Abstract
Breast cancer is the most common and the leading cause of cancer death in women worldwide; treating invasive breast carcinomas is challenging due to the side effects of chemotherapeutics. Compounds isolated from natural sources have been proposed as potential molecules for cancer therapy; for instance, the homoisoflavonoid brazilin has shown pharmacological properties, including anti-tumoral and anti-inflammatory activities. In this study, we isolated brazilin from the heartwood of Haematoxylum brasiletto; then, we performed a semi-synthesis by adding three methyl or acetyl groups to the core structure of brazilin. We confirmed the identity of brazilin and its derivatives by spectroscopic data (1H NMR and 13C NMR) and measured their purity by optical rotation. Then, we analyzed the effects of brazilin and its derivatives in three mammary gland-derived cell lines: the TNBC MDA-MB-231, the ERα(+) MCF7, and the non-tumorigenic MCF10A. We evaluated the cell viability by MTT assays, cell migration by wound-healing assays, and focal adhesion kinase (FAK) activation by Western blot. Regarding biological assays, the MTT assay showed that these compounds showed cytotoxic effects on the MCF7 and MDA-MB-231 breast cancer cells at 20 µM but was not toxic in non-tumorigenic MCF10A mammary epithelial cells. Specifically, the greatest effects found from treatment with the compounds were in the MDA-MB-231 cell line, where the IC50 of brazilin was 49.92 μM, and for MCF7, the brazilin-(OAc)3 was 49.97 μM. These effects were dose- and time-dependent, as well as being associated with a decrease in the levels of cell migration and FAK activation.
1. Introduction
The diversity of natural products has played and will continue to play an essential role in drug discovery and development [1,2]. According to Newman and collaborators, about 75 (41%) of the newly approved anti-cancer drugs from 1946 to 2019 were inspired or derived from natural sources [3]. Natural sources include microorganisms, algae, and plants; the isolated compounds may consist of tannins, stilbenes, lignans, phenolic acids, and flavonoids. However, flavonoids are among the most abundant and bioactive compounds [4]. The anti-cancer effects of numerous members of flavonoids have been extensively reviewed; they modulate reactive oxygen species (ROS) responses, arrest the cell cycle, induce cell death, and suppress the proliferation and invasiveness of cancer cells [5,6]. Moreover, isoflavonoids such as genistein, daidzein, biochanin A, and glycitein have been taken to the stage of clinical trials for the development of new anti-invasive chemotherapeutics [7].
Brazilin [(6aS, 11bR) -7, 11b-dihydro-6H-indeno [2,1-c] chromene-3, 6a, 9, 10-tetrol] is a natural tetracyclic homoisoflavonoid made of a chroman skeleton fused in cis with a 2,3-dihydro-1H indene residue [8]. Brazilin is the most abundant compound in the heartwood of Caesalpinia sappan L. (Leguminosae) (Southeast Asia) and was also isolated from the ethanolic extracts of the heartwood of Haematoxylum brasiletto (Fabaceae) [9]. H. brasiletto, regionally known as “Palo de Brasil”, is commonly used in beverages, infusions, or extracts in traditional medicine in the treatment of chronic diseases [8]. The cytotoxic effects of brazilin (H. brasiletto) were tested against the A549, LS180, HeLa, SiHa, MDA-MB-231, and NCI-H1299 cancer cell lines, showing a reduction in cell viability in all cancer cells [9]. Previous reports showed that brazilin regulates markers of the epithelial–mesenchymal transition by decreasing the expression of vimentin, Twist, and invasion in breast cancer cells [10]. Despite the growing evidence of the anti-tumoral activities of brazilin, its effects inhibiting breast cancer progression continue to be explored.
Breast cancer is the most diagnosed and the leading cause of death in women worldwide. In 2020, in women aged 45 to 55 years, the incidence of breast cancer was more than two million new cases and mortality was over a half million [11]. Among breast cancer subtypes, triple-negative breast cancer (TNBC) is the most aggressive; patients often develop metastasis and chemoresistance [12]. From the hallmarks of cancer, metastasis [13] is the leading cause of death in cancer patients [14]. Metastasis is a multi-step process in which tumor cells separate from neighboring cells by losing their cell–cell and cell–extracellular matrix (ECM) adhesions; then, the cells acquire migratory capacity, invade the stroma, and enter the bloodstream; and finally, by extravasation, the tumor cells grow in a distant organ, forming a secondary tumor [15]. Cell migration is one of the initial and more essential steps in the metastatic cascade and involves cytoskeleton rearrangements; actin filaments polarize and form protrusions that facilitate cell movement [16]. In addition, cell–ECM adhesions, e.g., focal adhesions (FAs), are fundamental to giving directionality and strength to the movement. Both actin cytoskeleton dynamics and FAs are regulated by the downstream signaling of kinases, such as focal adhesion kinase (FAK) and Src [17]. Despite the strategies to counteract breast cancer progression with the use of mastectomy, radiation, and hormonal and targeted therapy, chemotherapy is the primary approach for treating TNBC [18]. However, around 90% of women who have received chemotherapy have shown side effects [19,20]. Therefore, new therapeutic agents are needed for the treatment of invasive breast cancer.
Previous evidence suggests that brazilin prevents the migration of platelet-derived growth factor (PDGF)-stimulated vascular smooth muscle cells (VSMCs); the authors proposed a mechanism by inhibiting the platelet-derived growth factor receptor (PDGFR)–Src-ERK1/2-Akt signaling axis [21]. Brazilin in co-treatment with doxorubicin (DOX) inhibited cell migration through HER2-overexpressing MCF7 breast cancer cells (MCF7/HER2), possibly through the downregulation of matrix metalloprotease-2 (MMP-2), MMP-9, HER2, Rac1, and p120 protein expression [22]. Furthermore, a recent investigation described the effects of brazilin on the invasion of breast cancer cells in vitro and in vivo. They found that brazilin inhibited the proliferation, migration, and invasion of 4T1 and MDA-MB-231 breast cancer cells; also, brazilin-treated mice showed increased survival, fewer metastatic nodules, and smaller tumor volume [23].
Even though natural products are a very important source for obtaining biomolecules with therapeutic potential in cancer, they have shown some physicochemical mimics related to low bioavailability, solubility, biotransformation, and low systemic absorption that hinder drug development. Therefore, structural optimization is a crucial step in the drug development process [24]. Semi-synthesis is a strategy to perform structural modifications to improve activity and selectivity; it usually consists of the change, addition, or removal of functional groups to the core structure of a compound [25]. Such chemical modifications change the physicochemical and pharmacological properties of compounds, which can result in improved performance, as described before for cabazitaxel (Jevtana®, Sanofi, Hong Kong), which is a C-7- and C-10-methoxylated derivative of docetaxel [26]. Likewise, halichondrin B (Halichondria okadai), initially used as a chemotherapeutic for breast cancer, was structurally simplified to eribulin mesylate with better activity and manageable secondary effects [27,28].
The aim of the present study was to evaluate the anti-migratory activity in three mammary gland-derived cell lines of brazilin and two semisynthetic derivatives. Initially, we isolated brazilin from the heartwood of H. brasiletto and performed chemodiversification by semi-synthesis; we obtained brazilin-(OMe)3 and brazilin-(OAc)3. We investigated the biological activity of brazilin and methylated and acetylated derivatives on TNBC MDA-MB-231, ERα (+) MCF7 breast cancer cells, and the non-tumorigenic mammary epithelial MCF10A cell line. We also evaluated their effects on cell viability, cell migration, and FAK activation; our compounds showed differential effects against the tested cell lines, exhibiting a greater impact against the MDA-MB-231 breast cancer cells.
2. Materials and Methods
2.1. Chemistry General Information
All the materials and solvents were analytical grade and used as received unless otherwise noted. The reaction progress in the synthesis of all the compounds was monitored on Merck aluminum plates pre-coated with silica gel 60 F254 (Merck, KGaA, Dormstadt, Germany). Silica gel column flash chromatography was performed with Silica Flash 60 230–400 mesh (Merck, KGaA, Dormstadt, Germany), and two alternative reagents were used for visualization: (a) iodine and (b) KMnO4 in water.
The proton and carbon nuclear magnetic resonance (1H-NMR and 13C-NMR) spectra were recorded in CD3OD or CDCl3 on a Bruker (500 MHz) instrument using TMS as an internal standard. The chemical shifts (δ) were determined in parts per million (ppm), and the coupling constant (J) values are given in hertz (Hz). Standard abbreviations are used for the peak multiplicities. The melting points were determined with a MEL-TEMP® 3.0 apparatus and are uncorrected. The optical rotation was measured on a Perkin Elmer 341 polarimeter (Perkin Elmer, Waltham, MA, USA) at 589 nm, and the samples were dissolved in MeOH. High-resolution FAB+ mass spectra (HRMS) data of the new derivatives were obtained on a JEOL MStation MS-700 mass spectrometer (JEOL, Tokyo, Japan).
2.2. Compound Brazilin Isolation
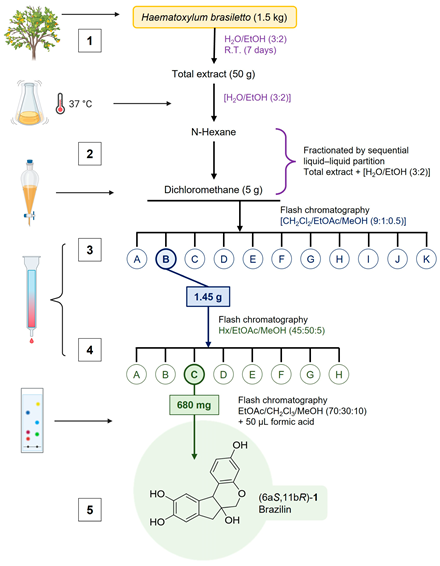
The heartwood of H. brasiletto was collected at Mochitlan, state of Guerrero, Mexico, at 99°21′19.03” W; 17°29′03.27” N to 1042 msnm. Purification of 6aS,11bR-1 or (+)-brazilin: (1) The ethanolic extract from the heartwood powder of H. brasiletto was obtained by maceration with EtOH at room temperature for seven days with regular stirring. The ethanol extracts were evaporated under reduced pressure on a rotary evaporator to yield the total extract. (2) The total extract was resuspended in 250 mL of an aqueous mixture (3:2 of H2O/EtOH), and the resulting suspension was fractionated by liquid–liquid separation with n-hexane and dichloromethane (CH2Cl2) to produce the corresponding fractions of low and low-media polarity, respectively. The fractions were stored at −4 °C in amber glass vials until use. (3) The brazilin was purified on flash chromatography using gradient elution with CH2Cl2/EtOAc/MeOH mixtures (9:1:0.5), yielding 11 main fractions (3A-3K) (Appendix B Figure A1b). (4) Fraction 3B was purified by flash chromatography eluting with n-hexane: EtOAc: MeOH (45:50:5) to yield 8 new fractions (4A-4H) (Appendix B Figure A1b). The final purification of fraction 4C using EtOAc: CH2Cl3: MeOH (70:30:10 + 50 µL formic acid) resulted in the isolation of (5) brazilin. See the workflow diagram described in Appendix A. The 1H and 13C NMR spectroscopic data for brazilin shown in Figure 1b, Supplementary Figure S1, and described below are similar to previous reports [29].
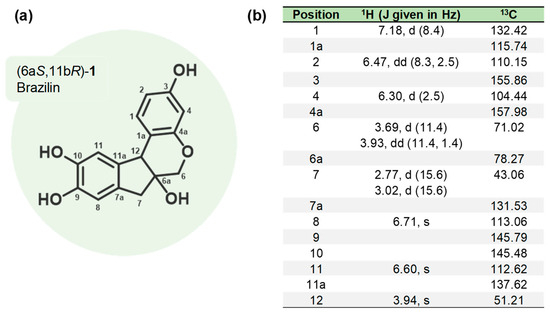
Figure 1.
Brazilin isolated from H. brasiletto. (a) Chemical structure and (b) spectroscopic data (1H NMR and 13C NMR) of brazilin.
Structural elucidation of brazilin-1 (6aS,11bR)-9,10-dihydroxy-6,6a,7,11b-tetrahydroindeno [2,1-c] chromene-3,6a-diol (+)-Brazilin (C16H14O5 Wt.: 286.08) (purity of 91%).
m.p.: 119–121 °C. +69.2 (c 1.00, MeOH), 1H-NMR (500 MHz, CD3OD): δ = 7.18 (d, J = 8.4 Hz, 1H), 6.71 (s, 1H), 6.60 (s, 1H), 6.47 (dd, J = 8.3, 2.5, Hz, 1H), 6.30 (d, J = 2.5 Hz, 1H), 3.96 (s, 1H), 3.93 (dd, J = 11.3, 1.4 Hz, 1H), 3.69 (d, J = 11.3 Hz, 1H), 3.02 (d, J = 15.6 Hz, 1H), and 2.77 (d, J = 15.6 Hz, 1H) ppm. 13C-NMR (125 MHz, CD3OD): δ = 157.98, 155.86, 145.79, 145.48, 137.62, 132.42, 131.53, 115.74, 113.06, 112.62, 110.15, 104.44, 78.27, 71.02, 51.21, and 43.06 ppm.
HRMS (FAB+): m/z [M + H]+ Calcd. for C16H15O5: 287.0919; found: 287.0991.
2.3. Semi-Synthesis of Brazilin-(OMe)3 and Brazilin-(OAc)3
3’,9’,10’-O-trimethylbrazilin or brazilin-(OMe)3: (6aS, 11bR)-3,9,10-trimethoxy-7,11b-dihydroindeno [2,1-c] chromene-6a (6H)-ol: A solution of brazilin (40 mg, 0.14 mmol) in acetone (3.5 mL) at room temperature was treated with K2CO3 (57.9 mg, 0.4191 mmol) and MeI (99.16 mg, 0.6986 mmol). The reaction mixture was stirred at room temperature for 24 h. The crude product was purified by column chromatography 80:20 (EtOAc-Hexane), giving the compound [28.8 mg (52%)] as a yellow oil. For the reaction conditions, see Table A1 in Appendix C.
1H NMR (500 MHz, CDCl3): δ = 7.28 (d, J = 8.5 Hz, 1H), 6.76 (s, 1H), 6.71 (s, 1H), 6.63 (dd, J = 8.4, 2.6, Hz, 1H), 6.46 (d, J = 2.6 Hz, 1H), 4.10 (s, 1H), 4.01 (dd, J = 11.3, 1.5 Hz, 1H), 3.89 (dd, J = 13.2, 2.0 Hz, 1H), 3.82 (s, 3H), 3.81 (s, 3H), 3.76 (s, 3H), 3.23 (d, J = 15.8 Hz, 1H), 2.86 (d, J = 15.8 Hz, 1H), and 2.52 (br s, 1H) ppm. 13C NMR (125 MHz, CDCl3): δ = 159.63, 154.59, 148.94, 148.65, 136.31, 131.31, 130.77, 114.60, 109.10, 108.66, 107.90, 102.21, 77.72, 70.50, 56.32, 56.28, 55.56, 50.73, and 41.57 ppm. See Supplementary Figure S2.
3’,9’,10’-O-triacetylbrazilin or brazilin-(OAc)3: (6aS, 11bR) -6a-hydroxy-6, 6a, 7,11b-tetrahydroindeno [2,1-c] chromene-3,9,10-triyl triacetate: A solution of brazilin (50 mg, 0.17 mmol) in THF (2.5 mL) at room temperature was treated with NaHCO3 (44 mg, 0.5238 mmol) and Ac2O (89.15 mg, 0.8733 mmol). The reaction mixture was stirred at room temperature for 6 h. The crude product was purified by column chromatography (EtOAc: hexane, 65:50), yielding the compound [24 mg (48%)] as a yellow oil. For the reaction conditions, see Table A2 in Appendix C.
1H NMR (500 MHz, CDCl3): δ = 7.34 (d, J = 8.4 Hz, 1H), 7.07 (d, J = 1.0 Hz, 1H), 7.03 (s, 1H), 6.79 (dd, J = 8.3, 2.4, Hz, 1H), 6.68 (d, J = 2.4 Hz, 1H), 4.19 (s, 1H), 4.04 (dd, J = 11.5, 1.6 Hz, 1H), 3.84 (d, J = 11.5 Hz, 1H), 3.30 (d, J = 16.3 Hz, 1H), 2.94 (d, J = 16.3 Hz, 1H), 2.58 (br s, 1H), 2.29 (s, 3H), and 2.26 (s, 6H) ppm. 13C NMR (125 MHz, CDCl3): δ = 169.59, 168.67, 168.57, 154.32, 150.47, 142.34, 141.69, 141.32, 137.60, 131.29, 120.45, 119.59, 119.08, 115.50, 111.02, 77.52, 70.17, 50.64, 41.23, 21.33, 20.86, and 20.81 ppm. See Supplementary Figure S3.
2.4. Cell Culture and Treatments
The non-tumorigenic mammary epithelial MCF10A cell line and the ERα(+) MCF7 and TNBC MDA-MB-231 cell lines (ATCC, Manassas, VA, USA) were cultured in DMEM/F12 medium (50:50, V:V; Sigma-Aldrich, St Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics (penicillin G/streptomycin; Gibco, Waltham, MA, USA) in a humidified atmosphere containing 5% CO2 at 37 °C.
The cell cultures were FBS-starved for 4 h (MCF10A) or 24 h (MDA-MB-231 and MCF7) and rinsed with sterile PBS prior to treatments. Brazilin (1), 3’,9’,10’-O-Trimethylbrazilin (2), or 3’,9’,10’-O-Triacetylbrazilin (3) were administered in concentrations from 2.5 µM to 40 µM in DMEM/F12 media for the time indicated in each section.
2.5. Viability Assays
The cells were seeded at 105 in a 96-well plate. After adherence overnight, the cells were incubated with the medium alone or with a serial dilution of brazilin, brazilin-(OMe)3, and brazilin-(OMe)3 starting with the highest concentration at 40 μM/mL for 48 h. Staurosporin and DMSO were used as the controls. Then, 20 µL of MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) solution was added to each well and mixed. After 4 h, the supernatants were removed, and 100 µL of the MTT solvent (acidified isopropanol) was added to each well to dissolve the formazan crystals. The cell viability was estimated by measuring the absorbance at 630 nm using a Thermo Scientific ™ Multiskan ™ FC microplate photometer. The percentage of cell viability was calculated as a function of the absorbance ratio between the cell culture treated with brazilin and the untreated control multiplied by 100, representing the cell viability (percentage of control).
2.6. Cell Migration Assays
MDA-MB-231, MCF7, and MCF10A cells were grown to confluence in 60 mm culture plates supplemented with complete DMEM/F12. The cells were FBS-starved and treated for 2 h with 10 µM Cytosine β-D-Arabinofuranoside (AraC) to inhibit cell proliferation during the experiment. After starvation, the cells were scratched using a sterile 200 μL pipet tip, the suspended cells were removed by washing twice with ice-cold sterile PBS, and the cultures were re-fed with DMEM/F12 in the absence (control cells or 0 condition) or presence 20 μM or 40 μM. The progress of wound healing was monitored using an EVOS FL microscope with a 10× objective. The cell cultures were photographed immediately after wounding and 48 h after the treatments; five images were analyzed per plate. The migration area was determined by measuring the total wound area using ImageJ 1.44p (NIH, Bethesda, MD, USA) software.
2.7. FAK Activation by Western Blot
After the treatments, the cells were solubilized in ice-cold lysis buffer/proteases and phosphatases inhibitor cocktail. The total protein (20 ng) was resolved in 10% SDS-PAGE and transferred to nitrocellulose membranes. Unspecific bindings were blocked with 5% non-fat milk solution incubation for 2 h at RT. The membranes were then incubated overnight at 4 °C with the primary anti-FAK (44-624G, Invitrogen, Waltham, MA, USA) (1:1000), total FAK (sc-558, Santa Cruz Biotechnology, Dallas, TX, USA) (1:1000), and GAPDH (AC027, ABclonal, Woburn, MA, USA) (1:5000 dilution). Next, the membranes were washed with TBS/0.1% Tween20 and incubated with a secondary HRP-conjugated anti-rabbit antibody (1:5000 dilution) for 2 h at RT. Proteins were detected using a Tanon High-sig ECL Western Blotting substrate (180-5001, ABclonal) and autoradiographic films. The images were processed using ImageJ 1.54 software (NIH, Bethesda, Rockville, MD, USA).
2.8. Statistical Analysis
The results are presented as the mean ± SE. The data were analyzed statistically by one-way ANOVA, and a Dunnett’s multiple comparison test was applied using the Prism 9.5.1 GraphPad software. A statistical probability of p< 0.05 was considered significant.
3. Results
3.1. Brazilin Purification from H. brasiletto and Semi-Synthesis of Brazilin-(OMe)3 and Brazilin-(OAc)3
In our study, the ground heartwood of H. brasiletto was used to obtain the homoisoflavonoid brazilin with a yield of 0.04% and a purity of 91% (Table 1). Brazilin was identified by spectroscopic 1H NMR and 13C NMR data (Figure 1) (Supplementary Figure S1).

Table 1.
Yield and purity of brazilin isolated from H. brasiletto heartwood.
Using brazilin (purified from H. brasiletto) as the raw material, we performed the semi-synthesis of two derivatives by the addition of three methyl or acetyl groups at 3’,9’, and 10’-OH groups (Figure 2). Therefore, the reaction of brazilin (Figure 2a) with methyl iodide and potassium carbonate in acetone at 25 °C for 24 h generated brazilin-(OMe)3 with a yield of 52% (Table 2; Figure 2b). On the other hand, the reaction of brazilin with acetic anhydride in the presence of sodium bicarbonate in tetrahydrofuran at 25 °C for 6 h provided brazilin-(OAc)3 with 48% yield (Table 2; Figure 2c). Brazilin-(OMe)3 and brazilin-(OAc)3 were identified by spectroscopic 1H NMR and 13C NMR data (Figure 3) (Supplementary Figures S2 and S3).
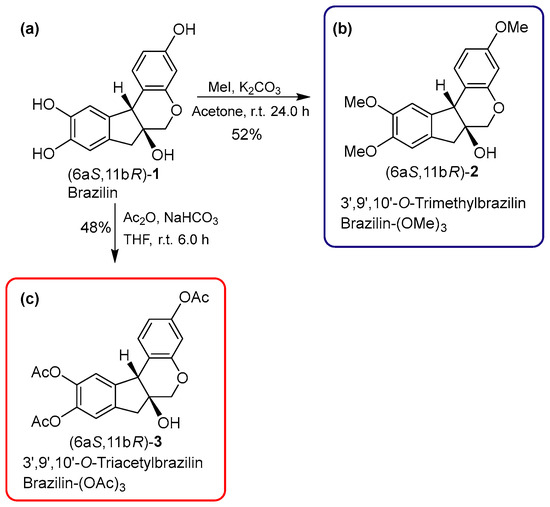
Figure 2.
Semisynthetic route and with the experimental conditions used for the chemodiversification of (a) brazilin to (b) brazilin-(OMe)3 and (c) brazilin-(OAc)3.

Table 2.
Yield of semi-synthesis of brazilin-(OMe)3 and brazilin-(OAc)3.
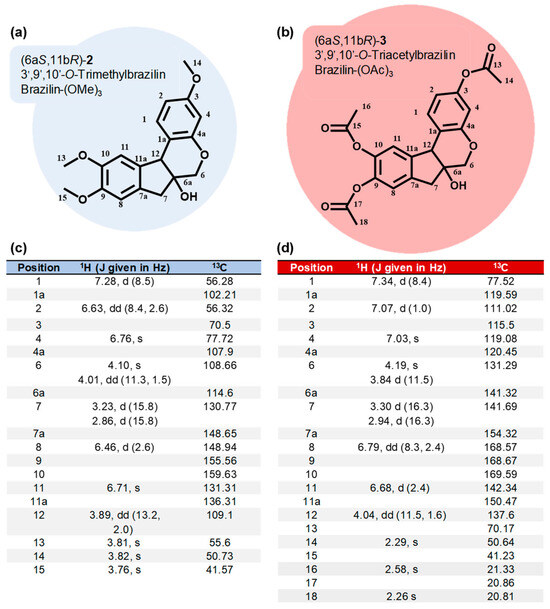
Figure 3.
Chemoderivatives of brazilin. Chemical structure and spectroscopic data (1H NMR and 13C NMR) of brazilin-(OMe)3 (a,c) and brazilin-(OAc)3 (b,d).
3.2. Brazilin, Brazilin-(OMe)3, and Brazilin-(OAc)3 Effects in Cell Viability
The impact of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on cell viability was evaluated in two breast cancer cell lines and as control the non-tumorigenic MCF10A mammary epithelial cell line, after 48 h. Selectivity was observed in the reduction of viability in the cancer cells, particularly in the MDA-MB-231 cell line, where a lower percentage of viability was recorded at concentrations of 20 µM of brazilin and 80 µM of the chemoderivatives (Table 3; Figure 4a). In the MCF7 cell line, viability was significantly decreased at concentrations of 40 µM of brazilin and brazilin-(OAc)3, compared to the control group (Table 3; Figure 4b). Furthermore, at 20 µM, brazilin also exerted an effect in decreasing the viability of the MCF10A cell line, while the compounds brazilin-(OMe)3 and brazilin-(OAc)3 showed their effect at concentrations higher than 40 µM (Table 3; Figure 4c).

Table 3.
IC50 of brazilin and derivatives in breast cancer and epithelial non-tumorigenic cells *.
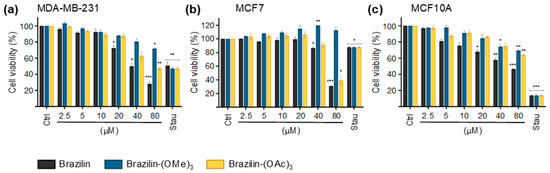
Figure 4.
Cell viability of MDA-MB-231 (a), and MCF7 (b) breast cancer cells and MCF10A (c) cells after treatment with brazilin for 24 h. Differences from the control were determined with one-way ANOVA and Dunnett’s multiple comparison test. Statistical significance: * p < 0.05), ** p < 0.01), and *** p < 0.001). Staurosporine (Stau) was used as an in vitro positive control for apoptosis.
3.3. Brazilin, Brazilin-(OMe)3, and Brazilin-(OAc)3 Inhibited Cell Migration of Breast Cancer Cell
Cell migration is an essential process during cancer cell invasion and metastasis and is an important target in cancer therapy [30]. Once the modified brazilin products were obtained, their biological effect on the cell migration of the breast cancer cell lines MDA-MB-231 and MCF7, as well as the non-tumorigenic breast epithelial cells MCF10A, was evaluated.
Using wound closure assays, the cell migration was evaluated by measuring the wound area of the cell cultures treated with different concentrations (0, 20, and 40 µM) of brazilin (Figure 5a), brazilin-(OMe)3 (Figure 5b), and brazilin-(OAc)3 (Figure 5c) for 24 h.
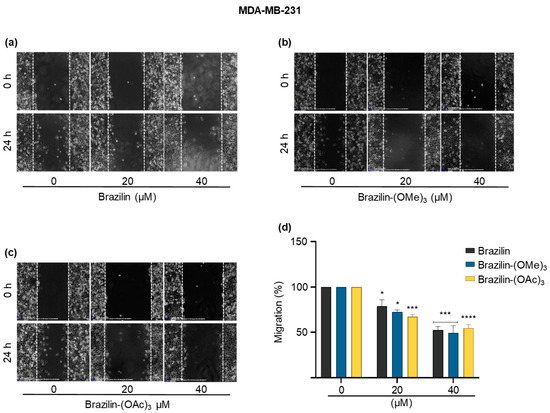
Figure 5.
Wound closure assays of the effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on cell migration of MDA-MB-231 cells. Cell cultures were exposed for 2 h with Ara-C to inhibit proliferation and treated with 20 µM and 40 µM of (a) brazilin, (b) brazilin-(OMe)3, and (c) brazilin-(OAc)3 for 24 h. Representative images of brightfield microscopy using 10x magnification; scale bars represent 400 μm. (d) Graphs represent the quantification of percent cell migration for each condition. The image is representative of three independent experiments. Differences with respect to control were determined by one-way ANOVA and Dunnett’s multiple comparison test. Statistical significance: * p < 0.05, *** p < 0.001, and **** p < 0.0001.
In the MDA-MB-231 cells, all three compounds had an inhibitory effect on cell migration in a concentration-dependent manner; as the concentration of the compounds increased, the cells decreased their migratory capacity compared to the control group (Figure 5d). Statistically, brazilin-(OAc) presented a more significant anti-migratory effect at a concentration of 20 µM compared to the other two compounds, decreasing cell migration by 30% compared to the control (Figure 5c); however, at 40 µM, the same effect was observed for all the compounds (Figure 5).
In the MCF7 cells (Figure 6), brazilin-(OAc)3 started to decrease the migration rate by about 50% from a 20 µM concentration (Figure 6c); however, brazilin-(OMe)3 had no activity at 20 µM, whereas, at the highest concentration, it was the compound that presented the greatest effect by decreasing 50% of the cell migration (Figure 6b).
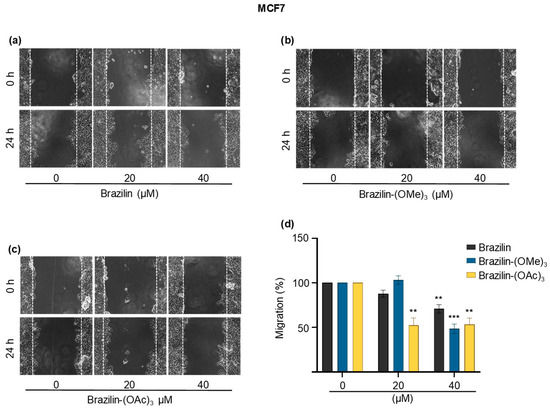
Figure 6.
Wound closure assays of the effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on cell migration of MCF7 cells. Cell cultures were exposed for 2 h with Ara-C to inhibit proliferation and treated with 20 µM and 40 µM of (a) brazilin, (b) brazilin-(OMe)3, and (c) brazilin-(OAc)3 for 24 h. Representative images of brightfield microscopy using 10x magnification; scale bars represent 400 μm. (d) Graphs represent the quantification of percent cell migration for each condition. The image is representative of three independent experiments. Differences with respect to control were determined by one-way ANOVA and Dunnett’s multiple comparison test. Statistical significance: ** p < 0.01); *** p < 0.001).
The migration rate of the MCF10A cells was considerably lower than the breast cancer cells. However, we observed that at 20 µM, the three compounds decreased cell migration by about 50% with respect to the control, while at 40 µM, the effect reduced approximately 20% of the cell migration (Figure 7).
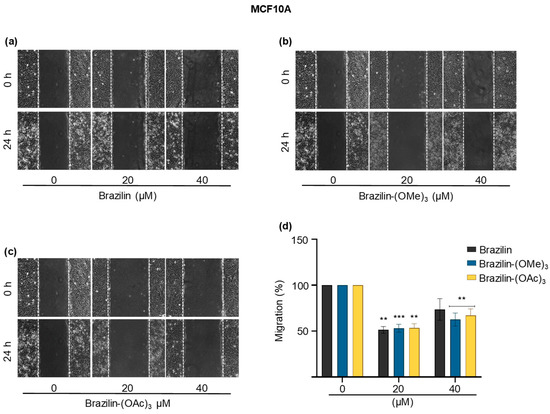
Figure 7.
Wound closure assays of the effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on cell migration of MCF10A cells. Cell cultures were exposed for 2 h with Ara-C to inhibit proliferation and treated with 20 µM and 40 µM of (a) brazilin, (b) brazilin-(OMe)3, and (c) brazilin-(OAc)3 for 24 h. Representative images of brightfield microscopy using 10x magnification; scale bars represent 400 μm. (d) Graphs represent quantification of percent cell migration for each condition. The image is representative of three independent experiments. Differences with respect to control were determined by one-way ANOVA and Dunnett’s multiple comparison test. Statistical significance: ** p < 0.01; *** p < 0.001.
3.4. Brazilin, Brazilin-(OMe)3, and Brazilin-(OAc)3 Inhibited Activation of FAK
Focal adhesion kinase (FAK) is a kinase involved in regulating cell migration in cancer and requires the formation of focal adhesions, a process regulated by adhesion molecules such as integrins and by various intracellular proteins [31]. FAK regulates the assembly and disassembly of adhesions during cell migration, and its activation is related to the autophosphorylation of the Y397; in addition, its expression is related to tumor progression and the clinical prognosis [32].
To determine the effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on FAK activation, we performed Western blotting of the total proteins obtained from the MDA-MB-231, MCF7, and MCF10A cells treated with 2.5, 5, 10, 20, and 40 µM of the different compounds for 24 h.
The results obtained in the MDA-MB-231 cells show a differential effect, where brazilin-(OMe)3 and brazilin-(OAc)3 increase FAK activation at 2.5 and 5 µM, but decrease considerably from 10 µM, showing a potential effect at 20 µM; however, in the brazilin, no changes were observed at low concentrations. Interestingly, we observed changes at 20 and 40 µM (Figure 8).
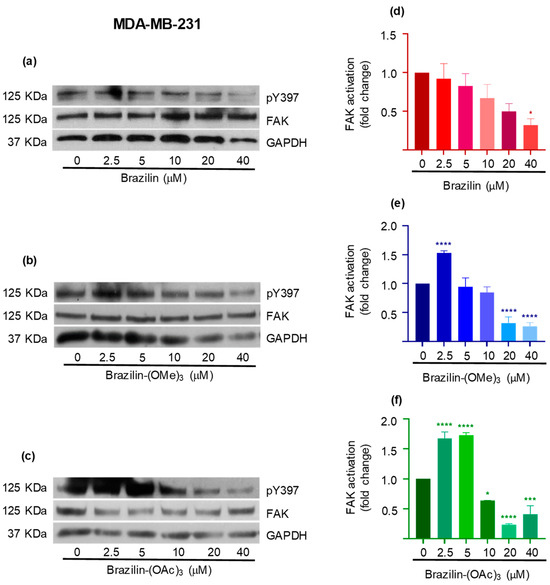
Figure 8.
Effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on FAK activation in MDA-MB-231 cells. Cell cultures were treated with the different compounds (2.5–40 μM) for 24 h. Western blot of FAK activation corresponding to treatments with (a) brazilin, (b) brazilin-(OMe)3, and (c) brazilin-(OAc)3 in MDA-MB-231 cells. (d–f) Graphs corresponding to the densitometric and statistical analysis of the bands obtained by WB. Statistical analysis was performed by one-way ANOVA and Dunnett’s multiple comparison test. Statistical significance: * p < 0.05, *** p < 0.001, and **** p < 0.0001.
In the MCF7 cells, different effects are observed with the three compounds. Brazilin promotes FAK activation at low concentrations, but at 20 and 40 µM, the FAK levels are reestablished with respect to the control (Figure 9a). Meanwhile brazilin-(OMe)3 at 2.5 µM decreases FAK activation; however, as the concentration increases, the two modified compounds increase FAK activation compared to the control (Figure 9d).
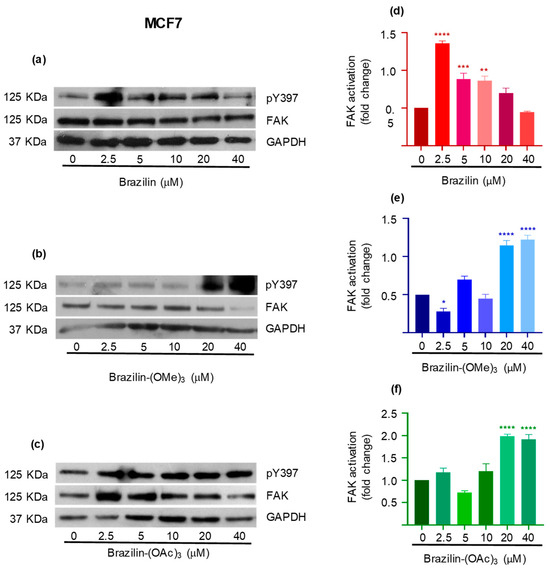
Figure 9.
Effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on FAK activation in MCF7 cells. Cell cultures were treated with the different compounds (2.5–40 μM) for 24 h. Western blot of FAK activation corresponding to treatments with (a) brazilin, (b) brazilin-(OMe)3, and (c) brazilin-(OAc)3 in MDA-MB-231 cells. (d–f) Graphs corresponding to the densitometric and statistical analysis of the bands obtained by WB. Statistical analysis was performed by one-way ANOVA and Dunnett’s multiple comparison test. Statistical significance: * p < 0.05, ** p < 0,01, *** p < 0.001, and **** p < 0.0001.
The results corresponding to the MCF10A cells show that brazilin and brazilin-(OMe)3 promote FAK activation with respect to the control (Figure 10a,b). Conversely, brazilin-(OAc)3 decreases FAK activation in a concentration-dependent manner (Figure 10d).
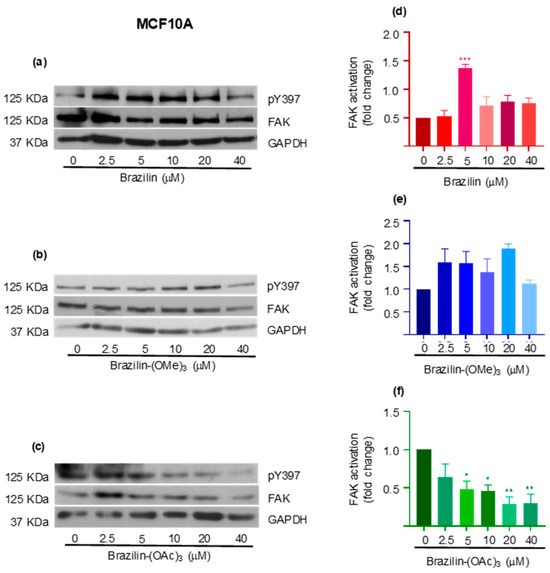
Figure 10.
Effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on FAK activation in MCF10A cells. Cell cultures were treated with the different compounds (2.5–40 μM) for 24 h. Western blot of FAK activation corresponding to treatments with (a) brazilin, (b) brazilin-(OMe)3, and (c) brazilin-(OAc)3 in MDA-MB-231 cells. (d–f) Graphs corresponding to the densitometric and statistical analysis of the bands obtained by WB. Statistical analysis was performed by one-way ANOVA and Dunnett’s multiple comparison test. Statistical significance: * p < 0.05, ** p < 0.01, and *** p < 0.001.
Our results show a differential effect on the three breast epithelial cell lines and the different compounds. However, all three compounds show a better effect on FAK activation in the MDA-MB-231 breast cancer cells.
4. Discussion
Despite the different treatments currently available, breast cancer continues to be one of the main causes of death worldwide, so it is important to continue the search for new therapeutic agents with potential effects against breast cancer [11,33]. In this regard, the study of phytochemicals has increased due to their potent antitumor activity and minimal toxicity, with alkaloids, taxanes, and flavonoids the most studied molecules [34].
Brazilin is an isoflavonoid isolated from Haematoxilum brasiletto [9] and Caesalpinia sappan [35]. It has been reported that brazilin displays a wide variety of antitumor effects on various cancer cell lines, inhibiting cell proliferation and cell cycle progression, decreasing cell migration, and inducing apoptosis [36,37]. However, its effects on breast cancer cells are poorly known. Therefore, in this study, we evaluated the effect of brazilin, extracted from the heartwood of H. brasiletto, and methoxylated and acetoxylated products of brazilin on cell migration and FAK activation in breast cancer cells.
The strategy for structural modification is to increase potency and selectivity; improve physicochemical, biochemical, and pharmacokinetic properties; eliminate or reduce adverse effects; and simplify the structural complexity of the candidate drug. It is described that flavonoids can act as enzyme inhibitors and receptor ligands [38,39].
In our study, we evaluated the potential cytotoxic effect of brazilin and its derivatives by MTT assays in MDA-MB-231 and MCF7 breast cancer cells and in non-tumorigenic MCF10A mammary epithelial cells. Interestingly, we observed specific effects in the IC50 according to the cell line and the compound tested, resulting in a higher activity of the three compounds in the MDA-MB-231 and MCF10A cells, whereas in the MCF7 cells, only brazilin and brazilin-(OAc)3 exhibited cytotoxic effects. Particularly, the greatest effects were in the MDA-MB-231 cell line where the IC50 of brazilin was 49.92 μM, while the IC50 value of brazilin-(OAc)3 was 49.97 μM against the MCF7 cells (Figure 4). These differences in the IC50 values of the derivatives in the tested cell lines could be related to the molecular and genetic characteristics of the breast cancer cell lines [40]. It has been described that in MDA-MB-231 cells, there is overexpression of EGFR, mutation in p53, loss of the tumor suppressor gene PTEN (involved in the regulation of the PI3K/AKT pathway that promotes cell survival and growth), and activation of NF-κB [41,42]. On the other hand, MCF7 cells are estrogen receptor (ER)- or progesterone receptor (PR)-positive (cell proliferation is regulated by these hormones) and overexpress Bcl-2, cyclin D, and the PI3K/AKT/mTOR pathway [41,42,43]. In this context, there is more significant dysregulation at the molecular level in MDA-MB-231 cells; therefore, brazilin and its derivatives may act through different targets, thus further decreasing viability in this breast cancer cell line.
According to our results on cell migration, a greater effect was observed with the modified compounds brazilin-(OMe)3 and brazilin-(OAc)3 than with the parent compound (Figure 5, Figure 6 and Figure 7). In this regard, the biological activity of the compounds shown here could be related to their aromatic rings, allowing for molecular (non-covalent) interactions with proteins showing strong dispersion interactions with non-polar amino acid [44]. Furthermore, hydrogen bond donors (HBDs), hydrogen bond acceptors (HBAs), and rotatable bonds in the compounds are critical determinants in the biological activity of drugs, as they significantly participate in the interaction with molecular targets, including proteins. Hydrogen bonds, formed by functional groups such as -OH, stabilize drug–protein complexes by facilitating specific interactions with crucial residues in active sites [45]. On the other hand, rotatable bonds affect the conformational flexibility of the ligand. High flexibility allows for better adaptation to the binding site, and optimization of this parameter is essential to design molecules with selective affinity toward targets with rigid cavities [46]. All these characteristics of the compounds allow for molecular interactions, essential for the differential biological activity observed in the cell viability and cell migration of breast cancer cells.
However, although we observed that these compounds have a potential effect in decreasing breast cancer cell migration, the mechanism of the inhibitory effect has not yet been elucidated; here, we decided to evaluate FAK activation in breast cancer and non-tumorigenic epithelial cell lines.
FAK is considered a central regulator of cell migration by promoting the formation of focal adhesions [47]. FAK phosphorylation at PY397 upon stimulation by integrins and growth factors promotes signaling pathway activation and its overexpression is related to tumor progression and poor prognosis in cancer [48].
Interestingly, in our study, a differential effect in FAK activation was found, both in the cell lines and in the concentrations of the treatments, because only at high concentrations of the three compounds did FAK activation decrease by about 50% in the MDA-MB-231 cells; remarkably, this result correlates with the results obtained for cell migration. Therefore, specifically in this TNBC cell line, brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 can decrease cell migration through FAK inhibition. However, at lower concentrations of brazilin-(OMe)3 and brazilin-(OAc)3, an increase in FAK activation was observed in the MDA-MB-231 cells (Figure 8). Likewise, in the MCF10A cells, FAK activation was also observed at all concentrations of brazilin and brazilin-(OMe)3 (Figure 10). These findings may be associated with a hormetic effect, where exposure to a low dose of a cytotoxic agent can induce oxidative stress in cancer cells and thus stimulate tumor proliferation and growth [49]. In this regard, an increase in FAK activation was also observed in the MCF7 cells at most concentrations of the three compounds (Figure 9). However, particularly in the MCF7 cell line, FAK activation at high concentrations of brazilin-(OMe)3 and brazilin-(OAc)3 (20 and 40 μM) can be related to their estrogenic positivity because, structurally, the isoflavonoids show similarities to estrogens, and they can bind to estrogen receptors with preferential affinity for estrogen receptor beta (ERβ), compete with 17β-estradiol for the ligand-binding domain of the receptor, and exhibit both estrogenic and anti-estrogenic activities [50,51]. Interestingly, estrogen, through the ER receptor, has been reported to promote FAK activation in ovarian cancer cells [52]. However, these results should be studied in depth to establish this relationship.
Furthermore, the chemical modifications to the original structure of brazilin improve the metabolic stability and allow for greater absorption and permeability across cell membranes, thus enhancing its potential effect [53]. There is evidence that methylation of flavonoids favors metabolic stability. Adding methyl groups (-CH3) increases hydrophobicity, favoring cell permeability and bioavailability, and improves metabolic stability by blocking reactive hydroxyl groups, prolonging the half-life of compounds. It can also modulate biological activity, increasing anti-inflammatory or antioxidant activity [54]. On the other hand, acetylation, which introduces acetyl groups (-COCH3), stabilizes compounds by reducing the reactivity of functional groups and can improve cell permeability, although in some cases it reduces antioxidant activity [55]. Both processes are crucial to optimize the use of natural product derivatives in therapeutic applications.
Our results indicate that all three compounds significantly affected cell migration and FAK activation in the MDA-MB-231 cells in a concentration-dependent manner. The MDA-MB-231 cell line corresponds to triple-negative breast cancer (TNBC), characterized by the absence of hormone receptors (ER-α/PR) and negativity for HER2, and is considered the most aggressive subtype, with early relapse and metastatic dissemination to several organs such as the lung, liver, and brain, being responsible for more than 50% of breast cancer deaths [56,57]. In this sense, these compounds have potential effects on this cell line, and they could be considered as potential new therapeutic agents in the treatment of breast cancer. In addition, it has been described that nanoformulations such as liposomes or nanoparticles can improve the bioavailability and targeting of natural compounds [58,59]; one perspective will be to test these derivatives and perform in vivo studies to confirm the efficacy and biosafety of these derivates.
However, it is important to deepen the investigation of the different mechanisms that brazilin and its derivates can be used to reduce tumor progression.
5. Conclusions
In this study, we observed that brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 had a greater effect on the triple-negative breast cancer cell line MDA-MB-231, while in the MCF7 estrogen receptor-positive tumor cells, brazilin-(OAc)3 had greater activity; however, the effect of the compounds was also time-specific and concentration-dependent. These results show the potential of these compounds, so it is important to continue their study, evaluating more processes and molecular targets that promote tumor progression because they may be promising compounds for treating breast cancer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/scipharm93010004/s1. Figure S1. Nuclear magnetic resonance spectrum of brazilin, 1H (a) and 13C (b); Figure S2. Nuclear magnetic resonance spectrum of brazilin-(OMe)3, 1H (a) and 13C (b); Figure S3. Nuclear magnetic resonance spectrum of brazilin-(OAc)3, 1H (a) and 13C (b).
Author Contributions
Conceptualization, A.H.-M., D.A.N.-T., J.B.-M. and N.N.-T.; methodology, A.H.-M., D.A.N.-T., M.D.Z.-E., M.O.-F., T.H.-M. and M.O.; validation, M.D.Z.-E., M.O.-F., T.H.-M., M.O. and A.E.Z.-G.; formal analysis, A.H.-M., D.A.N.-T., M.D.Z.-E., J.B.-M., M.O.-F., T.H.-M., A.E.Z.-G., M.A.M.-C. and N.N.-T.; investigation, M.O.-F., T.H.-M., A.E.Z.-G. and M.A.M.-C.; resources, J.B.-M. and N.N.-T.; writing—original draft preparation, A.H.-M., D.A.N.-T., M.D.Z.-E. and N.N.-T.; writing—review and editing, A.H.-M., D.A.N.-T., J.B.-M. and N.N.-T.; visualization, M.A.M.-C.; supervision, M.O. and N.N.-T.; project administration, N.N.-T.; funding acquisition, J.B.-M., M.O. and N.N.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from COCYTIEG awarded to J.B.-M. and SEP-PROMEP/103.5/14/11118 (UAGro-PTC-053) and SEP-CONACYT CB-2014-01-239870 awarded to N.N.-T.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
Alberto Hernandez-Moreno, Dania A. Nava-Tapia, and Miriam D. Zuñiga-Eulogio were supported by the CONAHCYT Predoctoral Training Grant.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 1H NMR | Proton Nuclear Magnetic Resonance |
| 13C NMR | Carbon-13 Nuclear Magnetic Resonance |
| AC2O | Acetic anhydride |
| CD3OD | Deuterated methanol |
| CDCl3 | Deuterated chloroform |
| CH2Cl2 | Methylene chloride |
| EtOAc | Ethyl acetate |
| EtOH | Ethanol |
| H2O | Water |
| K2CO3 | Potassium carbonate |
| KMnO4 | Potassium permanganate |
| m.p | Melting point |
| MeI | Iodomethane |
| MeOH | Methanol |
| NaHCO3 | Sodium bicarbonate |
| ppm | Parts per million |
| THF | Tetrahidrofuran |
| TMS | Tetramethylsilane |
Appendix A
Appendix B
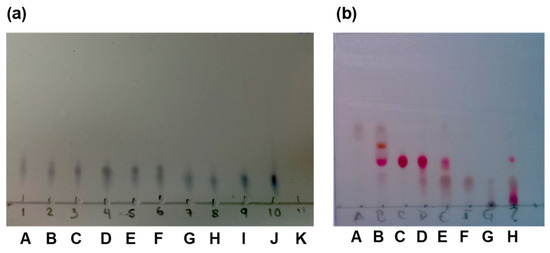
Figure A1.
(a) Brazilin purification scheme. (1) Obtaining the total extract, (2) sub-fractionation, (3,4) purification by column, (5) identification of brazilin. (b) Sub-fractions of brazilin isolation process. Thin layer chromatography of the fractions generated in (a) step 3 and (b) step 4 of the brazilin purification process.
Appendix C

Table A1.
Reaction conditions to obtain brazilin-(OMe)3.
Table A1.
Reaction conditions to obtain brazilin-(OMe)3.
| Information | |||||
|---|---|---|---|---|---|
| Reagents | P.M | E.Q | mg | µL | mmoles |
| Brazilin | 286.27 | 1 | 40 | 0.1397 | |
| K2CO3 | 138.21 | 3 | 57.93 | 0.4191 | |
| MeI | 141.94 | 5 | 99.16 | 0.6986 | |
| Acetone | 58.08 | 3500 | |||
K2CO3: potassium carbonate; MeI: Iodomethane.

Table A2.
Reaction conditions to obtain brazilin-(OAc)3.
Table A2.
Reaction conditions to obtain brazilin-(OAc)3.
| Information | |||||
|---|---|---|---|---|---|
| Reagents | P.M | E.Q | mg | µL | mmoles |
| Brazilin | 286.27 | 1 | 50 | 0.1746 | |
| Acetic anhydride | 102.09 | 5 | 89.15 | 83 | 0.8733 |
| NaHCO3 | 84.01 | 3 | 44 | 0.5238 | |
| THF | 72.11 | 2500 | |||
NaHCO3: sodium bicarbonate; THF: tetrahydrofuran.
References
- Bauer, A. Industrial Natural Product Chemistry for Drug Discovery and Development. Nat. Prod. Rep. 2014, 31, 35–60. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Hoskins, C. Drug Development: Lessons from Nature (Review). Biomed. Rep. 2017, 6, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Patil, V.M.; Masand, N. Anticancer Potential of Flavonoids: Chemistry, Biological Activities, and Future Perspectives. Stud. Nat. Prod. Chem. 2018, 59, 401–430. [Google Scholar]
- Cayetano-Salazar, L.; Olea-Flores, M.; Zuñiga-Eulogio, M.D.; Weinstein-Oppenheimer, C.; Fernández-Tilapa, G.; Mendoza-Catalán, M.A.; Zacapala-Gómez, A.E.; Ortiz-Ortiz, J.; Ortuño-Pineda, C.; Navarro-Tito, N. Natural Isoflavonoids in Invasive Cancer Therapy: From Bench to Bedside. Phytother. Res. 2021, 35, 4092–4110. [Google Scholar] [CrossRef]
- Nava-Tapia, D.A.; Cayetano-Salazar, L.; Herrera-Zúñiga, L.D.; Bello-Martínez, J.; Mendoza-Catalán, M.A.; Navarro-Tito, N. Brazilin: Biological Activities and Therapeutic Potential in Chronic Degenerative Diseases and Cancer. Pharmacol. Res. 2022, 175, 106023. [Google Scholar] [CrossRef]
- Bello-Martínez, J.; Jiménez-Estrada, M.; Rosas-Acevedo, J.L.; Avila-Caballero, L.P.; Vidal-Gutierrez, M.; Patiño-Morales, C.; Ortiz-Sánchez, E.; Robles-Zepeda, R.E. Antiproliferative Activity of Haematoxylum Brasiletto H. Karst. Pharmacogn. Mag. 2017, 13, S289–S293. [Google Scholar] [CrossRef]
- Cayetano-Salazar, L.; Hernandez-Moreno, J.A.; Bello-Martinez, J.; Olea-Flores, M.; Castañeda-Saucedo, E.; Ramirez, M.; Mendoza-Catalán, M.A.; Navarro-Tito, N. Regulation of Cellular and Molecular Markers of Epithelial-Mesenchymal Transition by Brazilin in Breast Cancer Cells. PeerJ 2024, 12, e17360. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Laporta, E.; Welsh, J. Modeling Vitamin D Actions in Triple Negative/Basal-like Breast Cancer. J. Steroid Biochem. Mol. Biol. 2014, 144, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Dillekås, H.; Rogers, M.S.; Straume, O. Are 90% of Deaths from Cancer Caused by Metastases? Cancer Med. 2019, 8, 5574–5576. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular Principles of Metastasis: A Hallmark of Cancer Revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Alblazi, K.M.O.; Siar, C.H. Cellular Protrusions—Lamellipodia, Filopodia, Invadopodia and Podosomes—And Their Roles in Progression of Orofacial Tumours: Current Understanding. Asian Pac. J. Cancer Prev. 2015, 16, 2187–2191. [Google Scholar] [CrossRef]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell Adhesion in Cancer: Beyond the Migration of Single Cells. J. Biol. Chem. 2020, 295, 2495–2505. [Google Scholar] [CrossRef]
- Nounou, M.I.; ElAmrawy, F.; Ahmed, N.; Abdelraouf, K.; Goda, S.; Syed-Sha-Qhattal, H. Breast Cancer: Conventional Diagnosis and Treatment Modalities and Recent Patents and Technologies. Breast Cancer 2015, 9s2, BCBCR.S29420. [Google Scholar] [CrossRef]
- Jia, J.B.; Lall, C.; Tirkes, T.; Gulati, R. Chemotherapy-Related Complications in the Kidneys and Collecting System: An Imaging Perspective. Insights Into Imaging 2015, 6, 479–487. [Google Scholar] [CrossRef]
- Grigorian, A.; Brien, C.B.O. Review Article Hepatotoxicity Secondary to Chemotherapy. J. Clin. Transl. Hepatol. 2014, 2, 95–102. [Google Scholar]
- Guo, J.; Li, L.; Wu, Y.-J.; Yan, Y.; Xu, X.-N.; Wang, S.-B.; Yuan, T.-Y.; Fang, L.-H.; Du, G.-H. Inhibitory Effects of Brazilin on the Vascular Smooth Muscle Cell Proliferation and Migration Induced by PDGF-BB. Am. J. Chin. Med. 2013, 41, 1283–1296. [Google Scholar] [CrossRef] [PubMed]
- Jenie, R.I.; Handayani, S.; Susidarti, R.A.; Udin, L.Z.; Meiyanto, E. The Cytotoxic and Antimigratory Activity of Brazilin-Doxorubicin on MCF-7/HER2 Cells. Adv. Pharm. Bull. 2018, 8, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liang, Y.; Zhao, L.; Chen, L.; Yang, Y.; Wang, J.; Yan, L.; Zhang, S.; Liu, X.; Zhang, H. Brazilin Inhibits the Invasion and Metastasis of Breast Cancer. Biol. Pharm. Bull. 2023, 46, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Xue, X. Medicinal Chemistry Strategies for the Modification of Bioactive Natural Products. Molecules 2024, 29, 689. [Google Scholar] [CrossRef]
- Yao, H.; Liu, J.; Xu, S.; Zhu, Z.; Xu, J. The Structural Modification of Natural Products for Novel Drug Discovery. Expert. Opin. Drug Discov. 2017, 12, 121–140. [Google Scholar] [CrossRef]
- Vrignaud, P.; Benning, V.; Beys, E.; Gupta, S.; Semiond, D.; Bouchard, H. Preclinical Profile of Cabazitaxel. Drug Des. Devel Ther. 2014, 8, 1851–1867. [Google Scholar] [CrossRef]
- Zheng, W.; Seletsky, B.M.; Palme, M.H.; Lydon, P.J.; Singer, L.A.; Chase, C.E.; Lemelin, C.A.; Shen, Y.; Davis, H.; Tremblay, L.; et al. Macrocyclic Ketone Analogues of Halichondrin B. Bioorg Med. Chem. Lett. 2004, 14, 5551–5554. [Google Scholar] [CrossRef]
- McBride, A.; Butler, S.K. Eribulin Mesylate: A Novel Halichondrin B Analogue for the Treatment of Metastatic Breast Cancer. Am. J. Health-Syst. Pharm. 2012, 69, 745–755. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Yang, X.; Zhao, J.; Pan, C. Enantioselective Total Synthesis of (+)-Brazilin, (−)-Brazilein and (+)-Brazilide A. Chem. Commun. 2013, 49, 5405–5407. [Google Scholar] [CrossRef]
- De la Fuente, I.M.; López, J.I. Cell Motility and Cancer. Cancers 2020, 12, 2177. [Google Scholar] [CrossRef]
- Pan, M.R.; Hou, M.F.; Ou-Yang, F.; Wu, C.C.; Chang, S.J.; Hung, W.C.; Yip, H.K.; Luo, C.W. FAK Is Required for Tumor Metastasis-Related Fluid Microenvironment in Triple-Negative Breast Cancer. J. Clin. Med. 2019, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Sanchez, R.; Ramirez-Ricardo, J.; Martinez-Baeza, E.; Cortes-Reynosa, P.; Candanedo-Gonzales, F.; Gomez, R.; Salazar, E.P. Bisphenol A Induces Focal Adhesions Assembly and Activation of FAK, Src and ERK2 via GPER in MDA-MB-231 Breast Cancer Cells. Toxicology in Vitro 2020, 66, 104871. [Google Scholar] [CrossRef] [PubMed]
- Park, M.N.; Song, H.S.; Kim, M.; Lee, M.J.; Cho, W.; Lee, H.J.; Hwang, C.H.; Kim, S.; Hwang, Y.; Kang, B.; et al. Review of Natural Product-Derived Compounds as Potent Antiglioblastoma Drugs. Biomed. Res. Int. 2017, 2017, 8139848. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting Cancer Signaling Pathways by Natural Products: Exploring Promising Anti-Cancer Agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information PubChem Compound Summary for CID 73384, Brazilin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Brazilin (accessed on 23 October 2024).
- Siraichi, J.T.G.; Felipe, D.F.; Brambilla, L.Z.S.; Gatto, M.J.; Terra, V.A.; Cecchini, A.L.; Cortez, L.E.R.; Rodrigues-Filho, E.; Cortez, D.A.G. Antioxidant Capacity of the Leaf Extract Obtained from Arrabidaea Chica Cultivated in Southern Brazil. PLoS ONE 2013, 8, e72733. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, I. Total Synthesis of Brazilin. J. Org. Chem. 2015, 80, 2001–2005. [Google Scholar] [CrossRef]
- Wainwright, C.L.; Teixeira, M.M.; Adelson, D.L.; Buenz, E.J.; David, B.; Glaser, K.B.; Harata-Lee, Y.; Howes, M.J.R.; Izzo, A.A.; Maffia, P.; et al. Future Directions for the Discovery of Natural Product-Derived Immunomodulating Drugs: An IUPHAR Positional Review. Pharmacol. Res. 2022, 177, 106076. [Google Scholar] [CrossRef]
- Das, B.; Baidya, A.T.K.; Mathew, A.T.; Yadav, A.K.; Kumar, R. Structural Modification Aimed for Improving Solubility of Lead Compounds in Early Phase Drug Discovery. Bioorg Med. Chem. 2022, 56, 116614. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, P.; Long, X. Differential Expression Profiles of the Transcriptome in Breast Cancer Cell Lines Revealed by Next Generation Sequencing. Cell. Physiol. Biochem. 2017, 44, 804–816. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, P.; Tang, J. Characterization of Triple-Negative Breast Cancer MDA-MB-231 Cell Spheroid Model. Onco Targets Ther. 2020, 13, 5395–5405. [Google Scholar] [CrossRef]
- Witt, B.L.; Tollefsbol, T.O. Molecular, Cellular, and Technical Aspects of Breast Cancer Cell Lines as a Foundational Tool in Cancer Research. Life 2023, 13, 2311. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.; Evangelista, A.; Macedo, T.; Oliveira, R.; Scapulatempo-Neto, C.; Vieira, R.; Marques, M. Molecular Characterization of Breast Cancer Cell Lines by Clinical Immunohistochemical Markers. Oncol. Lett. 2017, 13, 4708–4712. [Google Scholar] [CrossRef] [PubMed]
- Raghunathan, S.; Jaganade, T.; Priyakumar, U.D. Urea-Aromatic Interactions in Biology. Biophys. Rev. 2020, 12, 65–84. [Google Scholar] [CrossRef]
- Coimbra, J.T.S.; Feghali, R.; Ribeiro, R.P.; Ramos, M.J.; Fernandes, P.A. The Importance of Intramolecular Hydrogen Bonds on the Translocation of the Small Drug Piracetam through a Lipid Bilayer. RSC Adv. 2020, 11, 899–908. [Google Scholar] [CrossRef]
- Caron, G.; Digiesi, V.; Solaro, S.; Ermondi, G. Flexibility in Early Drug Discovery: Focus on the beyond-Rule-of-5 Chemical Space. Drug Discov. Today 2020, 25, 621–627. [Google Scholar] [CrossRef]
- Maziveyi, M.; Suresh, K. Alahari Cell Matrix Adhesions in Cancer: The Proteins That Form the Glue. Oncotarget 2017, 8, 48471–48487. [Google Scholar] [CrossRef]
- Katoh, K. FAK-Dependent Cell Motility and Cell Elongation. Cells 2020, 9, 192. [Google Scholar] [CrossRef]
- Bao, J.; Huang, B.; Zou, L.; Chen, S.; Zhang, C.; Zhang, Y.; Chen, M.; Wan, J.B.; Su, H.; Wang, Y.; et al. Hormetic Effect of Berberine Attenuates the Anticancer Activity of Chemotherapeutic Agents. PLoS ONE 2015, 10, e0139298. [Google Scholar] [CrossRef]
- Basu, P.; Maier, C. Phytoestrogens and Breast Cancer: In Vitro Anticancer Activities of Isoflavones, Lignans, Coumestans, Stilbenes and Their Analogs and Derivatives. Biomed. Pharmacother. 2018, 107, 1648–1666. [Google Scholar] [CrossRef]
- Lepri, S.R.; Luiz, R.C.; Zanelatto, L.C.; Da Silva, P.B.G.; Sartori, D.; Ribeiro, L.R.; Mantovani, M.S. Chemoprotective Activity of the Isoflavones, Genistein and Daidzein on Mutagenicity Induced by Direct and Indirect Mutagens in Cultured HTC Cells. Cytotechnology 2013, 65, 213–222. [Google Scholar] [CrossRef]
- Tsai, C.L.; Wu, H.M.; Lin, C.Y.; Lin, Y.J.; Chao, A.; Wang, T.H.; Hsueh, S.; Lai, C.H.; Wang, H.S. Estradiol and Tamoxifen Induce Cell Migration through GPR30 and Activation of Focal Adhesion Kinase (FAK) in Endometrial Cancers with Low or without Nuclear Estrogen Receptor α (ERα). PLoS ONE 2013, 8, e72999. [Google Scholar] [CrossRef] [PubMed]
- Koirala, N.; Thuan, N.H.; Ghimire, G.P.; Thang, D.; Van Sohng, J.K. Methylation of Flavonoids: Chemical Structures, Bioactivities, Progress and Perspectives for Biotechnological Production. Enzyme Microb. Technol. 2016, 86, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Nakanishi, I.; Ohkubo, K.; Ohba, Y.; Arai, T.; Mizuno, M.; Fukuzumi, S.; Matsumoto, K.; Fukuhara, K. Synthesis of Methylated Quercetin Analogues for Enhancement of Radical-Scavenging Activity. RSC Adv. 2017, 7, 17968–17979. [Google Scholar] [CrossRef]
- Hirpara, K.V.; Aggarwal, P.; Mukherjee, A.J.; Joshi, N.; Burman, A.C. Quercetin and Its Derivatives: Synthesis, Pharmacological Uses with Special Emphasis on Anti-Tumor Properties and Prodrug with Enhanced Bio-Availability. Anticancer. Agents Med. Chem. 2009, 9, 138–161. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.L.; Cardoso Nunes, N.C.; Izetti, P.; de Mesquita, G.G.; de Melo, A.C. Triple Negative Breast Cancer: A Thorough Review of Biomarkers. Crit. Rev. Oncol. Hematol. 2020, 145, 102855. [Google Scholar] [CrossRef]
- Simu, S.; Marcovici, I.; Dobrescu, A.; Malita, D.; Dehelean, C.A.; Coricovac, D.; Olaru, F.; Draghici, G.A.; Navolan, D. Insights into the Behavior of Triple-Negative Mda-Mb-231 Breast Carcinoma Cells Following the Treatment with 17β-Ethinylestradiol and Levonorgestrel. Molecules 2021, 26, 2776. [Google Scholar] [CrossRef]
- Hba, S.; Ghaddar, S.; Wahnou, H.; Pinon, A.; El Kebbaj, R.; Pouget, C.; Sol, V.; Liagre, B.; Oudghiri, M.; Limami, Y. Natural Chalcones and Derivatives in Colon Cancer: Pre-Clinical Challenges and the Promise of Chalcone-Based Nanoparticles. Pharmaceutics 2023, 15, 2718. [Google Scholar] [CrossRef]
- Wahnou, H.; Liagre, B.; Sol, V.; El Attar, H.; Attar, R.; Oudghiri, M.; Duval, R.E.; Limami, Y. Polyphenol-Based Nanoparticles: A Promising Frontier for Enhanced Colorectal Cancer Treatment. Cancers 2023, 15, 3826. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).