Resveratrol: Extraction Techniques, Bioactivity, and Therapeutic Potential in Ocular Diseases
Abstract
:1. Introduction
2. Natural Sources and Extraction Methods of RV
3. Mechanisms of Action and Bioactivities of RV
3.1. Bioactive Properties and Molecular Pathways
3.2. Evidence from Clinical Studies of RV
3.3. Therapeutic Effects and Synergy with Other Active Molecules
4. Resveratrol in Ocular Disease
4.1. Role and Mechanisms
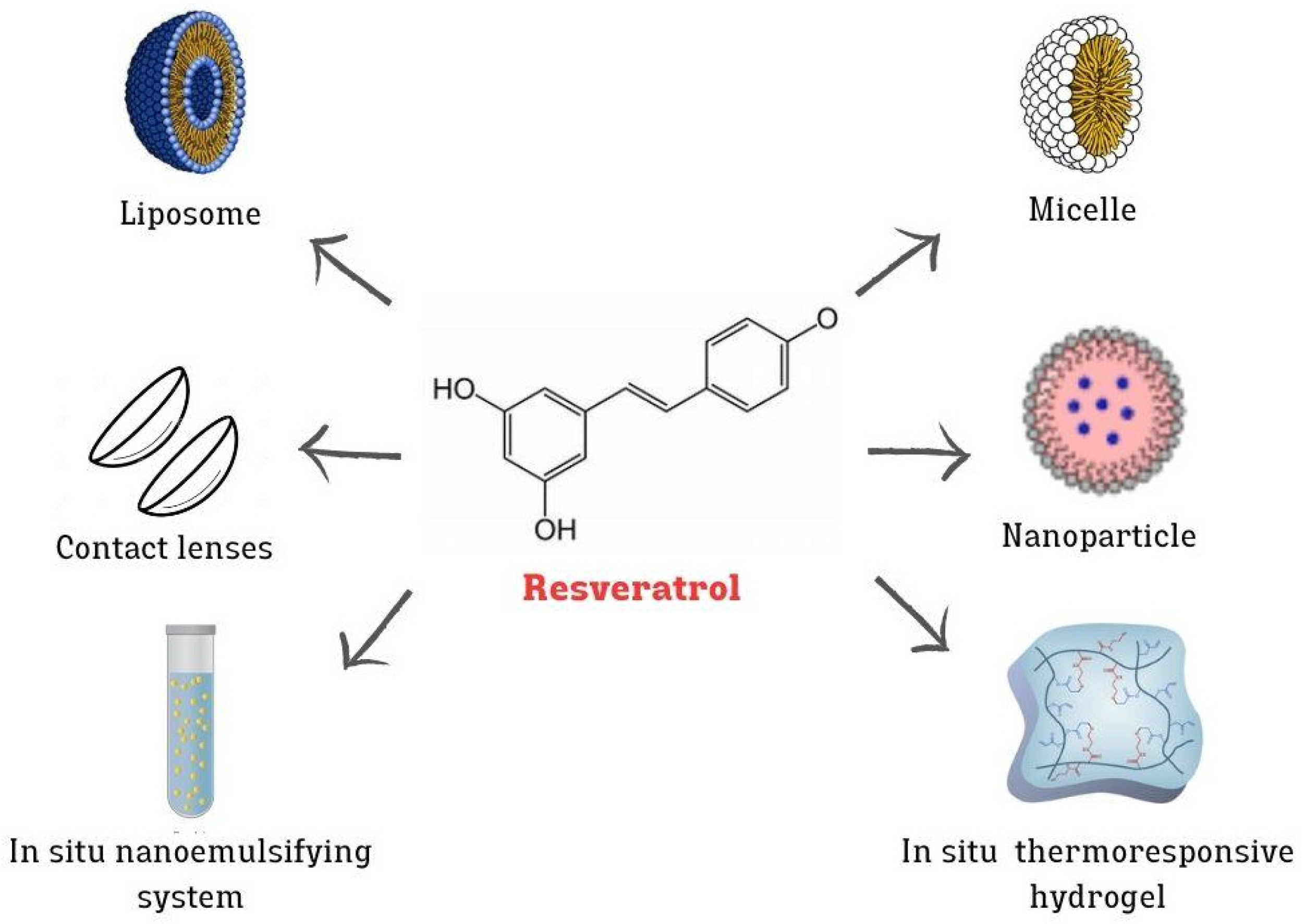
4.2. Advanced Nanotechnologies for the Ocular Delivery of RV
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMD | age-related macular degeneration |
| CS | chitosan |
| COX | cyclooxygenase |
| DED | dry eye disease |
| DES | deep eutectic solvent |
| DOX | doxazosin |
| EAE | enzyme-assisted extraction |
| eEF2 | eukaryotic elongation factor-2 |
| HLEC | human lens epithelial cells |
| IL | interleukins |
| iNOS | inducible NO synthase |
| IOP | intraocular pressure |
| iROS | intracellular reactive oxygen species |
| LDL-C | low-density lipoprotein cholesterol |
| LP | liposome |
| MAE | microwave-assisted extraction |
| MPC | 2-methacryloyloxyethyl phosphorylcholine |
| MSPD | matrix solid-phase dispersion |
| NG | nanogel |
| NO | nitric oxide |
| NP | nanoparticle |
| PCL | poly(ε-caprolactone) |
| PEG | poly ethylene glycol |
| PG | prostaglandin |
| PKC | protein kinase C |
| PLGA | poly(lactic-co-glycolic acid) |
| PLGA-PEI | acetylated polyethyleneimine-poly(lactic-co-glycolic acid) |
| RGNP | gold nanoparticles encapsulated resveratrol |
| ROP | retinopathy of prematurity |
| ROS | reactive oxygen species |
| RPE | retinal pigment epithelial |
| RV | resveratrol |
| SE | solvent extraction |
| SFE | supercritical fluid extraction |
| SNEDDSs | self-nanoemulsifying drug delivery systems |
| TMC | trimethylated chitosan |
| UAE | ultrasound-assisted extraction |
| VEGF | vascular endothelial growth factor |
References
- Akinwumi, B.C.; Bordun, K.A.M.; Anderson, H.D. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef]
- Duta-Bratu, C.G.; Nitulescu, G.M.; Mihai, D.P.; Olaru, O.T. Resveratrol and Other Natural Oligomeric Stilbenoid Compounds and Their Therapeutic Applications. Plants 2023, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, J. Resveratrol: A Review of Plant Sources, Synthesis, Stability, Modification and Food Application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, Alcohol, Platelets, and the French Paradox for Coronary Heart Disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Tang, H.; Hou, H.; Song, L.; Tian, Z.; Liu, W.; Xia, T.; Wang, A. The Role of MTORC1/TFEB Axis Mediated Lysosomal Biogenesis and Autophagy Impairment in Fluoride Neurotoxicity and the Intervention Effects of Resveratrol. J. Hazard. Mater. 2024, 467, 133634. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, K.; Wang, N.; Xiao, X.; Leng, Y.; Fan, J.; Du, Y.; Wang, S. Sulfur Dioxide-Free Wine with Polyphenols Promotes Lipid Metabolism via the Nrf2 Pathway and Gut Microbiota Modulation. Food Chem. X 2024, 21, 101079. [Google Scholar] [CrossRef]
- Bai, Y.; Sui, R.; Zhang, L.; Bai, B.; Zhu, Y.; Jiang, H. Resveratrol Improves Cognitive Function in Post-Stroke Depression Rats by Repressing Inflammatory Reactions and Oxidative Stress via the Nrf2/HO-1 Pathway. Neuroscience 2024, 541, 50–63. [Google Scholar] [CrossRef]
- Golestaneh, E.; Dehkordi, A.H.; Yalameha, B.; Noorshargh, P.; Nasri, P.; Nasri, H. Comparative Study of Nephroprotective Effects of Resveratrol and Silymarin in Diabetic Rats; an Experimental Histopathologic Study. J. Nephropharmacol. 2024, 13, e10381–e10385. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, L.; Liu, Y.; Zhang, A.; Wang, W. Resveratrol alleviates perinatal methylmercury-induced neurobehavioral impairments by modulating the gut microbiota composition and neurotransmitter disturbances. Environ. Toxicol. 2024, 39, 329–340. [Google Scholar] [CrossRef]
- Delmas, D.; Cornebise, C.; Courtaut, F.; Xiao, J.; Aires, V. New Highlights of Resveratrol: A Review of Properties against Ocular Diseases. Int. J. Mol. Sci. 2021, 22, 1295. [Google Scholar] [CrossRef]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef] [PubMed]
- Zingale, E.; Bonaccorso, A.; D’Amico, A.G.; Lombardo, R.; D’Agata, V.; Rautio, J.; Pignatello, R. Formulating Resveratrol and Melatonin Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) for Ocular Administration Using Design of Experiments. Pharmaceutics 2024, 16, 125. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Chen, P.; Liu, X.; Lian, Y.; Xi, J.; Li, J.; Song, J.; Li, X. Trimethylated Chitosan-Coated Flexible Liposomes with Resveratrol for Topical Drug Delivery to Reduce Blue-Light-Induced Retinal Damage. Int. J. Biol. Macromol. 2023, 252, 126480. [Google Scholar] [CrossRef] [PubMed]
- De Luca, I.; Di Cristo, F.; Conte, R.; Peluso, G.; Cerruti, P.; Calarco, A. In-Situ Thermoresponsive Hydrogel Containing Resveratrol-Loaded Nanoparticles as a Localized Drug Delivery Platform for Dry Eye Disease. Antioxidants 2023, 12, 993. [Google Scholar] [CrossRef]
- Li, W.; Yuan, H.; Liu, Y.; Wang, B.; Xu, X.; Xu, X.; Hussain, D.; Ma, L.; Chen, D. Current analytical strategies for the determination of resveratrol in foods. Food Chem. 2024, 431, 137182. [Google Scholar] [CrossRef]
- Angelov, G.; Boyadzhiev, L.; Georgieva, S. Useful Bioactive Substances from Wastes: Recovery of Trans-Resveratrol from Grapevine Stems. Open Chem. Eng. J. 2016, 10, 4–9. [Google Scholar] [CrossRef]
- Piyaratne, P.S.; Leblanc, R.; Myracle, A.D.; Cole, B.J.W.; Fort, R.C. Extraction and Purification of (E)-Resveratrol from the Bark of Conifer Species. Processes 2022, 10, 647. [Google Scholar] [CrossRef]
- Jitrangsri, K.; Chaidedgumjorn, A.; Satiraphan, M. Supercritical Fluid Extraction (SFE) Optimization of Trans-Resveratrol from Peanut Kernels (Arachis hypogaea) by Experimental Design. J. Food Sci. Technol. 2020, 57, 1486–1494. [Google Scholar] [CrossRef]
- Akhtar, I.; Javad, S.; Yousaf, Z.; Iqbal, S.; Jabeen, K. Microwave Assisted Extraction of Phytochemicals an Efficient and Modern Approach for Botanicals and Pharmaceuticals. Pak. J. Pharm. Sci. 2019, 32, 223–230. [Google Scholar]
- Piñeiro, Z.; Marrufo-Curtido, A.; Vela, C.; Palma, M. Microwave-Assisted Extraction of Stilbenes from Woody Vine Material. Food Bioprod. Process. 2017, 103, 18–26. [Google Scholar] [CrossRef]
- Sun, H.; Lin, Q.; Wei, W.; Qin, G. Ultrasound-Assisted Extraction of Resveratrol from Grape Leaves and Its Purification on Mesoporous Carbon. Food Sci. Biotechnol. 2018, 27, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Zhang, M.; Shao, H.; Zhang, M.; Wang, X.; Wang, Q.; Bao, Z.; Fan, X.; Li, H. Efficient Enzyme-Assisted Extraction and Conversion of Polydatin to Resveratrol from Polygonum Cuspidatum Using Thermostable Cellulase and Immobilized β-Glucosidase. Front. Microbiol. 2019, 10, 445. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.A.; Kuo, C.H.; Chen, B.Y.; Li, Y.; Liu, Y.C.; Chen, J.H.; Shieh, C.J. A Novel Enzyme-Assisted Ultrasonic Approach for Highly Efficient Extraction of Resveratrol from Polygonum Cuspidatum. Ultrason. Sonochem 2016, 32, 258–264. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Mikkelsen, L.H.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. A Combined Approach Based on Matrix Solid-Phase Dispersion Extraction Assisted by Titanium Dioxide Nanoparticles and Liquid Chromatography to Determine Polyphenols from Grape Residues. J. Chromatogr. A 2021, 1644, 462128. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, N.V.; Dai, H.; Roos, K.L.T.; Evanson, N.K.; Tomsik, J.; Elton, T.S.; Simmons, D.L. COX-3, a Cyclooxygenase-1 Variant Inhibited by Acetaminophen and Other Analgesic/Antipyretic Drugs: Cloning, Structure, and Expression. Proc. Natl. Acad. Sci. USA 2002, 99, 13926–13931. [Google Scholar] [CrossRef]
- Szewczuk, L.M.; Forti, L.; Stivala, L.A.; Penning, T.M. Resveratrol Is a Peroxidase-Mediated Inactivator of COX-1 but Not COX-2: A Mechanistic Approach to the Design of COX-1 Selective Agents. J. Biol. Chem. 2004, 279, 22727–22737. [Google Scholar] [CrossRef]
- Murias, M.; Handler, N.; Erker, T.; Pleban, K.; Ecker, G.; Saiko, P.; Szekeres, T.; Jäger, W. Resveratrol Analogues as Selective Cyclooxygenase-2 Inhibitors: Synthesis and Structure-Activity Relationship. Bioorg. Med. Chem. 2004, 12, 5571–5578. [Google Scholar] [CrossRef]
- Subbaramaiah, K.; Chung, W.J.; Michaluart, P.; Telang, N.; Tanabe, T.; Inoue, H.; Jang, M.; Pezzuto, J.M.; Dannenberg, A.J. Resveratrol Inhibits Cyclooxygenase-2 Transcription and Activity in Phorbol Ester-Treated Human Mammary Epithelial Cells. J. Biol. Chem. 1998, 273, 21875–21882. [Google Scholar] [CrossRef]
- Chen, F.; Castranova, V.; Shi, X. Review New Insights into the Role of Nuclear Factor-B in Cell Growth Regulation. Am. J. Pathol. 2001, 159, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wu, F.; Jin, Z.; Zhai, Z.; Wang, Y.; Tu, B.; Yan, W.; Tang, T. Plumbagin Inhibits LPS-Induced Inflammation through the Inactivation of the Nuclear Factor-Kappa B and Mitogen Activated Protein Kinase Signaling Pathways in RAW 264.7 Cells. Food Chem. Toxicol. 2014, 64, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.D.; Morrissey, R.L.; Usborne, A.L.; Kapetanovic, I.; Crowell, J.A.; Muzzio, M.; McCormick, D.L. Subchronic oral toxicity and cardiovascular safety pharmacology studies of resveratrol, a naturally occurring polyphenol with cancer preventive activity. Food Chem. Toxicol. 2011, 49, 3319–3327. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Garza, S.L.; Laveriano-Santos, E.P.; Marhuenda-Muñoz, M.; Storniolo, C.E.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Health Effects of Resveratrol: Results from Human Intervention Trials. Nutrients 2018, 10, 1892. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, Y. Effects of resveratrol therapy on glucose metabolism, insulin resistance, inflammation, and renal function in the elderly patients with type 2 diabetes mellitus: A randomized controlled clinical trial protocol. Medicine 2022, 101, e30049. [Google Scholar] [CrossRef]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Effect of Resveratrol on Markers of Oxidative Stress and Sirtuin 1 in Elderly Adults with Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 7422. [Google Scholar] [CrossRef]
- Gu, J.; Li, Z.; Chen, H.; Xu, X.; Li, Y.; Gui, Y. Neuroprotective Effect of Trans-Resveratrol in Mild to Moderate Alzheimer Disease: A Randomized, Double-Blind Trial. Neurol. Ther. 2021, 10, 905–917. [Google Scholar] [CrossRef]
- Gimblet, C.J.; Kruse, N.T.; Geasland, K.; Michelson, J.; Sun, M.; Mandukhail, S.R.; Wendt, L.H.; Eyck, P.T.; Pierce, G.L.; Jalal, D.I. Effect of Resveratrol on Endothelial Function in Patients with CKD and Diabetes: A Randomized Controlled Trial. Clin. J. Am. Soc. Nephrol. 2024, 19, 161–168. [Google Scholar] [CrossRef]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, D.; Jiang, P.; Tang, X.; Lang, Q.; Yu, Q.; Zhang, S.; Che, Y.; Feng, X. Resveratrol Synergizes with Low Doses of L-DOPA to Improve MPTP-Induced Parkinson Disease in Mice. Behav. Brain Res. 2019, 367, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Zhou, X.; Gu, M.; Jiao, W.; Yu, M.; Wang, Y.; Liu, S.; Yang, J.; Ji, F. Resveratrol Synergizes with Cisplatin in Antineoplastic Effects against AGS Gastric Cancer Cells by Inducing Endoplasmic Reticulum Stress-Mediated Apoptosis and G2/M Phase Arrest. Oncol. Rep. 2020, 44, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Alhayali, A.S.A.; Hasan, W.A.; Salah, F.S. Autophagy Induction Using Resveratrol Enhances the Anti-Cancer Efficacy of Doxazosin in Breast Cancer Cells. Bionatura 2023, 8, 63. [Google Scholar] [CrossRef]
- Toniolo, L.; Fusco, P.; Formoso, L.; Mazzi, A.; Canato, M.; Reggiani, C.; Giacomello, E. Resveratrol Treatment Reduces the Appearance of Tubular Aggregates and Improves the Resistance to Fatigue in Aging Mice Skeletal Muscles. Exp. Gerontol. 2018, 111, 170–179. [Google Scholar] [CrossRef]
- Hussain, S.A.; Marouf, B.H.; Ali, Z.S.; Ahmmad, R.S. Efficacy and Safety of Co-Administration of Resveratrol with Meloxicam in Patients with Knee Osteoarthritis: A Pilot Interventional Study. Clin. Interv. Aging 2018, 13, 1621–1630. [Google Scholar] [CrossRef]
- Farrokhi, E.; Ghatreh-Samani, K.; Salehi-Vanani, N.; Mahmoodi, A. The Effect of Resveratrol on Expression of Matrix Metalloproteinase 9 and Its Tissue Inhibitors in Vascular Smooth Muscle Cells. ARYA Atheroscler. 2018, 14, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health Benefits of Resveratrol Administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Corpas, R.; Griñán-Ferré, C.; Rodríguez-Farré, E.; Pallàs, M.; Sanfeliu, C. Resveratrol Induces Brain Resilience Against Alzheimer Neurodegeneration Through Proteostasis Enhancement. Mol. Neurobiol. 2019, 56, 1502–1516. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, K.; Wan, W.; Cheng, Y.; Pu, X.; Ye, X. Resveratrol Provides Neuroprotection by Regulating the JAK2/STAT3/PI3K/AKT/MTOR Pathway after Stroke in Rats. Genes Dis. 2018, 5, 245–255. [Google Scholar] [CrossRef]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by Resveratrol: A Human Clinical Trial in Patients with Stable Coronary Artery Disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef]
- Romain, C.; Gaillet, S.; Carillon, J.; Vidé, J.; Ramos, J.; Izard, J.C.; Cristol, J.P.; Rouanet, J.M. Vineatrol and Cardiovascular Disease: Beneficial Effects of a Vine-Shoot Phenolic Extract in a Hamster Atherosclerosis Model. J. Agric. Food Chem. 2012, 60, 11029–11036. [Google Scholar] [CrossRef]
- Bryl, A.; Falkowski, M.; Zorena, K.; Mrugacz, M. The Role of Resveratrol in Eye Diseases—A Review of the Literature. Nutrients 2022, 14, 2974. [Google Scholar] [CrossRef] [PubMed]
- Doganay, S.; Borazan, M.; Iraz, M.; Cigremis, Y. The Effect of Resveratrol in Experimental Cataract Model Formed by Sodium Selenite. Curr. Eye Res. 2006, 31, 147–153. [Google Scholar] [CrossRef]
- Wakamatsu, T.H.; Dogru, M.; Matsumoto, Y.; Kojima, T.; Kaido, M.; Ibrahim, O.M.A.; Sato, E.A.; Igarashi, A.; Ichihashi, Y.; Satake, Y.; et al. Evaluation of Lipid Oxidative Stress Status in Sjögren Syndrome Patients. Investig. Ophthalmol. Vis. Sci. 2013, 54, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Anekonda, T.S.; Adamus, G. Resveratrol Prevents Antibody-Induced Apoptotic Death of Retinal Cells through Upregulation of Sirt1 and Ku70. BMC Res. Notes 2008, 1, 122. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Dace, D.S.; Ryazanov, A.G.; Kelly, J.; Apte, R.S. Resveratrol Regulates Pathologic Angiogenesis by a Eukaryotic Elongation Factor-2 Kinase-Regulated Pathway. Am. J. Pathol. 2010, 177, 481–492. [Google Scholar] [CrossRef]
- Shetty, R.; Joshi, P.D.; Mahendran, K.; Jayadev, C.; Das, D. Resveratrol for Dry Eye Disease—Hope or Hype? Indian. J. Ophthalmol. 2023, 71, 1270–1275. [Google Scholar] [CrossRef]
- Luna, C.; Li, G.; Liton, P.B.; Qiu, J.; Epstein, D.L.; Challa, P.; Gonzalez, P. Resveratrol Prevents the Expression of Glaucoma Markers Induced by Chronic Oxidative Stress in Trabecular Meshwork Cells. Food Chem. Toxicol. 2009, 47, 198–204. [Google Scholar] [CrossRef]
- Razali, N.; Agarwal, R.; Agarwal, P.; Froemming, G.R.A.; Tripathy, M.; Ismail, N.M. IOP Lowering Effect of Topical Trans-Resveratrol Involves Adenosine Receptors and TGF-Β2 Signaling Pathways. Eur. J. Pharmacol. 2018, 838, 1–10. [Google Scholar] [CrossRef]
- Bhatt, P.; Fnu, G.; Bhatia, D.; Shahid, A.; Sutariya, V. Nanodelivery of Resveratrol-Loaded PLGA Nanoparticles for Age-Related Macular Degeneration. AAPS PharmSciTech 2020, 21, 291. [Google Scholar] [CrossRef] [PubMed]
- Natesan, S.; Pandian, S.; Ponnusamy, C.; Palanichamy, R.; Muthusamy, S.; Kandasamy, R. Co-Encapsulated Resveratrol and Quercetin in Chitosan and Peg Modified Chitosan Nanoparticles: For Efficient Intra Ocular Pressure Reduction. Int. J. Biol. Macromol. 2017, 104, 1837–1845. [Google Scholar] [CrossRef]
- Buosi, F.S.; Alaimo, A.; Di Santo, M.C.; Elías, F.; García Liñares, G.; Acebedo, S.L.; Castañeda Cataña, M.A.; Spagnuolo, C.C.; Lizarraga, L.; Martínez, K.D.; et al. Resveratrol Encapsulation in High Molecular Weight Chitosan-Based Nanogels for Applications in Ocular Treatments: Impact on Human ARPE-19 Culture Cells. Int. J. Biol. Macromol. 2020, 165, 804–821. [Google Scholar] [CrossRef]
- El-Haddad, M.E.; Hussien, A.A.; Saeed, H.M.; Farid, R.M. Down Regulation of Inflammatory Cytokines by the Bioactive Resveratrol-Loaded Chitoniosomes in Induced Ocular Inflammation Model. J. Drug Deliv. Sci. Technol. 2021, 66, 102787. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Luo, L.J.; Yang, C.J.; Lai, J.Y. Highly Retina-Permeating and Long-Acting Resveratrol/Metformin Nanotherapeutics for Enhanced Treatment of Macular Degeneration. ACS Nano 2023, 17, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Lopez, M.; Sparacino, C.; Quelle-Regaldie, A.; Sánchez, L.; Candal, E.; Barreiro-Iglesias, A.; Huete-Toral, F.; Carracedo, G.; Otero, A.; Concheiro, A.; et al. Pluronic®/Casein Micelles for Ophthalmic Delivery of Resveratrol: In Vitro, Ex Vivo, and in Vivo Tests. Int. J. Pharm. 2022, 628, 122281. [Google Scholar] [CrossRef]
- Dong, Y.; Wan, G.; Yan, P.; Qian, C.; Li, F.; Peng, G. Fabrication of Resveratrol Coated Gold Nanoparticles and Investigation of Their Effect on Diabetic Retinopathy in Streptozotocin Induced Diabetic Rats. J. Photochem. Photobiol. B 2019, 195, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gu, P.; Liu, X.; Hu, S.; Zheng, H.; Liu, T.; Li, C. Gold Nanoparticles Encapsulated Resveratrol as an Anti-Aging Agent to Delay Cataract Development. Pharmaceuticals 2023, 16, 26. [Google Scholar] [CrossRef]
- Vivero-Lopez, M.; Pereira-Da-Mota, A.F.; Carracedo, G.; Huete-Toral, F.; Parga, A.; Otero, A.; Concheiro, A.; Alvarez-Lorenzo, C. Phosphorylcholine-Based Contact Lenses for Sustained Release of Resveratrol: Design, Antioxidant and Antimicrobial Performances, and in Vivo Behavior. ACS Appl. Mater. Interfaces 2022, 14, 55431–55446. [Google Scholar] [CrossRef]
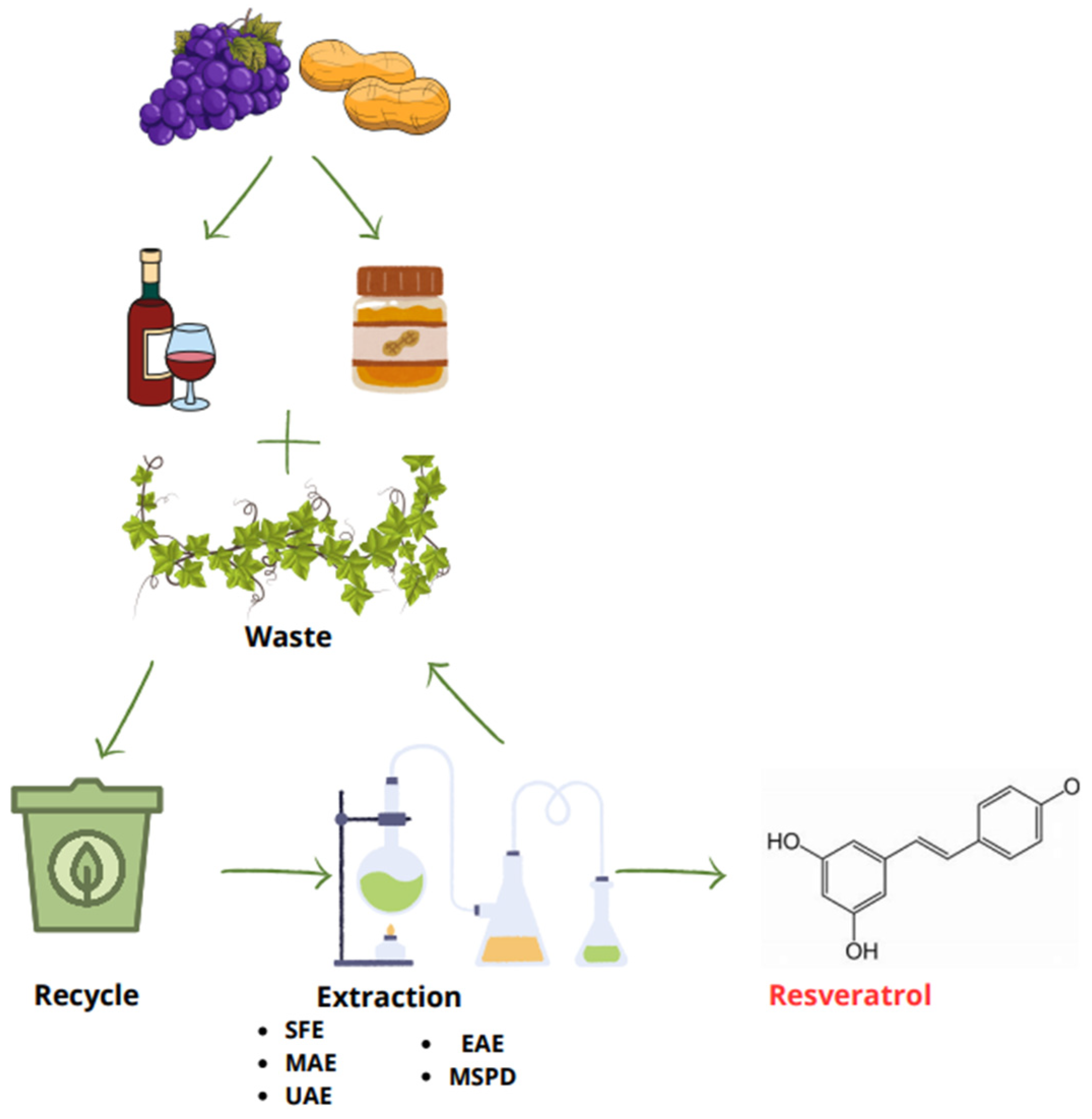
| Method of Extraction | Solvent | Extraction Condition | Natural Source | Ref. |
|---|---|---|---|---|
| SFE | Ethanol | 70 °C, 48 MPa of pressure for 50 min | Peanut kernels | [18] |
| MAE | Ethanol/water (80%) | 125 °C and 750 W for 5 min | Grape stem and canes | [20] |
| UAE | Ethanol/water (40%) | 50 °C in ultrasonic bath (40 kHz, 100 W) | Grape leaves | [21] |
| EAE | Citric acid–NaOH buffer (50 mM, pH 5.0), 27.0 U cellulase; Methanol | 25–50 °C, with shaking at 150 rpm | Polygonum cuspidatum roots | [23] |
| MSPD | TiO2 NPs and diatomaceous earth | Room temperature and pressure | Grape pomace and grape fruit | [24] |
| Nanosystem | Loaded Molecules | Composition | Major Outcome | Cell Line/ Animal Model | Reference |
|---|---|---|---|---|---|
| NPs | RV | Poly (lactic-co-glycolic acid) (PLGA) | <VEGF expression | In vitro (ARPE-19) | [61] |
| RV and quercetin | Polyethylene glycols (PEGs) modified chitosan (CS) | <IOP | In vivo (rabbit) | [62] | |
| RV and metformin | Biopolymer poly(ε-caprolactone) (PCL) | Antioxidant, anti-inflammatory, and antiangio-genic activities | In vitro (HUVECs)/ in vivo (rats) | [65] | |
| RV | Gold | <mRNA expression of VEGF-1, TNFα, adhesion molecules, IL-6, IL-1β | In vivo (rat)/ ex vivo | [67] | |
| RV | Gold | <ROS, expression levels of senescence markers (p16, p21, BAX, BCL-2 and SASP) >GSH | In vitro (HLECB3)/ in vivo (rats) | [68] | |
| In situ thermoresponsive hydrogel | RV | Acetylated polyethyleneimine-modified poly lactic-co-glycolic acid- (PLGA-PEI) NPs into poloxamer 407 hydrogel | Antioxidant and anti-inflammatory effects | In vitro (HCECs) | [14] |
| NG | RV | High weight chitosan | Anti-inflammatory effects | In vitro (ARPE-19) | [63] |
| LPs | RV | Trimethylated chitosan-coated | <H2O2-induced damage | In vitro (ARPE-19), in vivo (mouse) | [13] |
| Niosomes | RV | Chitosan-coated | <TNFα, IL-6 | In vivo | [64] |
| Micelle | RV | Pluronic® F127 and casein | >Solubilize RV, preserve antioxidant properties, <biofilm development | In vitro (HCECs), ex vivo (porcine eye), in vivo (rabbit) | [66] |
| SNEEDS | RV and melatonin | Capryol® PGMC, Tween® 80, and Transcutol® P | Optimization of formulations for ocular administration | In vitro (SIRC) | [12] |
| Contact lenses | RV | Daily contact lens coating with 2-methacryloyloxyethyl phosphorylcholine (MPC) | <Inflammation and biofilm development, antibiofouling | In vitro (THP-1), in vivo (rabbit) | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Accomasso, G.; Turku, F.; Sapino, S.; Chirio, D.; Peira, E.; Gallarate, M. Resveratrol: Extraction Techniques, Bioactivity, and Therapeutic Potential in Ocular Diseases. Sci. Pharm. 2024, 92, 59. https://doi.org/10.3390/scipharm92040059
Accomasso G, Turku F, Sapino S, Chirio D, Peira E, Gallarate M. Resveratrol: Extraction Techniques, Bioactivity, and Therapeutic Potential in Ocular Diseases. Scientia Pharmaceutica. 2024; 92(4):59. https://doi.org/10.3390/scipharm92040059
Chicago/Turabian StyleAccomasso, Giulia, Flavia Turku, Simona Sapino, Daniela Chirio, Elena Peira, and Marina Gallarate. 2024. "Resveratrol: Extraction Techniques, Bioactivity, and Therapeutic Potential in Ocular Diseases" Scientia Pharmaceutica 92, no. 4: 59. https://doi.org/10.3390/scipharm92040059
APA StyleAccomasso, G., Turku, F., Sapino, S., Chirio, D., Peira, E., & Gallarate, M. (2024). Resveratrol: Extraction Techniques, Bioactivity, and Therapeutic Potential in Ocular Diseases. Scientia Pharmaceutica, 92(4), 59. https://doi.org/10.3390/scipharm92040059








