itapira, Brazil) and Isoflurane (BioChimico, Itatiaia, Brazil). For the experiments, the cannabis extract, with 95% purity, and the acetylsalicylic acid (ASA) were freshly prepared in DMSO minutes before the tests.
2.3. Experimental Model
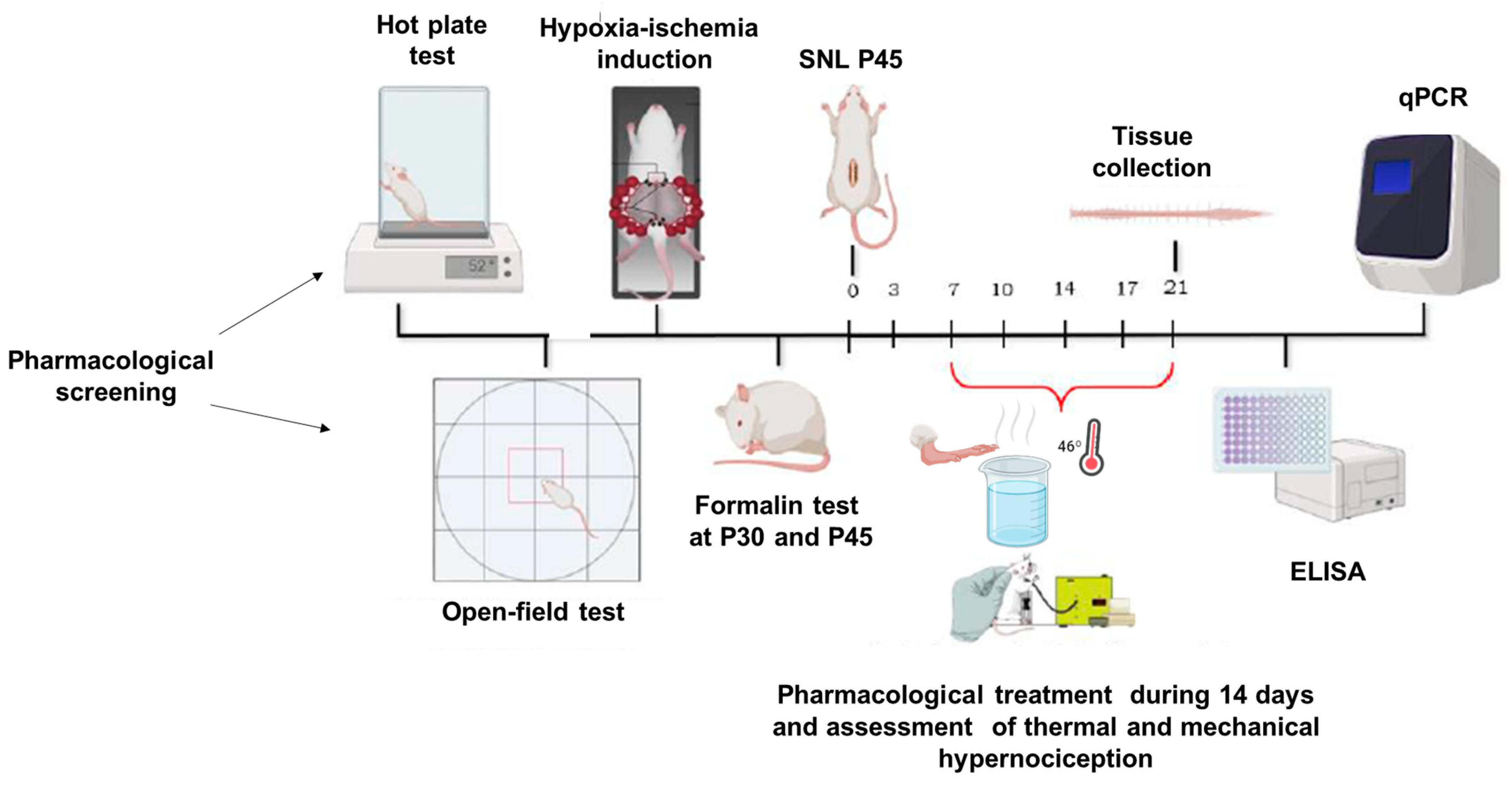
In this study, the initial phase involved a pharmacological screening using both mice and rats. Mice were evaluated using the hot plate test, while rats were subjected to locomotor assessments. For randomization, the animals were divided into two groups: one receiving the vehicle (DMSO) and the other treated with a CBG-rich cannabis extract. From this point, only rats were utilized in the subsequent experiments. The next phase involved inducing a perinatal hypoxic-ischemic (HI) model, where offspring were tested with the formalin assay on postnatal days 30 (P30) and 45 (P45). Animals were administered DMSO, CBG, or ASA via oral (using a gavage). In the following stage, a distinct group of P45 HI and sham rats underwent spinal nerve ligation surgery (SNL) to induce a chronic pain model. Nociceptive responses were monitored for 14 days, with animals receiving CBG or DMSO orally by gavage throughout this period. Finally, molecular analyses were conducted on spinal cord tissues collected post-experimentation. The full timeline of experimental procedures is illustrated in Figure 1.
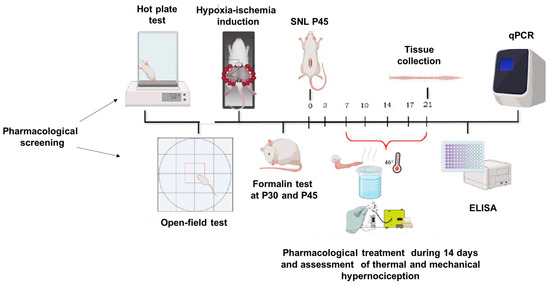
Figure 1.
Timeline of experimental methods, behavioral data collection, and sample collection.
2.3.1. Pharmacological Screening
Hot Plate Test
The central analgesic activity was evaluated using the hot plate test, which was also employed as a pharmacological screening tool. In brief, Swiss mice of both sexes were placed on a hot plate maintained at 52 ± 1 °C (Letica, Letica Scientific Instruments, Barcelona, Spain). The latency of their nociceptive threshold was recorded based on the time until licking or shaking of one of their paws, or jumping, was observed. The maximum duration of the animals on the hot plate was set at 22 s to prevent paw damage. The animal’s response was assessed before and 5–120 min after treatment, following oral administration (by gavage) of either vehicle or CBG (50 mg/kg), with the dose chosen based on data from previous studies showing that CBG at different doses does not alter the locomotor activity of the animals [30], and that the 50 mg/kg dose exhibits anti-inflammatory activity [31]. Antinociceptive efficacy was indexed as a percentage of the maximum possible effect (%MPE) according to the following Equation (1):
% MPE = (postdrug latency) − (predrug latency) × 100% (22 s) − (predrug latency)
Locomotor Activity
To exclude the possibility of the extract affecting motor activity, we used the open-field test to assess the animals’ ambulatory behavior [32,33]. The open field consisted of a high-resistance acrylic circle (100 × 80 × 50 cm; diameter: 60 cm) with transparent walls and a floor divided into 16 squares. Both male and female rats were included in the assessment. The animals were acclimated to the open field for 15 min. After acclimation, they received either CBG (50 mg/kg) or vehicle orally via gavage. Thirty-five minutes post-administration, each rat was placed in the central square of the field, and ambulation (the number of squares crossed) was recorded for 5 min. This ambulation served as a measure of the rats’ locomotor activity.
2.3.2. Induction of Prenatal Hypoxic-Ischemic Insult
Female rats weighing between 250 and 300 g were paired with males in a 2:3 or 1:2 male-to-female ratio for mating at 6:00 p.m. They were kept together in the same cage for 12 h. After this period, the females were identified, weighed, and then transferred to individual cages until the surgical procedure. Pregnancy was identified by monitoring weight gain after 17 days. The HI procedure was based on the model of occlusion of the uterine and ovarian arteries on the 18th day of pregnancy (E18), as described by Savignon et al. [34]. The pregnant rats were anesthetized with isoflurane, using an initial dose of 4% in 2 min/L of oxygen until they lost their reflexes, such as paw sensitivity. Throughout the entire procedure, they were maintained on a heating pad and gas mask with 1.5–2% isoflurane in 0.8 min/L of oxygen. After laparotomy, the uterine horns were exposed, and aneurysm clips were used to clamp the ovarian and uterine arteries for 45 min (HI group). After this, the clips were removed, and the abdominal cavity was sutured. The control group was obtained by exposing the uterine horns without occluding the arteries (SHAM group). The pups were born at term without surgical intervention, and the offspring animals were used in experiments when they completed 30 or 45 days after birth (P30 or P45).
2.3.3. Formalin Test
The formalin test was conducted following a previously established protocol designed for the quantitative assessment of antinociceptive effects [35]. The formalin was administered to promote two phases of nociceptive behavior. The first phase (0–5 min after injection) is referred to as the neurogenic phase. This phase is followed by a short period of quiescence (5–15 min) preceding the second phase (15–45 min after injection), which includes an inflammatory response. Pain was induced by intraplantar injection of formalin solution (50 µL, 2.5%) into the right hind paw. The SHAM and HI rat (P30 and P45) groups, of both sexes, were used in the test. The animals were treated orally using a gavage with either the vehicle, CBG (50 mg/kg), or ASA (300 mg/kg). Both CBG and ASA were dissolved in DMSO, which is our control group. After 40 min of treatment with the substances, the animals received intraplantar formalin injection, and the time spent by the animals licking, scratching, or biting is an indicative of nociceptive response, which it was observed during 45 min.
2.3.4. Chronic Nociception Model
Chronic nociception was induced by spinal nerve ligation (SNL) as previously described [36]. Briefly, after rats (HI or sham—P45) had been anesthetized with a mixture of xylazine (20 mg/kg, i.p.) and ketamine (100 mg/kg, i.p.), their skin was disinfected with povidone-iodine 10% and the lateral laminae of the lower lumbar and upper sacral vertebrae were exposed by a dorsal incision. The right L6 transverse process was removed and the right L5 and L6 spinal nerves were isolated and ligated tightly using 6-0 silk sutures. Sham-operated groups submitted the same process with the exception of the nerve ligation. Seven days after surgery, SNL animals developed thermal and mechanical hypernociception and the treatment was then initiated and maintained for 14 days.
2.3.5. Thermal and Mechanical Hypernociception
Thermal hypernociception was assessed using the paw immersion method in which the paw is placed in water heated to 46 °C. In this experiment, the parameter observed was the time (s) of paw withdrawal from the water, designated as the thermal nociceptive threshold. A cutoff of 15 s was established to avoid paw injury [37]. For the evaluation of thermal hypernociception in the chronic pain model, 3 groups of 10 animals were used: 1 group of HI animals (P45) subjected to SNL surgery and treated orally with CBG gavage (50 mg/kg), a group subjected to HI and SNL surgery and treated orally with vehicle gavage, and a group that was only subjected to the stress caused by the HI (sham) and sham surgery (sham–sham group) and treated with the vehicle orally via gavage.
The assessment of mechanical hypernociception was measured using a digital analgesimeter (Insight—Brazil). The equipment applies a force to the dorsal surface of the hind paw, which increases at a constant rate (a determined number of grams per second). This force is continuously monitored by an indicator moving along the scale and through an electronic panel. The moment when the animal withdraws its paw is observed, indicating nociception. Upon observing the animal’s nociceptive response, the experimenter stops the pressure applied to the paw, and the value indicated on the device’s scale, expressed in grams (g), corresponds to the nociceptive threshold. To assess mechanical hypernociception in the chronic pain model, 3 groups of 10 animals were used: 1 group of HI animals at P45 underwent SNL surgery and received CBG (50 mg/kg), another group underwent the same surgery but received the vehicle, and a control group was subjected to HI (sham) and sham surgery, with all treatments administered orally via gavage.
2.4. Tissue Collection
After the final day of hypernociception testing, conducted 21 days after the SNL surgery, the animals were anesthetized (90 mg/kg of ketamine and 4.5 mg/kg of xylazine, administered intraperitoneally, i.p.) and euthanized for tissue collection. The spinal cord was dissected between the L1 and L5 segments, the tissues were weighed, immediately frozen in liquid nitrogen, and stored at −80 °C prior to RNA or protein extraction.
2.5. Quantification of IFN-Gamma by ELISA
For IFN-gamma measurements, the spinal cord portion was homogenized in a cold RIPA buffer (150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris Base, 2 mM PMSF, pH = 8) and the supernatant was collected after centrifugation at 14,000× g for 10 min at 4 °C. The protein concentration was determined using the BCA Protein Assay (Thermo Scientific, Waltham, MA, USA). Samples diluted 1:10 in the dilution reagent were used for the detection of IFN-gamma (R&D Systems, Minneapolis, MN, USA, AF-585-NA) by ELISA according to the manufacturer’s instructions. The results were expressed as pg/μg of protein.
2.6. Gene Expression Evaluation
Total RNA was isolated using the PureLink™ RNA Mini Kit (Thermo Fisher, Waltham, MA, USA) according to the manufacturer’s instructions. The quantification and quality assessment of the material were performed using the NanoVue® spectrophotometer (GE Healthcare, London, UK). cDNA synthesis was performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystem, Waltham, MA, USA) and the purified mRNA according to the manufacturer’s instructions. The samples were stored at −80 °C until use. Quantitative real-time PCR (qPCR) reactions were performed using the QuantStudio™ 3 Real-Time PCR System. The primers used were designed using the online PrimerQuest Tool (IDT-Integrated DNA Technologies, Coralville, IA, USA), were obtained from the literature, or were already available (Table 1). Briefly, the qPCR reactions contained specific primers, 1× Quantifast SYBR Green PCR master mix (Life Technologies, Carlsbad, CA, USA), 1 µL (10 ng) of template cDNA, in a final reaction volume of 10 µL. The relative expression difference, known as fold change, was calculated using the Livak method (2−ΔΔCt).

Table 1.
Primers used along with their respective sense and antisense sequences.
2.7. Statistical Analysis
Tests and graphs were generated using GraphPad Prism® software, version 8.1 (GraphPad Software Incorporated, San Diego, CA, USA). To assess whether the data followed a normal distribution, we used the Shapiro–Wilk test. Results conforming to a parametric distribution were analyzed by one-way Analysis of Variance (ANOVA) followed by Dunnett’s post hoc test; two-way ANOVA followed by Sidak post hoc test, or an unpaired Student’s t-test. Experimental groups were compared with their respective control groups. Values were expressed as mean ± standard error of the mean (SEM), with p-values less than 0.05 considered statistically significant. Symbols (* or #) denote the degree of significance.
3. Results
3.1. Hot Plate Test
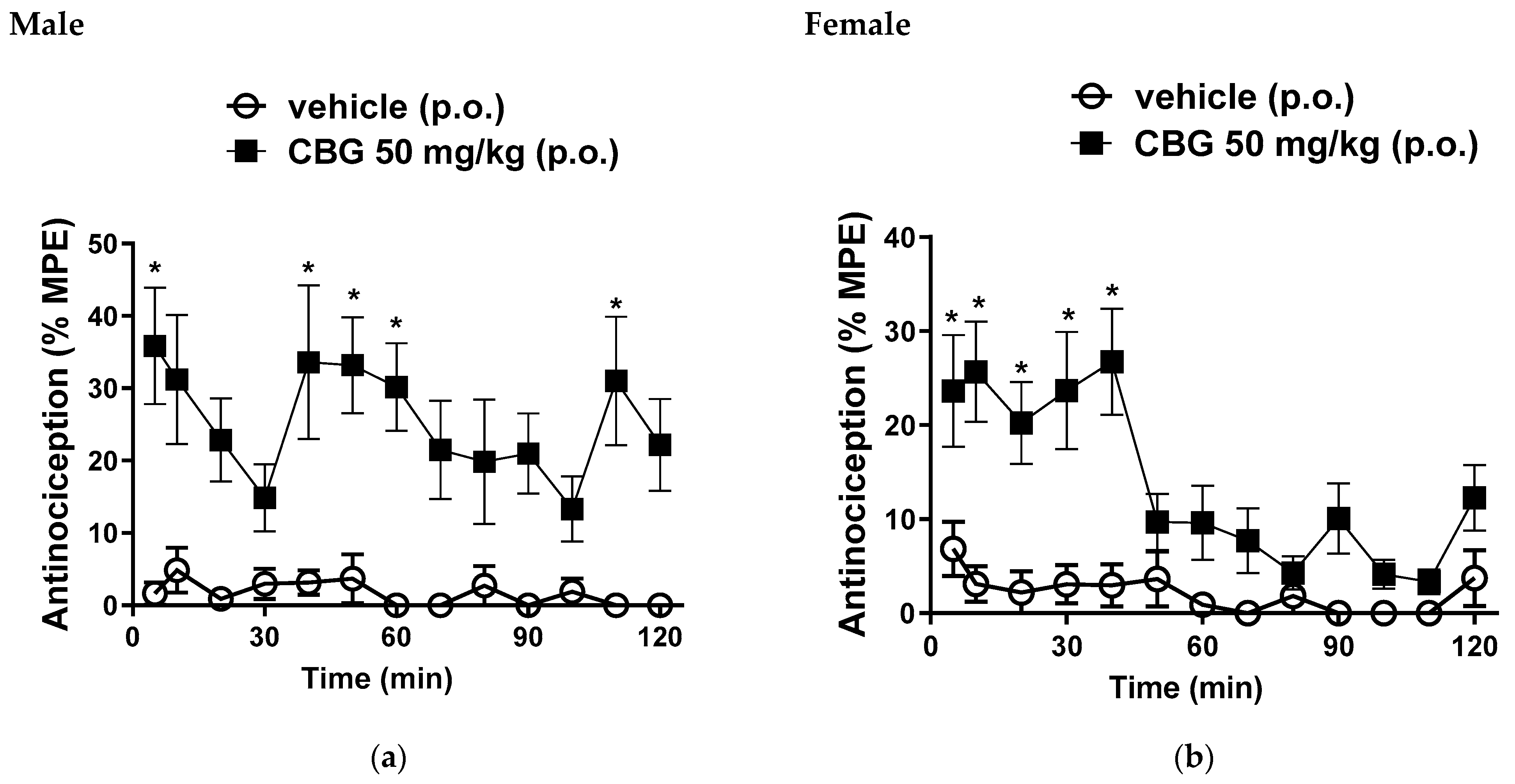
In male mice, we observed a mean %MPE of 35.8 ± 8%; 33.6 ± 10.6%; 33.1 ± 6.6%; 30.1 ± 6.6% and 31 ± 8.8% at 5, 40, 50, 60 and 110 min, respectively, following oral administration of CBG at a dose of 50 mg/kg (Figure 2a). Female mice exhibited %MPE values of 23.2 ± 5.9%; 25.6 ± 5.3%; 20.2 ± 4.3%; 23.6 ± 6% and 26.7 ± 5.6% at 5, 10, 20, 30 and 40 min, respectively, with the same 50 mg/kg oral dose of CBG (Figure 2b). These results are significant when compared to the vehicle control group, confirming the central antinociceptive activity of this CBG dose.
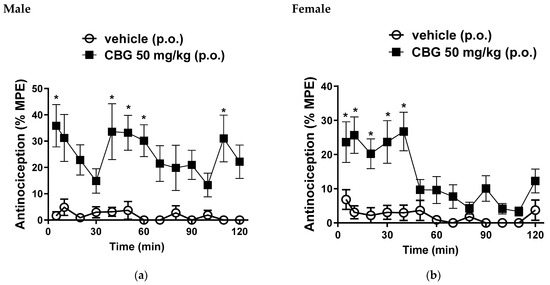
Figure 2.
Effects of oral gavage administration of vehicle or high-CBG cannabis extract (50 mg/kg) in the hot plate test (a) male and (b) female mice. Data are expressed as mean ± SEM (n = 10 per group). * p < 0.05 vs. vehicle, two-way ANOVA followed by Sidak test.
3.2. Locomotor Activity
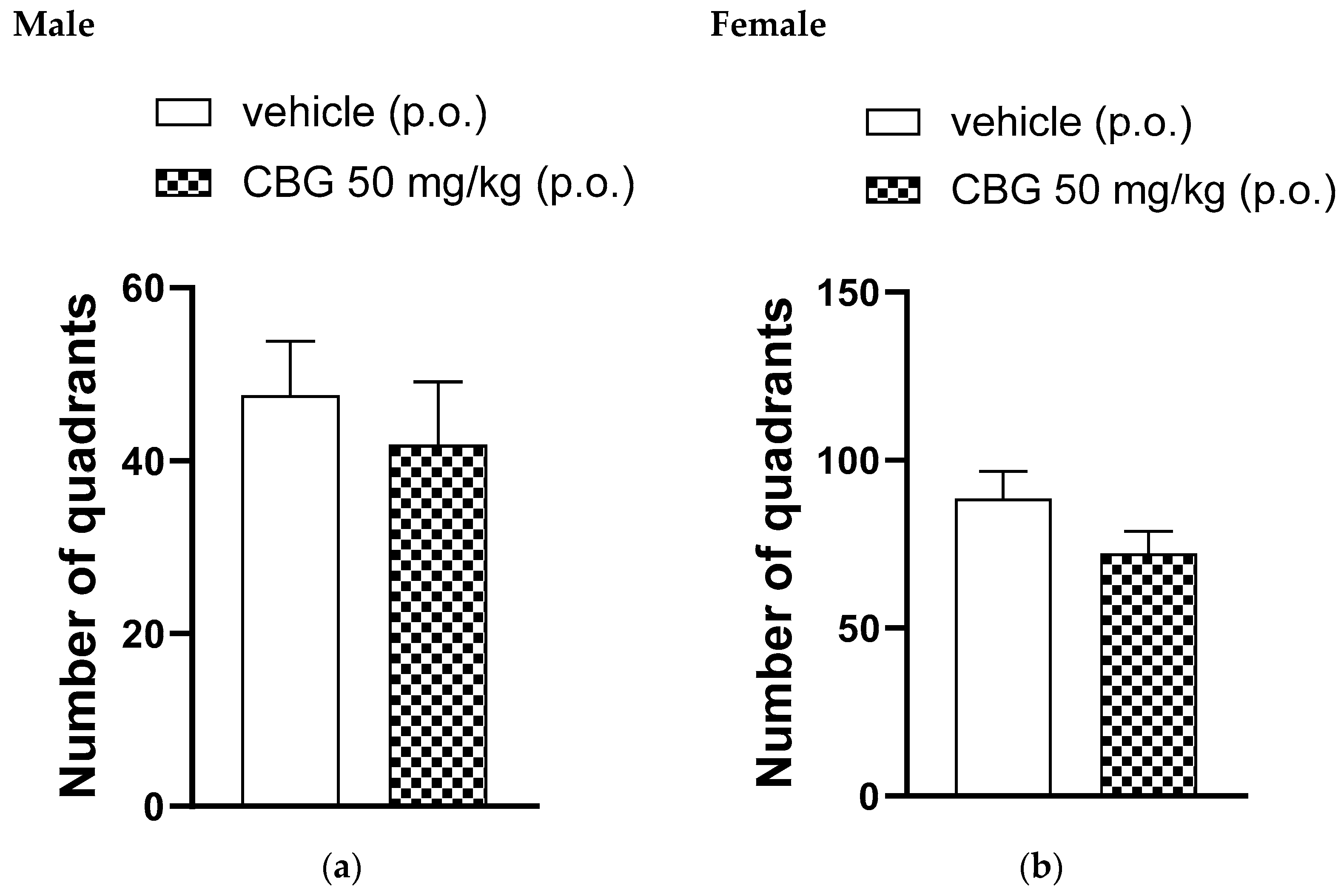
In the open-field test, a single oral administration of 50 mg/kg of CBG or a vehicle was given. Figure 3 shows the number of quadrants crossed by the animals 35 min after administration. The number of quadrants crossed by the animals in the open field was observed for 5 min and showed no significant differences between the CBG-treated group and the vehicle-treated group for both male and female rats.
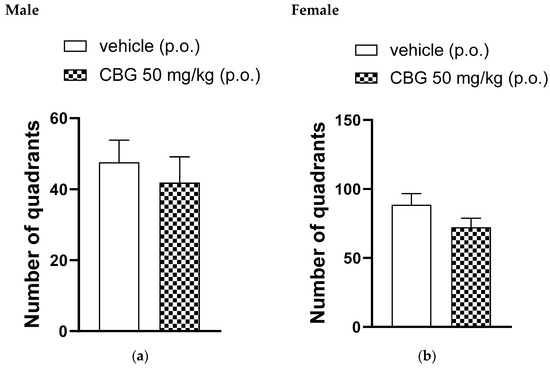
Figure 3.
Effect of locomotor activity of animals assessed in the open-field test, high-CBG cannabis extract (50 mg/kg) or vehicle were orally administered to Wistar rats (a) male and (b) female. Data are presented as mean ± SEM (n = 7 per group). The unpaired Student’s t-test was applied and there were no statistically significant differences between groups.
3.3. Formalin Test
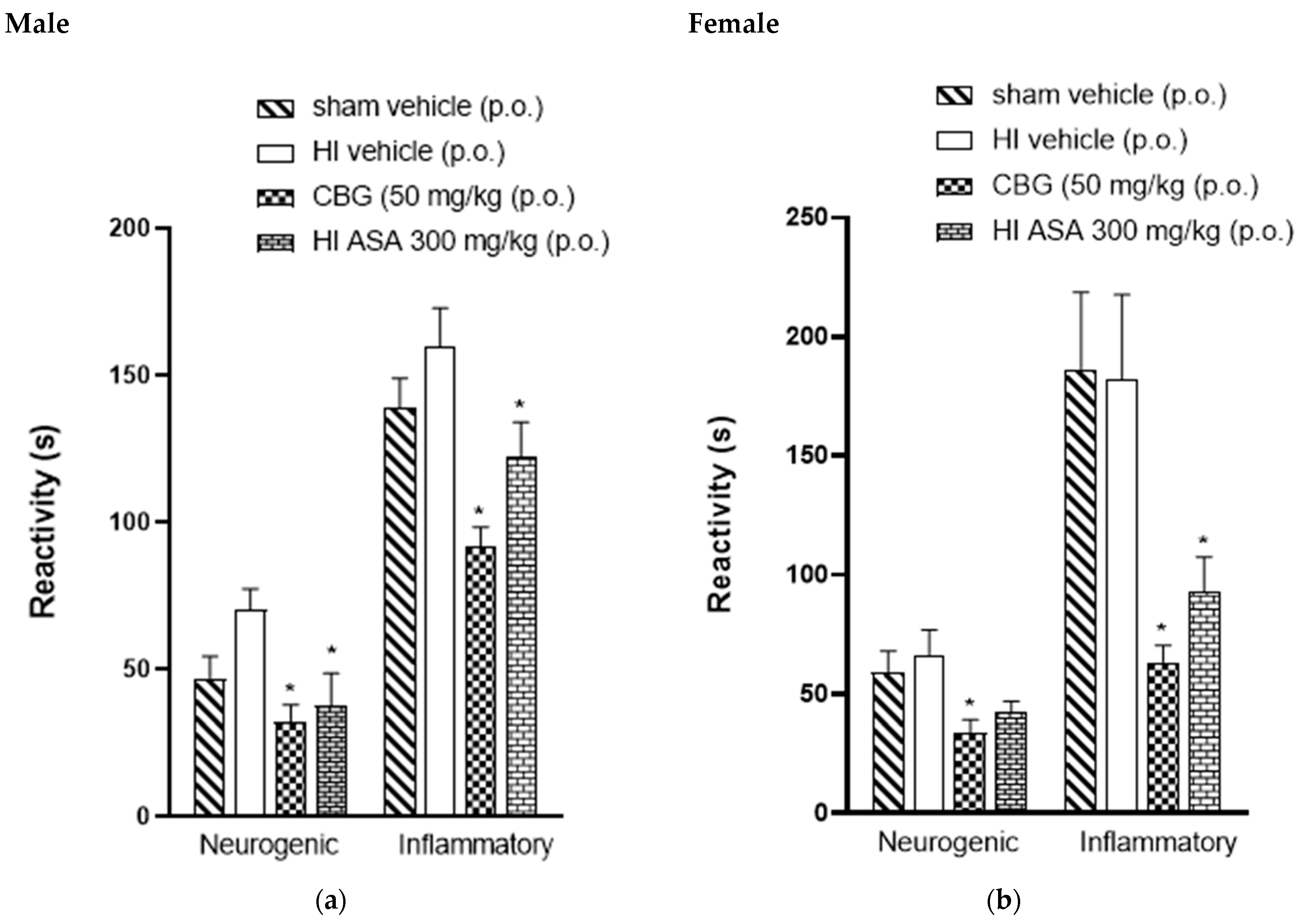
The acute peripheral antinociceptive effect was investigated using the formalin test. Figure 4 presents the neurogenic and inflammatory phase responses following formalin injection in HI (P30) animals treated orally with vehicle, acetylsalicylic acid (ASA 300 mg/kg), and CBG (50 mg/kg), as well as sham-operated HI animals. It was observed that the formalin response time during the neurogenic phase in the CBG-treated group was significantly reduced to 31.6 ± 3.7 s and 35.3 ± 1.4 s for male and female rats, respectively, compared to 41.2 ± 4.3 s and 52.7 ± 6.0 s in the vehicle group for male and female rats, respectively. Therefore, during the inflammatory phase of the formalin test, HI animals treated with CBG at a dose of 50 mg/kg showed a decrease in formalin response time from 125.3 ± 18.9 s and 147 ± 18.5 s (vehicle) to 97.8 ± 18.2 s and 76.8 ± 6.6 s for male and female rats, respectively, as shown in Figure 4.
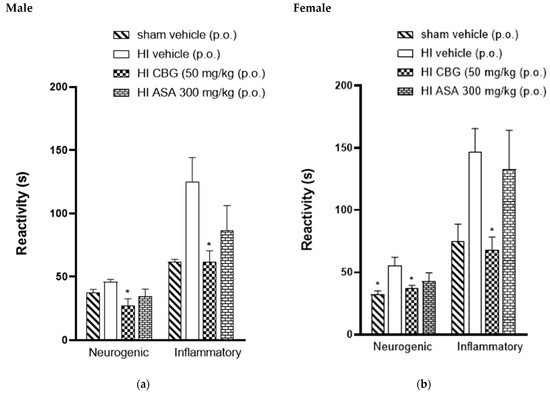
Figure 4.
Effect of oral administration of high-CBG cannabis extract (50 mg/kg), ASA (300 mg/kg) or vehicle during the neurogenic and inflammatory phases of the formalin test in Wistar rats (P30) (a) male and (b) female. Data are expressed as mean ± SEM (n = 8 per group). One-way ANOVA was performed followed by Dunnett’s test, * p < 0.05 compared to the vehicle group.
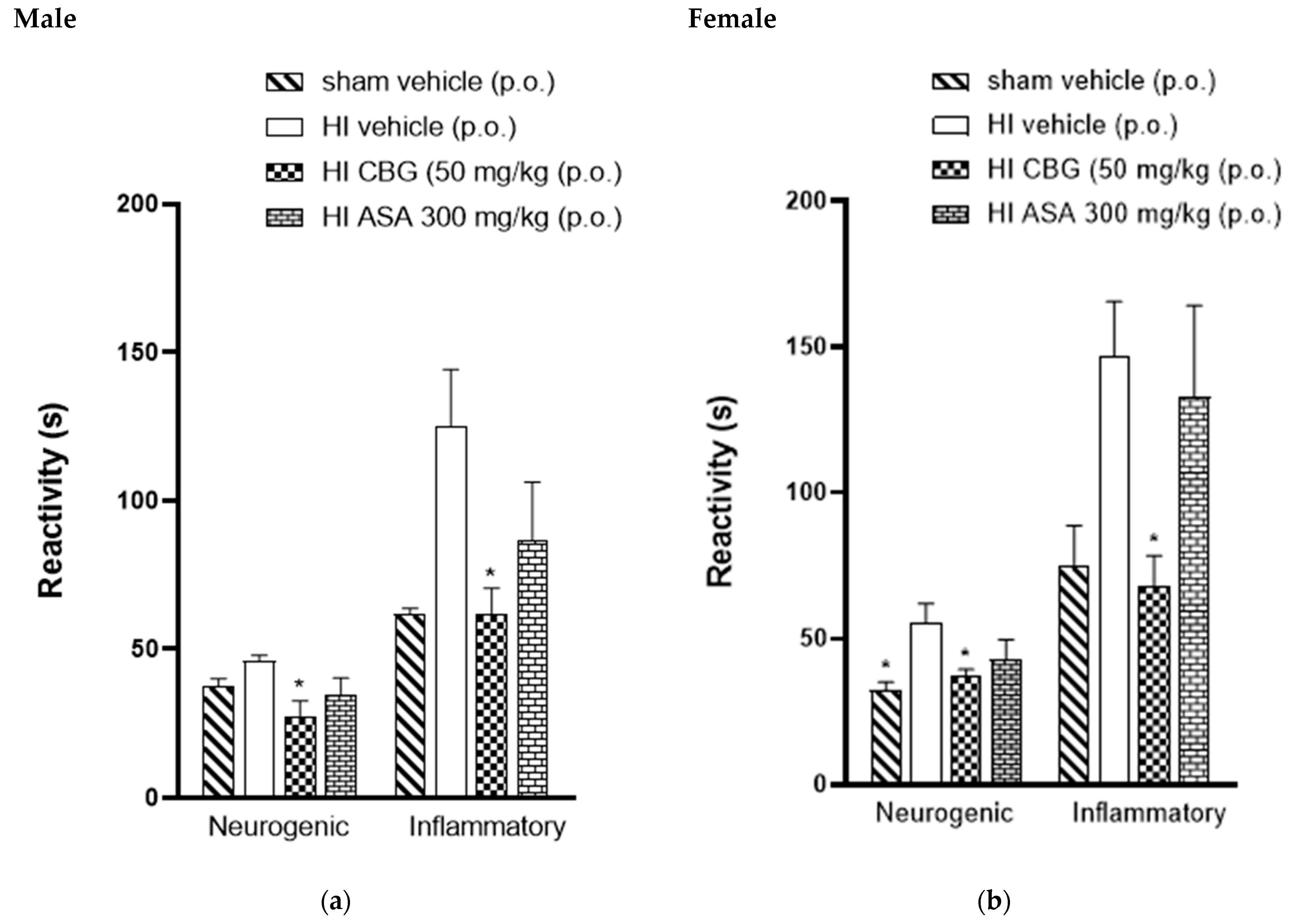
The antinociceptive activity was also investigated in P45-aged animals to observe if they exhibited the same response profile as the P30 animals. Figure 5 shows data for male and female rats (P45). The animals treated with CBG at a dose of 50 mg/kg showed a significant decrease in formalin response time from 66.5 ± 10.3 s to 33.8 ± 6.6 s and from 70.1 ± 7.2 s to 32 ± 6.0 s for male and female rats, respectively, compared to the HI vehicle group. During the inflammatory phase, CBG-treated animals exhibited a reduction in formalin response time from 182.1 ± 35.6 s to 63.3 ± 7.03 s and from 160 ± 12.9 s to 91.5 ± 6.8 s for male and female rats, respectively, compared to the HI vehicle group.
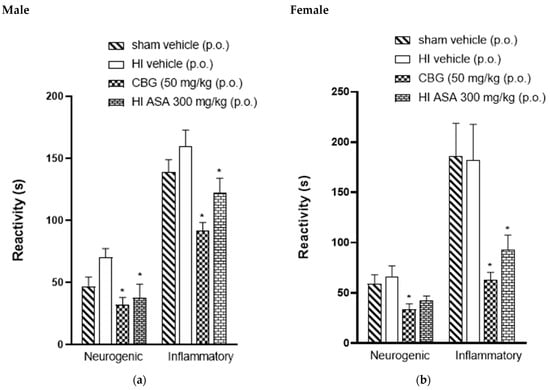
Figure 5.
Effect of oral administration of high-CBG cannabis extract (50 mg/kg), ASA (300 mg/kg) or vehicle during the neurogenic and inflammatory phases of the formalin test in Wistar rats (P45) (a) male and (b) female. Data are expressed as mean ± SEM (n = 8 per group). One-way ANOVA was performed followed by Dunnett’s test, * p < 0.05 compared to the vehicle group.
3.4. Thermal and Mechanical Hypernociception
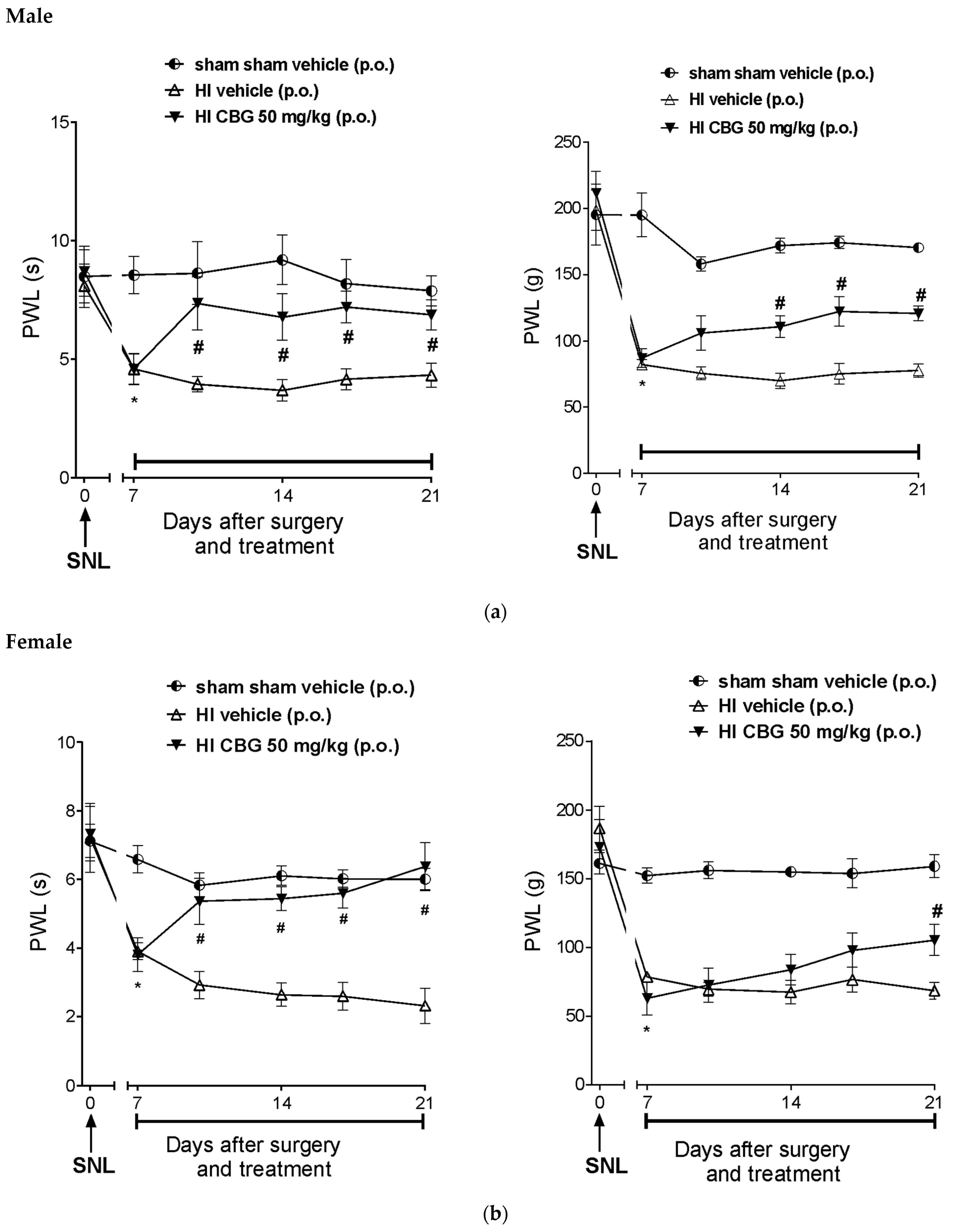
Figure 6a,b show that SNL significantly reduced the paw withdrawal latency in response to thermal stimuli 7 days after surgery, indicating thermal and mechanical hypernociception. There was no hypernociception in the sham group (HI and SNL surgery). The paw withdrawal latency (PWL) of SNL rats decreased from 6.3 ± 1.2 s and 5.1 ± 0.9 s; 121.8 ± 16.1 g and 124.5 ± 2 g (baseline of the day 0) to 3.4 ± 0.2 s and 2.8 ± 0.4 s; 41.42 ± 3.3 g and 30.8 ± 1.9 g (day 7) in thermal and mechanical hypernociception for male and female rats, respectively. Treatment with CBG (50 mg/kg) increased the latency (p < 0.05) to 7.3 ± 0.5 and 5.2 ± 0.7 s; 78.4 ± 6.6 and 81.6 ± 7.2 g in the evaluation of thermal and mechanical hypernociception for male and female rats, respectively. The reversal effect of thermal and mechanical hypernociception was observed after the 10th and 17th days post-surgery, respectively.
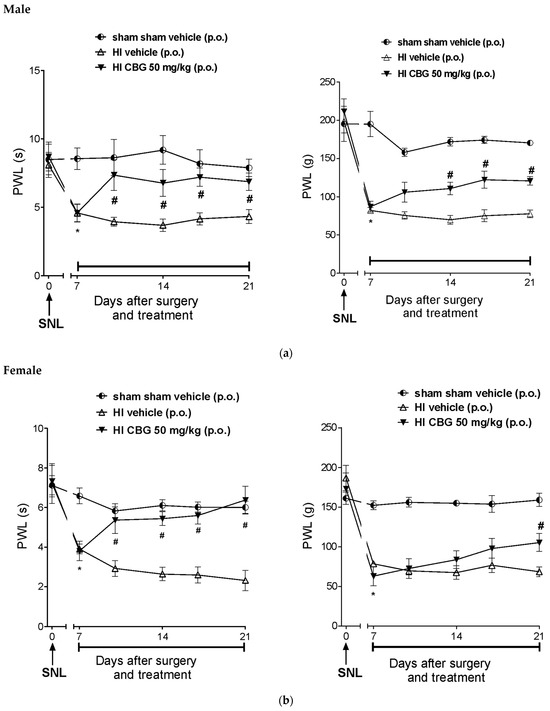
Figure 6.
Effect of 14-day oral treatment with vehicle or high-CBG Cannabis extract (50 mg/kg) on (a) male rats and (b) female rats with HI that developed thermal and mechanical hyperalgesia 7 days post SNL surgery. Data are expressed as mean ± SEM (n = 10 per group). Two-way ANOVA followed by Sidak test was performed, * p < 0.05 vs. 0 day, before SNL, # p < 0.05 when compared to the vehicle group throughout the treatment.
3.5. Evaluation of Inflammatory Cytokines
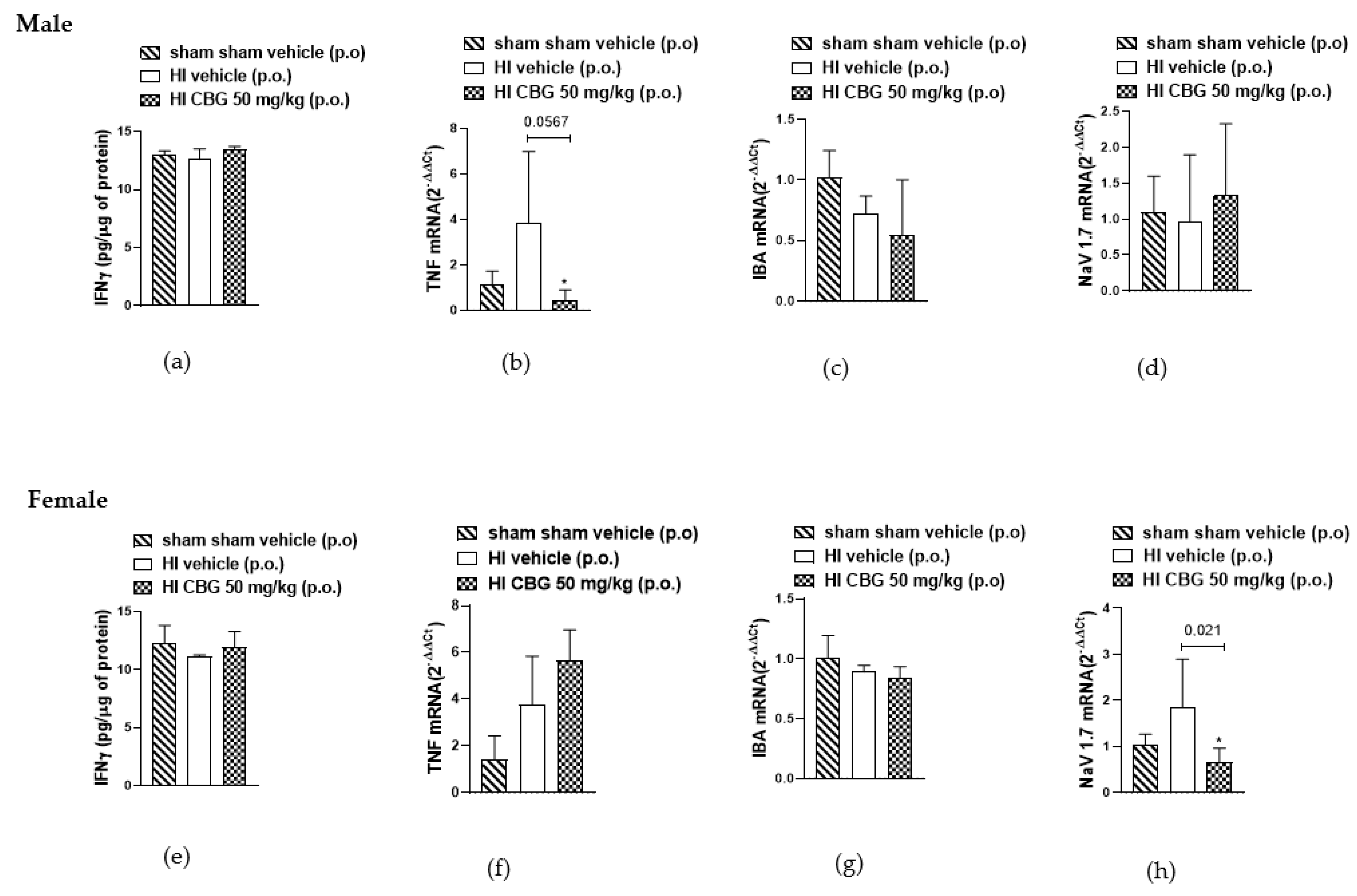
Inflammatory cytokines, the direct mediators of local inflammation, play a crucial role in the transmission of motor and sensory information in the spinal cord [38]. It is known that pro-inflammatory cytokines such as IFN-gamma and TNFα and Nav1.7 are significantly upregulated during chronic pain [39,40,41,42]. In this study, we aimed to evaluate the levels of both cytokines in the spinal cord between the L1 and L5 segments of rats in different experimental groups. No difference was detected in the protein levels of IFN-gamma in the spinal cords of both experimental groups. We observed an increase in TNFα mRNA levels in the HI vehicle group of male rats, with a reduction noted in the CBG-treated group. However, no reduction in TNFα levels was observed in female rats following treatment. Additionally, a decrease in Nav1.7 mRNA levels was observed in the spinal cord of females in the CBG-treated group, whereas no decrease was observed in males treated compared to the vehicle group. No differences were observed in IBA levels between males and females, nor between animals treated with vehicle or CBG (Figure 7).
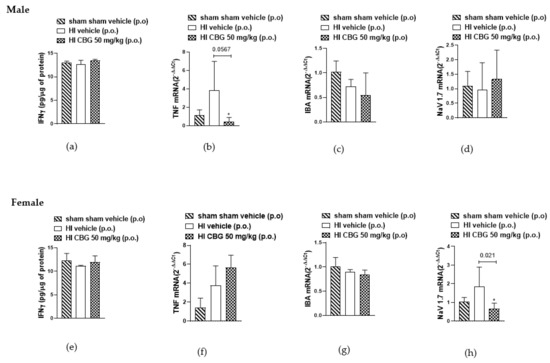
Figure 7.
Interferon gamma (IFN-γ) concentration in the spinal cord of (a) male rats and (e) female rats measured by ELISA method, and gene expression analysis of TNFα, IBA, and Nav1.7 in the spinal cord of (b–d) male rats and (f–h) female rats determined by qPCR method. Data are expressed as mean ± SEM (n = 5 per group). One-way ANOVA followed by Dunnett’s test was performed, * p < 0.05 when compared to the vehicle group.
4. Discussion
Pain significantly impacts patients’ quality of life and can lead to psychological trauma, posing a global health challenge [43,44]. Current first-line therapies, such as nonsteroidal anti-inflammatory drugs (NSAIDs), often lack long-term effectiveness and may present unfavorable risk–benefit profiles [45]. For example, non-selective NSAIDs are associated with gastrointestinal ulcers, bleeding, increased cardiovascular risks, and kidney disease [46,47,48]. In addition, opioids also are used to relieve pain, but they also induce an exaggerated sense of well-being and, if used excessively, can lead to addiction and high hospitalization economic costs associated [49,50,51]. As a result of the limitation of actuality treatment for pain, there is an increasing imperative to investigate alternative or supplementary drugs for managing pain.
Current randomized controlled trials support the effectiveness of cannabis for pain treatment [52]. Piper et al. [53] evaluated the chronic pain perspectives of 984 north-eastern U.S. patients suffering from various conditions such as neuropathic pain, back pain, arthritis, post-surgical pain, headaches, and abdominal pain regarding their use of medical cannabis. Most patients reported pain relief as the primary benefit, but also noted disadvantages such as economic costs and stigma. On the other hand, preclinical studies reveal the complexities of its antinociceptive effects [54]. Research into the anti-inflammatory and analgesic properties of CBG has gained momentum, giving it unique and overlapping mechanisms with THC and CBD [54,55,56]. It is already known that CBG, a non-psychoactive phytocannabinoid [57], shows potential antinociceptive agent, though few preclinical studies investigated this in detail [58]. Here, our results demonstrate the antinociceptive effects of orally administered CBG-rich cannabis extract in acute and chronic pain rodent models.
The hot plate and formalin tests assess spontaneous nociceptive behaviors that engage higher brain centers [59]. We observed that CBG reduced acute hypersensitivity in hot plate and formalin tests in mice and rats, respectively. The hot plate test, sensitive to central antinociceptive mechanisms, showed a 36% analgesic effect compared to the vehicle group, without affecting locomotion, consistent with Brierley et al. [30]. CBG did not produce any significant alteration in locomotor functions of the animals when compared with the DMSO-treated control group; hence, the substance did not show any motor effect. In HI P30 and P45 animals, 50 mg/kg CBG reduced formalin-induced nociceptive behavior in both neurogenic and inflammatory phases. Interestingly, Sepulveda et al. observed that CBD (10 mg/kg, i.p.) effectively reduced nociceptive behaviors in C57BL/6 wild-type male mice, but only during phase I of the formalin test. However, it did not reduce nociceptive behaviors in female mice during the same test. These findings highlight the potential cannabis compounds differences in the antinociceptive effects, underscoring the need for further research to fully understand their mechanisms of action.
As found in our model, other preclinical studies have also highlighted CBG as a promising antinociceptive agent for alleviating inflammatory and neuropathic pain. A recent study conducted by Wen et al. [60] demonstrated that CBG had a superior antinociceptive effect in the carrageenan-induced inflammatory pain model, surpassing cannabichromene (CBC) and CBD, and its efficacy was comparable to some positive control drugs. However, CBG exhibited a fast onset but a short duration of sustained analgesia. While CBG had no effect on arthritis inflammation, it showed a better antinociceptive effect when administered locally, indicating its effectiveness after topical peripheral administration in the collagen-induced arthritis (CIA) model. Additionally, the data showed that CBG can also alleviate neuropathic pain. Another important study observed that CBG significantly reduced mechanical hypersensitivity in a mouse model of cisplatin-induced peripheral neuropathy (CIPN) after 7 and 14 days of treatment, without causing tolerance or adverse effects. The treatment decreased pain sensitivity by 60–70% and was effective in both male and female mice, regardless of the estrous cycle [61].
Here, we demonstrated the antinociceptive effects of CBG in HI animals using the SNL model, a well-established method for studying chronic neuropathic pain. Furthermore, CBG’s reduction of TNFα expression in HI male animals supports its role in modulating inflammatory responses, potentially affecting central pain modulation pathways. In HI female animals, we already observed the decrease in Nav1.7 mRNA levels with CBG. Hormonal fluctuations in females are known to affect cannabinoid receptor expression and efficacy [62,63,64,65,66,67], and estrogen’s pro-inflammatory effects contribute to the higher incidence of chronic pain in females. Our data reinforce the complexity of interactions between sex, the endocannabinoid system, and immune response.
Some studies indicate that SNL leads to upregulation of TNF-α and Nav1.7 expression in the DRG, highlighting these as key mechanisms involved in pain modulation following nerve injury [68,69,70]. Furthermore, the reduction in inflammatory mediators may contribute to the observed decrease in pain sensitivity [71]. TNFα, produced by microglia, promotes the release of other inflammatory mediators, enhancing pain perception [41,72,73,74]. Using a TNFα antibody or TNF receptor antagonist can reduce pain hypersensitivity in various pathological pain models [75,76,77]. Beyond its role in regulating TRPV1 membrane translocation, TNFα has also been found to elevate TRPV1 total protein levels through translational regulation [78]. Wang et al. [79] demonstrated that TNFα sensitizes TRPV1 by upregulating its expression, leading to mechanical allodynia and thermal hyperalgesia in vincristine-treated rats. Although phytocannabinoids have been proposed as an anti-inflammatory and analgesic agent [80,81], studies evaluating TNF expression after CBG treatment are still controversial. In RAW264.7 cells, CBG did not reduce IL-6, iNOS, TNFα, and NLRP3 mRNA levels [82]. However, Calapai et al. [83] observed that CBG pre-treatment reduced inflammation, as evidenced by lower IL-1β, TNFα, IFN-γ, and PPAR protein levels in NSC-34 cells treated with the medium from RAW264.7 cells stimulated with lipopolysaccharide (LPS).
The expression of Nav1.7 in nociceptive sensory neurons of the DRG has become a critical focus in understanding pain modulation [84,85]. Additionally, human genetic studies have underscored Nav1.7 as a key regulator of pain modulation, emphasizing its crucial involvement in pain perception mechanisms [86,87,88]. Consequently, Nav1.7 has emerged as a promising target for pain relief in preclinical models, leading to the development of several sodium channel blockers. Among these, Compound 33, a quinoline amide, demonstrated potent inhibitory activity against Nav1.7 and efficacy in rat models of inflammatory and neuropathic pain [89]. Another compound, N-[(R)-1-((R)-7-chloro-1-isopropyl-2-oxo-2,3,4,5-tetrahydro-1H-benzo[b]azepin-3-ylcarbamoyl)-2-(2-fluorophenyl)-ethyl]-4-fluoro-2-trifluoromethyl-benzamide (BZP), selectively targets Nav1.7 with high affinity, providing significant anti-nociceptive effects in both inflammatory and neuropathic pain models while avoiding CNS side effects [90]. Furthermore, studies employing a pharmacokinetic–pharmacodynamic approach confirmed that the in vivo and ex vivo inhibition of ectopic activity in the spinal nerve ligation model correlates well with in vitro Nav1.7 inhibition, suggesting these models are useful for predicting clinical efficacy and guiding the development of new Nav1.7-targeting analgesics [91].
Huang et al. [92] demonstrate that CBD interacts with Nav1.7 channels at sub-micromolar concentrations in a state-dependent manner. They identify specific binding sites for CBD, and mutations in these residues significantly diminish CBD’s state-dependent inhibition. This suggests that CBD stabilizes the inactivated state of Nav1.7 channels through direct binding. Previous studies have explored the interactions of Nav channels also with CBG, revealing that CBG is more effective at inhibiting maximal sodium conductance (Gmax) than at stabilizing inactivation [80]. Interestingly, Ghovanloo et al. [93] found that Cannabinol (CBN) is slightly less effective at inhibiting Nav1.7 compared to either CBD or CBG. In our study, we observed a restoration of Nav1.7 mRNA levels to normal in spinal cord samples (L1-L6 region) following treatment with CBG in the spinal nerve ligation model. Corroborating our findings, Fukuoka et al. [94] observed an increase in Nav1.7 mRNA levels in the L5 after SNL. Notably, our study is the first to demonstrate that CBG, a phytocannabinoid, can reduce Nav1.7 expression in a pain model.
5. Limitations of the Study
While this study provides valuable insights into the antinociceptive effects of CBG in acute and chronic pain models, it is not without limitations. First, the study primarily used rodent models, which, although widely accepted in preclinical research, may not fully replicate human pain conditions. Thus, the translational applicability of the results to clinical settings requires further validation. Second, while the study explored both male and female subjects, sex-related differences in cannabinoid receptor expression and hormonal variations, particularly in females, could have introduced variability in responses. A more comprehensive analysis of the role of sex hormones in CBG’s effects is needed to better understand this variability. Additionally, the dosing regimen focused on a single dose of 50 mg/kg CBG. Exploring a wider range of doses could provide more nuanced information regarding its efficacy and safety. Furthermore, the pharmacokinetic profile of CBG was not assessed, leaving potential gaps in understanding its bioavailability and metabolism in vivo. Finally, while molecular analyses were performed, the study did not explore the potential long-term effects of CBG on neural tissues beyond the 14-day treatment period. Further studies are warranted to investigate the sustained efficacy and safety of CBG in chronic pain models over an extended period. These limitations should be addressed in future research to strengthen the understanding of CBG’s therapeutic potential in pain management.
6. Conclusions
In conclusion, our study provides evidence for the antinociceptive and anti-inflammatory effects of CBG-rich cannabis extract in animals subjected to prenatal hypoxia-ischemia in both acute and chronic pain models. These findings support the potential of CBG as a therapeutic agent for pain management, emphasizing the need for further research to elucidate its mechanisms and optimize its clinical use. Given the regulatory landscape in Brazil, continued scientific investigation is crucial to ensure that safe and effective cannabis-based therapies are available to patients.
Author Contributions
Conceptualization, P.C.B., F.L.F.-D. and G.C.M.; methodology, B.R., F.A., F.E.A.d.C., V.M.d.S.G., B.C.C.A.d.O., G.G.N. and Y.B.d.S.; software, B.R. and K.L.M.; validation, B.R., P.C.B., F.L.F.-D. and G.C.M.; formal analysis, F.L.F.-D. and G.C.M.; investigation, B.R.; resources, F.A. and B.R.; data curation, K.L.M. and B.R.; writing—original draft preparation, B.R.; writing—review and editing, F.L.F.-D. and G.C.M.; visualization, P.C.B., B.R., F.L.F.-D. and G.C.M.; supervision, F.L.F.-D. and G.C.M.; project administration, F.L.F.-D. and G.C.M.; funding acquisition, G.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro FAPERJ N° Processo SEI-260003/015605/2021—APQ1 (E-26/211.795/2021) and N° Processo E-26/201.515/2023—BOLSA. The funder had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of the Instituto de Biologia Roberto Alcantara Gomes- UERJ (protocol code 010/2022 and date of approval 14 June 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting reported results can be found at http://sucupira-legado.capes.gov.br/sucupira/public/consultas/coleta/trabalhoConclusao/viewTrabalhoConclusao.jsf?popup=true&id_trabalho=15128055.
Acknowledgments
We thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Apoio à Pesquisa e Pacientes de Cannabis Medicinal (APEPI).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Woolf, C.J. What is this thing called pain? J. Clin. Investig. 2010, 120, 3742–3744. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Woolf, C.J. Pain. Curr. Biol. 1999, 9, R429–R431. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.C.; Mullally, W.J. Pain Neurology. Am. J. Med. 2020, 133, 273–280. [Google Scholar] [CrossRef]
- Desantana, J.M.; Perissinotti, D.M.N.; Oliveira Junior, J.O.D.; Correia, L.M.F.; Oliveira, C.M.D.; Fonseca, P.R.B.D. Definition of pain revised after four decades. Braz. J. Pain. 2020, 3, 197–198. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Muley, M.M.; Krustev, E.; McDougall, J.J. Preclinical Assessment of Inflammatory Pain. CNS Neurosci. Ther. 2016, 22, 88–101. [Google Scholar] [CrossRef]
- Engidawork, E.; Chen, Y.; Dell’Anna, E.; Goiny, M.; Lubec, G.; Ungerstedt, U.; Andersson, K.; Herrera-Marschitz, M. Effect of perinatal asphyxia on systemic and intracerebral pH and glycolysis metabolism in the rat. Exp. Neurol. 1997, 145, 390–396. [Google Scholar] [CrossRef] [PubMed]
- de Haan, M.; Wyatt, J.S.; Roth, S.; Vargha-Khadem, F.; Gadian, D.; Mishkin, M. Brain and cognitive-behavioural development after asphyxia at term birth. Dev. Sci. 2006, 9, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Biarge, M.; Madero, R.; González, A.; Quero, J.; García-Alix, A. Perinatal morbidity and risk of hypoxic-ischemic encephalopathy associated with intrapartum sentinel events. Am. J. Obstet. Gynecol. 2012, 206, 148.e1–148.e7. [Google Scholar] [CrossRef]
- du Plessis, A.J.; Johnston, M.V. Hypoxic-ischemic brain injury in the newborn. Cellular mechanisms and potential strategies for neuroprotection. Clin. Perinatol. 1997, 24, 627–654. [Google Scholar] [CrossRef]
- Gross, J.; Andersson, K.; Chen, Y.; Müller, I.; Andreeva, N.; Herrera-Marschitz, M. Effect of perinatal asphyxia on tyrosine hydroxylase and D2 and D1 dopamine receptor mRNA levels expressed during early postnatal development in rat brain. Brain Res. Mol. Brain Res. 2005, 134, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Rumajogee, P.; Bregman, T.; Miller, S.P.; Yager, J.Y.; Fehlings, M.G. Rodent Hypoxia-Ischemia Models for Cerebral Palsy Research: A Systematic Review. Front. Neurol. 2016, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, L.S.; Cunha-Rodrigues, M.C.; Araujo, P.C.; de Almeida, O.M.; Barradas, P.C. Effects of prenatal hypoxia-ischemia on male rat periaqueductal gray matter: Hyperalgesia, astrogliosis and nitrergic system impairment. Neurochem. Int. 2023, 164, 105500. [Google Scholar] [CrossRef] [PubMed]
- Von Adamovich, G.M.G.; Bastos Torres, J.A.G.; Vianna, F.S.; Barradas, P.C.; Alves de Oliveira, B.F.; Villela, N.R.; De Rodrigues, M.C.C.; Montes, G.C. Evaluation of Pain Prevalence in Children Who Experienced Perinatal Hypoxia-Ischemia Events: Characteristics and Associations With Sociodemographic Factors. Cureus 2023, 15, e46359. [Google Scholar] [CrossRef] [PubMed]
- Collier, R. A short history of pain management. CMAJ 2018, 190, E26–E27. [Google Scholar] [CrossRef]
- Alles, S.R.A.; Smith, P.A. Etiology and Pharmacology of Neuropathic Pain. Pharmacol. Rev. 2018, 70, 315–347. [Google Scholar] [CrossRef]
- Ji, R.R.; Xu, Z.Z.; Gao, Y.J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Moseley, G.L.; Butler, D.S. Fifteen Years of Explaining Pain: The Past, Present, and Future. J. Pain. 2015, 16, 807–813. [Google Scholar] [CrossRef]
- Fine, P.G.; Rosenfeld, M.J. The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Med. J. 2013, 4, e0022. [Google Scholar] [CrossRef]
- Legare, C.A.; Raup-Konsavage, W.M.; Vrana, K.E. Therapeutic Potential of Cannabis, Cannabidiol, and Cannabinoid-Based Pharmaceuticals. Pharmacology 2022, 107, 131–149. [Google Scholar] [CrossRef]
- Urits, I.; Charipova, K.; Gress, K.; Li, N.; Berger, A.A.; Cornett, E.M.; Kassem, H.; Ngo, A.L.; Kaye, A.D.; Viswanath, O. Adverse Effects of Recreational and Medical Cannabis. Psychopharmacol. Bull. 2021, 51, 94–109. [Google Scholar] [PubMed]
- Anand, P.; Whiteside, G.; Fowler, C.J.; Hohmann, A.G. Targeting CB2 receptors and the endocannabinoid system for the treatment of pain. Brain Res. Rev. 2009, 60, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.R.; Ali, D.W. Pharmacology of Medical Cannabis. Adv. Exp. Med. Biol. 2019, 1162, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Bass, B.; Padwa, H.; Khurana, D.; Urada, D.; Boustead, A. Adult use cannabis legalization and cannabis use disorder treatment in California, 2010–2021. J. Subst. Use Addict. Treat. 2024, 162, 209345. [Google Scholar] [CrossRef]
- Pantoja-Ruiz, C.; Restrepo-Jimenez, P.; Castañeda-Cardona, C.; Ferreirós, A.; Rosselli, D. Cannabis and pain: A scoping review. Braz. J. Anesthesiol. 2022, 72, 142–151. [Google Scholar] [CrossRef]
- McPartland, J.M.; Glass, M.; Matias, I.; Norris, R.W.; Kilpatrick, C.W. A shifted repertoire of endocannabinoid genes in the zebrafish (Danio rerio). Mol. Genet. Genom. 2007, 277, 555–570. [Google Scholar] [CrossRef]
- Ndong, C.; O’Donnell, D.; Ahmad, S.; Groblewski, T. Cloning and pharmacological characterization of the dog cannabinoid CB₂ receptor. Eur. J. Pharmacol. 2011, 669, 24–31. [Google Scholar] [CrossRef]
- Ye, L.; Cao, Z.; Wang, W.; Zhou, N. New Insights in Cannabinoid Receptor Structure and Signaling. Curr. Mol. Pharmacol. 2019, 12, 239–248. [Google Scholar] [CrossRef]
- Brierley, D.I.; Samuels, J.; Duncan, M.; Whalley, B.J.; Williams, C.M. Cannabigerol is a novel, well-tolerated appetite stimulant in pre-satiated rats. Psychopharmacology 2016, 233, 3603–3613. [Google Scholar] [CrossRef]
- Robaina Cabrera, C.L.; Keir-Rudman, S.; Horniman, N.; Clarkson, N.; Page, C. The anti-inflammatory effects of cannabidiol and cannabigerol alone, and in combination. Pulm. Pharmacol. Ther. 2021, 69, 102047. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. Methods Mol. Biol. 2019, 1916, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Khatian, N.; Aslam, M. Effect of Ganoderma lucidum on memory and learning in mice. Clin. Phytoscience 2019, 5, 4. [Google Scholar] [CrossRef]
- Savignon, T.; Costa, E.; Tenorio, F.; Manhães, A.C.; Barradas, P.C. Prenatal hypoxic-ischemic insult changes the distribution and number of NADPH-diaphorase cells in the cerebellum. PLoS ONE 2012, 7, e35786. [Google Scholar] [CrossRef]
- Dubuisson, D.; Dennis, S.G. The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 1977, 4, 161–174. [Google Scholar] [CrossRef]
- Ho Kim, S.; Mo Chung, J. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992, 50, 355–363. [Google Scholar] [CrossRef]
- Lolignier, S.; Amsalem, M.; Maingret, F.; Padilla, F.; Gabriac, M.; Chapuy, E.; Eschalier, A.; Delmas, P.; Busserolles, J. Nav1.9 channel contributes to mechanical and heat pain hypersensitivity induced by subacute and chronic inflammation. PLoS ONE 2011, 6, e23083. [Google Scholar] [CrossRef]
- Ren, H.; Chen, X.; Tian, M.; Zhou, J.; Ouyang, H.; Zhang, Z. Regulation of Inflammatory Cytokines for Spinal Cord Injury Repair Through Local Delivery of Therapeutic Agents. Adv. Sci. 2018, 5, 1800529. [Google Scholar] [CrossRef]
- Donnelly, C.R.; Jiang, C.; Andriessen, A.S.; Wang, K.; Wang, Z.; Ding, H.; Zhao, J.; Luo, X.; Lee, M.S.; Lei, Y.L.; et al. STING controls nociception via type I interferon signalling in sensory neurons. Nature 2021, 591, 275–280. [Google Scholar] [CrossRef]
- Ferrara, V.; Toti, A.; Ghelardini, C.; Di Cesare Mannelli, L. Interferon-gamma and neuropathy: Balance between pain and neuroprotection. Neural Regen. Res. 2022, 17, 2700–2701. [Google Scholar]
- Leung, L.; Cahill, C.M. TNF-alpha and neuropathic pain—A review. J. Neuroinflamm. 2010, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Shiers, S.; Funk, G.; Cervantes, A.; Horton, P.; Dussor, G.; Hennen, S.; Price, T.J. NaV1.7 mRNA and protein expression in putative projection neurons of the human spinal dorsal horn. bioRxiv, 2023; preprint. [Google Scholar]
- Rainer, T.H.; Cheng, C.H.; Janssens, H.J.; Man, C.Y.; Tam, L.S.; Choi, Y.F.; Yau, W.H.; Lee, K.H.; Graham, C.A. Oral Prednisolone in the Treatment of Acute Gout: A Pragmatic, Multicenter, Double-Blind, Randomized Trial. Ann. Intern. Med. 2016, 164, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, A.; Croft, P.; Langford, R.M.; Donaldson, L.J.; Jones, G.T. Prevalence of chronic pain in the UK: A systematic review and meta-analysis of population studies. BMJ Open 2016, 6, e010364. [Google Scholar] [CrossRef]
- Taneja, A.; Della Pasqua, O.; Danhof, M. Challenges in translational drug research in neuropathic and inflammatory pain: The prerequisites for a new paradigm. Eur. J. Clin. Pharmacol. 2017, 73, 1219–1236. [Google Scholar] [CrossRef]
- Day, R.O.; Graham, G.G. The vascular effects of COX-2 selective inhibitors. Aust. Prescr. 2004, 27, 142–145. [Google Scholar] [CrossRef]
- Bally, M.; Dendukuri, N.; Rich, B.; Nadeau, L.; Helin-Salmivaara, A.; Garbe, E.; Brophy, J.M. Risk of acute myocardial infarction with NSAIDs in real world use: Bayesian meta-analysis of individual patient data. BMJ 2017, 357, j1909. [Google Scholar] [CrossRef] [PubMed]
- Lanas, A.; Chan, F.K.L. Peptic ulcer disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef]
- Hsu, D.J.; McCarthy, E.P.; Stevens, J.P.; Mukamal, K.J. Hospitalizations, costs and outcomes associated with heroin and prescription opioid overdoses in the United States 2001-12. Addiction 2017, 112, 1558–1564. [Google Scholar] [CrossRef]
- Han, B.; Compton, W.M.; Blanco, C.; Crane, E.; Lee, J.; Jones, C.M. Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and Health. Ann. Intern. Med. 2017, 167, 293–301. [Google Scholar] [CrossRef]
- Corder, G.; Castro, D.C.; Bruchas, M.R.; Scherrer, G. Endogenous and Exogenous Opioids in Pain. Annu. Rev. Neurosci. 2018, 41, 453–473. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.P. Medical Marijuana for Treatment of Chronic Pain and Other Medical and Psychiatric Problems: A Clinical Review. JAMA 2015, 313, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Piper, B.J.; Beals, M.L.; Abess, A.T.; Nichols, S.D.; Martin, M.W.; Cobb, C.M.; DeKeuster, R.M. Chronic pain patients’ perspectives of medical cannabis. Pain 2017, 158, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.P.; Palastro, M.D.; Johnson, B.; Ditre, J.W. Cannabis and Pain: A Clinical Review. Cannabis Cannabinoid Res. 2017, 2, 96–104. [Google Scholar] [CrossRef]
- Nichols, J.M.; Kaplan, B.L.F. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef]
- Kogan, N.M.; Lavi, Y.; Topping, L.M.; Williams, R.O.; McCann, F.E.; Yekhtin, Z.; Feldmann, M.; Gallily, R.; Mechoulam, R. Novel CBG Derivatives Can Reduce Inflammation, Pain and Obesity. Molecules 2021, 26, 5601. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Evans, F.J. Cannabinoids: The separation of central from peripheral effects on a structural basis. Planta Med. 1991, 57, S60–S67. [Google Scholar] [CrossRef]
- Vogel, H.G. Drug Discovery and Evaluation: Pharmacological Assays; Springer: Berlin/Heidelberg, Germany, 2002; pp. 670–773. [Google Scholar]
- Wen, Y.; Wang, Z.; Zhang, R.; Zhu, Y.; Lin, G.; Li, R.; Zhang, J. The antinociceptive activity and mechanism of action of cannabigerol. Biomed. Pharmacother. 2023, 158, 114163. [Google Scholar] [CrossRef] [PubMed]
- Nachnani, R.; Sepulveda, D.E.; Booth, J.L.; Zhou, S.; Graziane, N.M.; Raup-Konsavage, W.M.; Vrana, K.E. Chronic Cannabigerol as an Effective Therapeutic for Cisplatin-Induced Neuropathic Pain. Pharmaceuticals 2023, 16, 1442. [Google Scholar] [CrossRef]
- Blanton, H.L.; Barnes, R.C.; McHann, M.C.; Bilbrey, J.A.; Wilkerson, J.L.; Guindon, J. Sex differences and the endocannabinoid system in pain. Pharmacol. Biochem. Behav. 2021, 202, 173107. [Google Scholar] [CrossRef] [PubMed]
- Britch, S.C.; Goodman, A.G.; Wiley, J.L.; Pondelick, A.M.; Craft, R.M. Antinociceptive and Immune Effects of Delta-9-Tetrahydrocannabinol or Cannabidiol in Male Versus Female Rats with Persistent Inflammatory Pain. J. Pharmacol. Exp. Ther. 2020, 373, 416–428. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fertil. Res. Pract. 2020, 6, 5. [Google Scholar] [CrossRef]
- Shulman, L.M.; Spritzer, M.D. Changes in the sexual behavior and testosterone levels of male rats in response to daily interactions with estrus females. Physiol. Behav. 2014, 133, 8–13. [Google Scholar] [CrossRef]
- Mogil, J.S. Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 2012, 13, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Fillingim, R.B.; King, C.D.; Ribeiro-Dasilva, M.C.; Rahim-Williams, B.; Riley, J.L., 3rd. Sex, gender, and pain: A review of recent clinical and experimental findings. J. Pain. 2009, 10, 447–485. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, S.J.; Yang, L.; Fang, X.L.; Hu, H.F.; Zhao, M.Y.; Li, L.; Guo, Y.Y.; Shao, J.P. Voltage-gated sodium channel 1.7 expression decreases in dorsal root ganglia in a spinal nerve ligation neuropathic pain model. Kaohsiung J. Med. Sci. 2019, 35, 493–500. [Google Scholar] [CrossRef]
- Kim, S.H.; Nam, J.S.; Choi, D.K.; Koh, W.W.; Suh, J.H.; Song, J.G.; Shin, J.W.; Leem, J.G. Tumor Necrosis Factor-alpha and Apoptosis Following Spinal Nerve Ligation Injury in Rats. Korean J. Pain. 2011, 24, 185–190. [Google Scholar] [CrossRef]
- Cao, J.; Li, Z.; Zhang, Z.; Ren, X.; Zhao, Q.; Shao, J.; Li, M.; Wang, J.; Huang, P.; Zang, W. Intrathecal injection of fluorocitric acid inhibits the activation of glial cells causing reduced mirror pain in rats. BMC Anesthesiol. 2014, 14, 119. [Google Scholar] [CrossRef]
- Rhen, T.; Cidlowski, J.A. Anti-inflammatory mechanisms of glucocorticoids in chronic inflammatory diseases: A critical overview. J. Clin. Investig. 2005, 115, 1384–1392. [Google Scholar]
- Ren, K.; Dubner, R. Neuron-glia crosstalk gets serious: Role in pain hypersensitivity. Curr. Opin. Anaesthesiol. 2008, 21, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.R.; Milligan, E.D.; Maier, S.F. Glial proinflammatory cytokines mediate exaggerated pain states: Implications for clinical pain. Adv. Exp. Med. Biol. 2003, 521, 1–21. [Google Scholar] [PubMed]
- Watkins, L.R.; Milligan, E.D.; Maier, S.F. Glial activation: A driving force for pathological pain. Trends Neurosci. 2001, 24, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, N.; Maeda, T.; Kobayashi, Y.; Saika, F.; Kishioka, S. Involvement of inflammatory mediators in neuropathic pain caused by vincristine. Int. Rev. Neurobiol. 2009, 85, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Kochukov, M.Y.; McNearney, T.A.; Yin, H.; Zhang, L.; Ma, F.; Ponomareva, L.; Abshire, S.; Westlund, K.N. Tumor necrosis factor-alpha (TNF-alpha) enhances functional thermal and chemical responses of TRP cation channels in human synoviocytes. Mol. Pain. 2009, 5, 49. [Google Scholar] [CrossRef]
- Sasaki, M.; Hashimoto, S.; Sawa, T.; Amaya, F. Tumor necrosis factor-alpha induces expression of C/EBP-beta in primary afferent neurons following nerve injury. Neuroscience 2014, 279, 1–9. [Google Scholar] [CrossRef]
- Hensellek, S.; Brell, P.; Schaible, H.G.; Bräuer, R.; Segond von Banchet, G. The cytokine TNFalpha increases the proportion of DRG neurones expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Mol. Cell Neurosci. 2007, 36, 381–391. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, C.; He, H.; He, J.; Wang, J.; Li, X.; Wang, S.; Li, W.; Hou, J.; Liu, T.; et al. Sensitization of TRPV1 receptors by TNF-α orchestrates the development of vincristine-induced pain. Oncol. Lett. 2018, 15, 5013–5019. [Google Scholar] [CrossRef]
- Ghovanloo, M.R.; Dib-Hajj, S.D.; Goodchild, S.J.; Ruben, P.C.; Waxman, S.G. Non-psychotropic phytocannabinoid interactions with voltage-gated sodium channels: An update on cannabidiol and cannabigerol. Front. Physiol. 2022, 13, 1066455. [Google Scholar] [CrossRef]
- Jan, T.R.; Farraj, A.K.; Harkema, J.R.; Kaminski, N.E. Attenuation of the ovalbumin-induced allergic airway response by cannabinoid treatment in A/J mice. Toxicol. Appl. Pharmacol. 2003, 188, 24–35. [Google Scholar] [CrossRef]
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The Pharmacological Case for Cannabigerol. J. Pharmacol. Exp. Ther. 2021, 376, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Calapai, F.; Cardia, L.; Esposito, E.; Ammendolia, I.; Mondello, C.; Lo Giudice, R.; Gangemi, S.; Calapai, G.; Mannucci, C. Pharmacological Aspects and Biological Effects of Cannabigerol and Its Synthetic Derivatives. Evid. Based Complement. Alternat Med. 2022, 2022, 3336516. [Google Scholar] [CrossRef] [PubMed]
- Dib-Hajj, S.D.; Yang, Y.; Black, J.A.; Waxman, S.G. The Na(V)1.7 sodium channel: From molecule to man. Nat. Rev. Neurosci. 2013, 14, 49–62. [Google Scholar] [CrossRef]
- Rush, A.M.; Cummins, T.R.; Waxman, S.G. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J. Physiol. 2007, 579, 1–14. [Google Scholar] [CrossRef]
- Drenth, J.P.; Waxman, S.G. Mutations in sodium-channel gene SCN9A cause a spectrum of human genetic pain disorders. J. Clin. Investig. 2007, 117, 3603–3609. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Cummins, T.R.; Black, J.A.; Waxman, S.G. From genes to pain: Na v 1.7 and human pain disorders. Trends Neurosci. 2007, 30, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Djouhri, L.; Newton, R.; Levinson, S.R.; Berry, C.M.; Carruthers, B.; Lawson, S.N. Sensory and electrophysiological properties of guinea-pig sensory neurones expressing Nav 1.7 (PN1) Na+ channel alpha subunit protein. J. Physiol. 2003, 546, 565–576. [Google Scholar] [CrossRef]
- Yang, S.W.; Ho, G.D.; Tulshian, D.; Bercovici, A.; Tan, Z.; Hanisak, J.; Brumfield, S.; Matasi, J.; Sun, X.; Sakwa, S.A.; et al. Bioavailable pyrrolo-benzo-1,4-diazines as Na(v)1.7 sodium channel blockers for the treatment of pain. Bioorg. Med. Chem. Lett. 2014, 24, 4958–4962. [Google Scholar] [CrossRef]
- McGowan, E.; Hoyt, S.B.; Li, X.; Lyons, K.A.; Abbadie, C. A peripherally acting Na(v)1.7 sodium channel blocker reverses hyperalgesia and allodynia on rat models of inflammatory and neuropathic pain. Anesth. Analg. 2009, 109, 951–958. [Google Scholar] [CrossRef]
- Kalezic, I.; Luo, L.; Lund, P.E.; Eriksson, A.B.; Bueters, T.; Visser, S.A. In vivo and ex vivo inhibition of spinal nerve ligation-induced ectopic activity by sodium channel blockers correlate to in vitro inhibition of NaV1.7 and clinical efficacy: A pharmacokinetic-pharmacodynamic translational approach. Pharm. Res. 2013, 30, 1409–1422. [Google Scholar] [CrossRef]
- Huang, J.; Fan, X.; Jin, X.; Jo, S.; Zhang, H.B.; Fujita, A.; Bean, B.P.; Yan, N. Cannabidiol inhibits Nav channels through two distinct binding sites. Nat. Commun. 2023, 14, 3613. [Google Scholar] [CrossRef] [PubMed]
- Ghovanloo, M.-R.; Effraim, P.R.; Tyagi, S.; Zhao, P.; Dib-Hajj, S.D.; Waxman, S.G. Functionally-selective inhibition of threshold sodium currents and excitability in dorsal root ganglion neurons by cannabinol. Commun. Biol. 2024, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, T.; Kobayashi, K.; Noguchi, K. Laminae-specific distribution of alpha-subunits of voltage-gated sodium channels in the adult rat spinal cord. Neuroscience 2010, 169, 994–1006. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).