Abstract
The organic chemistry occurring in interstellar environments may lead to the production of complex molecules that are relevant to the emergence of life. Therefore, in order to understand the origins of life itself, it is necessary to probe the chemistry of carbon-bearing molecules under conditions that simulate interstellar space. Several of these regions, such as dense molecular cores, are exposed to ionizing radiation in the form of galactic cosmic rays, which may act as an important driver of molecular destruction and synthesis. In this paper, we report the results of a comparative and systematic study of the irradiation of CH4:H2O ice mixtures by 1 MeV protons and 2 keV electrons at 20 K. We demonstrate that our irradiations result in the formation of a number of new products, including both simple and complex daughter molecules such as C2H6, C3H8, C2H2, CH3OH, CO, CO2, and probably also H2CO. A comparison of the different irradiation regimes has also revealed that proton irradiation resulted in a greater abundance of radiolytic daughter molecules compared to electron irradiation, despite a lower radiation dose having been administered. These results are important in the context of the radiation astrochemistry occurring within the molecular cores of dense interstellar clouds, as well as on outer Solar System objects.
1. Introduction
A comprehensive understanding of the chemistry of carbon-bearing molecules in space is necessary in order to quantitatively study the extent of organic chemistry in extra-terrestrial environments, including reactions that may lead to the formation of complex organic molecules of inherent interest to biology and biochemistry. A number of carbon-bearing molecules have been detected in interstellar space, ranging in structural complexity from methylidyne radicals, CH, to fullerenes such as buckminsterfullerene, C60, and rugbyballene, C70 [1]. Methane, CH4, is the simplest saturated organic molecule that is known to exist in the interstellar medium, having originally been observed in both the solid and gas phases towards the young stellar object NGC 7538 IRS 9 [2].
The initial observations of interstellar CH4 found that a large fraction was locked away in interstellar icy grain mantles, thus suggesting a synthesis pathway that relied upon reactions in the solid phase. Indeed, recent experimental studies have demonstrated that carbon and hydrogen atom addition reactions on the surfaces of interstellar dust grains are able to produce CH4, and that the rate of formation is twice as high when CH4 is formed in an ice that is rich in solid water, H2O [3]. Such experimental results complement the previously observed correlations between CH4 and H2O ices in interstellar icy grain mantles observed towards low-mass young stellar objects [4]. CH4 has also been observed in comets [5,6,7], implying the possible survival of interstellar CH4 during the processes of stellar and planetary system formation. Cometary impacts could indeed have played a key role in the delivery of CH4 to various planetary-like bodies in the Solar System, such as Titan or Pluto [8,9].
Interstellar and outer Solar System environments are characterized by chemistry driven by ionizing radiation mediated by galactic cosmic rays or the solar wind. As such, it is conceivable that the radiation chemistry of CH4 in interstellar or outer Solar System ices could be a driver towards the formation of complex organic molecules in these environments. Such radiation chemistry is known to be driven by the release of several thousand low-energy secondary electrons along the track of the irradiating particle [10,11]. Previous laboratory studies have investigated the radiation chemistry of pure CH4 astrophysical ice analogues using keV-MeV ions and electrons [12,13,14,15,16], and have demonstrated the formation of various daughter hydrocarbons, including ethane, C2H6; propane, C3H8; ethene, C2H4; and ethyne, C2H2, as well as several radical species sourced from their mono-dehydrogenation. When mixed with other molecules, irradiated CH4-containing ices yield even more complex molecules: electron-irradiated mixtures with phosphine, PH3, yield methylphosphanes as large as CH3P8H9 [17], while the formation of higher-order carboxylic acids such as decanoic acid, C9H19COOH, has been reported in irradiated mixtures with carbon dioxide, CO2 [18]. The inclusion of nitrogen-bearing molecules, such as ammonia, NH3, into electron-irradiated CH4:CO2 mixed ices has also been shown to result in the formation of glycine [19], a proteinogenic amino acid of direct relevance to biology.
Of interest to this present study is the radiation chemistry occurring in CH4:H2O mixed ices due to the ubiquity of H2O ices within astronomical environments [20], as well as the apparent association of CH4 formation with H2O-rich environments [2,3]. This radiation chemistry has been considered by previous experimental studies [21,22,23,24], which have shown that alcohols, higher hydrocarbons, and methanal, H2CO, are among the readily produced closed-shell daughter molecules. For instance, the study of Wada et al. [22] considered the low-energy electron irradiation of CH4:H2O ices at 10 K and found that methanol, CH3OH, is the major daughter molecule of this process and is formed by the combination of hydroxyl, OH, and methyl, CH3, radicals, or alternatively by the insertion reaction between methylene radicals, CH2 and H2O. In their study on the irradiation of CH4:H2O ices by 40 MeV nickel ions at 15 K, Mejía et al. [23] were able to additionally demonstrate the formation of ethanol, C2H5OH; ethanal, CH3CHO; and methanoic acid, HCOOH, along with simpler molecules such as carbon monoxide, CO; CO2, and H2CO. Similar results were reported by Krim and Jonusas [24], who also reported the formation of a number of ether molecules, as well as glycolaldehyde, HOCH2CHO, after the irradiation of a CH4:H2O ice by vacuum-ultraviolet photons at 3 K.
To the best of our knowledge, however, no previous study has comparatively investigated the radiation chemistry induced in CH4:H2O ices by different types of energetic processing, despite the evident astrochemical importance of such ices. Understanding the potential differences in the radiation chemistries induced by different processing types is important since different astronomical environments are subject to different combinations of ion, electron, and ultraviolet photon irradiations and the predominance of one type of energetic processing over another may lead to the production of distinctive daughter molecules in varying abundances. Indeed, the work of Moore and Hudson [25] demonstrated that several daughter molecules produced after the proton irradiation of CH4 ices mixed with either CO or molecular nitrogen, N2, are not produced after similar irradiations using ultraviolet photons. Baratta et al. [12] also performed a comparative study in which they investigated the irradiation of pure CH4 ices by 30 keV He+ and 10.2 eV photons and found that similar administered doses resulted in a greater extent of CH4 destruction and daughter molecule formation in the case of the He+ irradiation. Indeed, they observed that C2H6, itself formed from the dissociation of the CH4 parent species, began to decay after a dose of approximately 30 eV per 16 u had been administered via He+ irradiation; such an observation was not made during irradiation with 10.2 eV photons.
In this paper, we present the results of a systematic and comparative study looking into the irradiation of CH4:H2O mixed ices using 1 MeV protons and 2 keV electrons so as to characterize the differences in daughter molecule formation and abundances between the different types of energetic processing. The experimental methodology used to carry out this study is described in Section 2, while our results are presented in Section 3 and are discussed in the context of their applicability to astrochemistry. A summative conclusion is given in Section 4.
2. Methodology
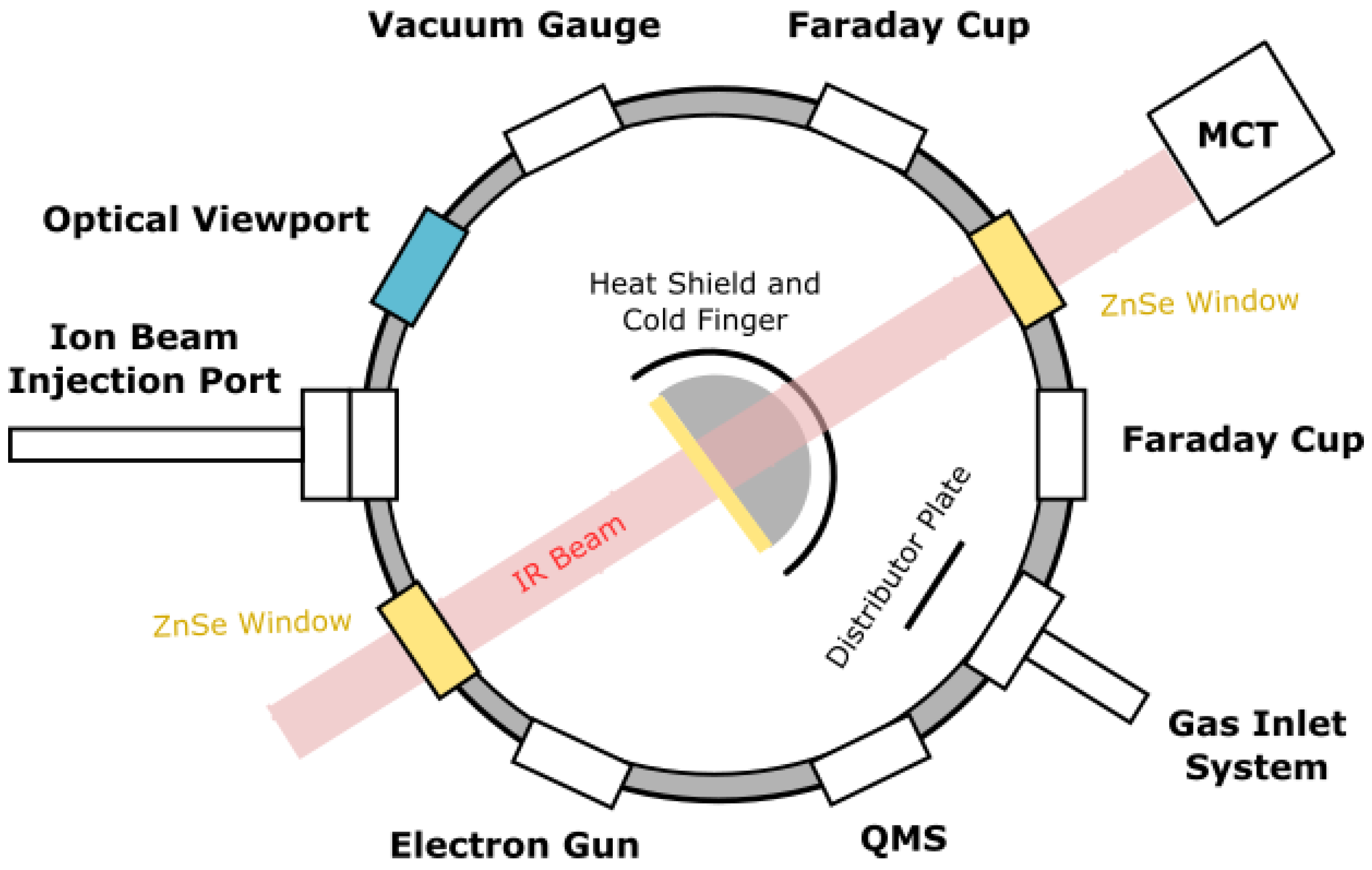
The experimental work was carried out using the Ice Chamber for Astrophysics–Astrochemistry (ICA) at the Institute for Nuclear Research (Atomki) in Debrecen, Hungary. This set-up has been described in great detail in previous publications [26,27] and so, in this paper, we limit ourselves to only the most salient details. The ICA is an ultrahigh-vacuum compatible steel chamber with a base pressure of a few 10−9 mbar, which is achieved through the combined action of a turbomolecular pump and a dry rough pump. In the center of the chamber is a gold-coated oxygen-free high-conductivity copper sample holder into which a maximum of four zinc selenide, ZnSe, substrates may be mounted (Figure 1). The sample holder and the substrates may be cooled to 20 K through contact with the cold finger of a closed-cycle helium cryostat, although an operational temperature range of 20–300 K is available.
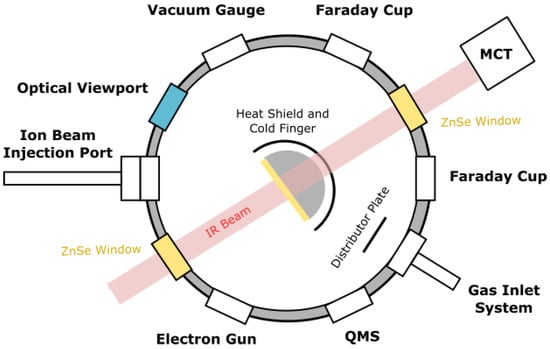
Figure 1.
Top-view schematic diagram of the ICA set-up. Figure reproduced from Mifsud et al. [27] with the kind permission of the European Physical Journal (EPJ).
CH4:H2O astrophysical ice analogues were prepared on the ZnSe substrates by background deposition of a gas mixture at 20 K. The gas mixture was first prepared in a mixing line with the desired stoichiometric ratio (CH4:H2O = 5:2) by introducing CH4 gas (Linde; 99.5% purity) and H2O vapor (from a high-purity liquid sample that had been de-gassed by performing several freeze–pump–thaw cycles using liquid nitrogen). The gas mixture was then dosed into the main chamber at a pressure of a few 10−6 mbar until ices of approximately 0.25 μm thickness were prepared. The deposition and growth of the ices were monitored in situ using Fourier-transform mid-infrared transmission absorption spectroscopy (resolution = 1 cm−1; spectral range = 4000–650 cm−1) and an external MCT detector. Once deposited, a pre-irradiation mid-infrared spectrum was acquired, which allowed us to quantify the column densities (N, molecules cm−2) of the deposited molecules via Equation (1):
where P is the area of a characteristic absorption band (cm−1) of the species of interest and Aν is its associated integrated band strength constant (cm molecule−1). The factor of ln(10) allows for the relation of P (which is measured from spectra on an absorbance scale) to Aν (which is measured on an optical depth scale). Deposited CH4:H2O ices were then exposed either to a 1 MeV proton beam delivered by a Tandetron particle accelerator [28,29] or to a 2 keV electron beam delivered by an electron gun mounted directly to one of the side ports of the ICA [27]. The homogeneities, profiles, and currents of the proton and electron beams were respectively assessed using the methods described in Herczku et al. [26] and Mifsud et al. [27] prior to commencing the irradiation of the ices. Beam currents were measured to be 290 nA in the case of the proton beam and 5.7 μA in the case of the electron beam. Additional mid-infrared spectra were collected throughout the irradiation process so as to identify the formation of daughter molecules. A summary of the experiments conducted in this study (including information on the physical parameters required to perform quantitative analysis) is provided in Table 1.

Table 1.
Summary of the experiments performed in this study.
3. Results and Discussion
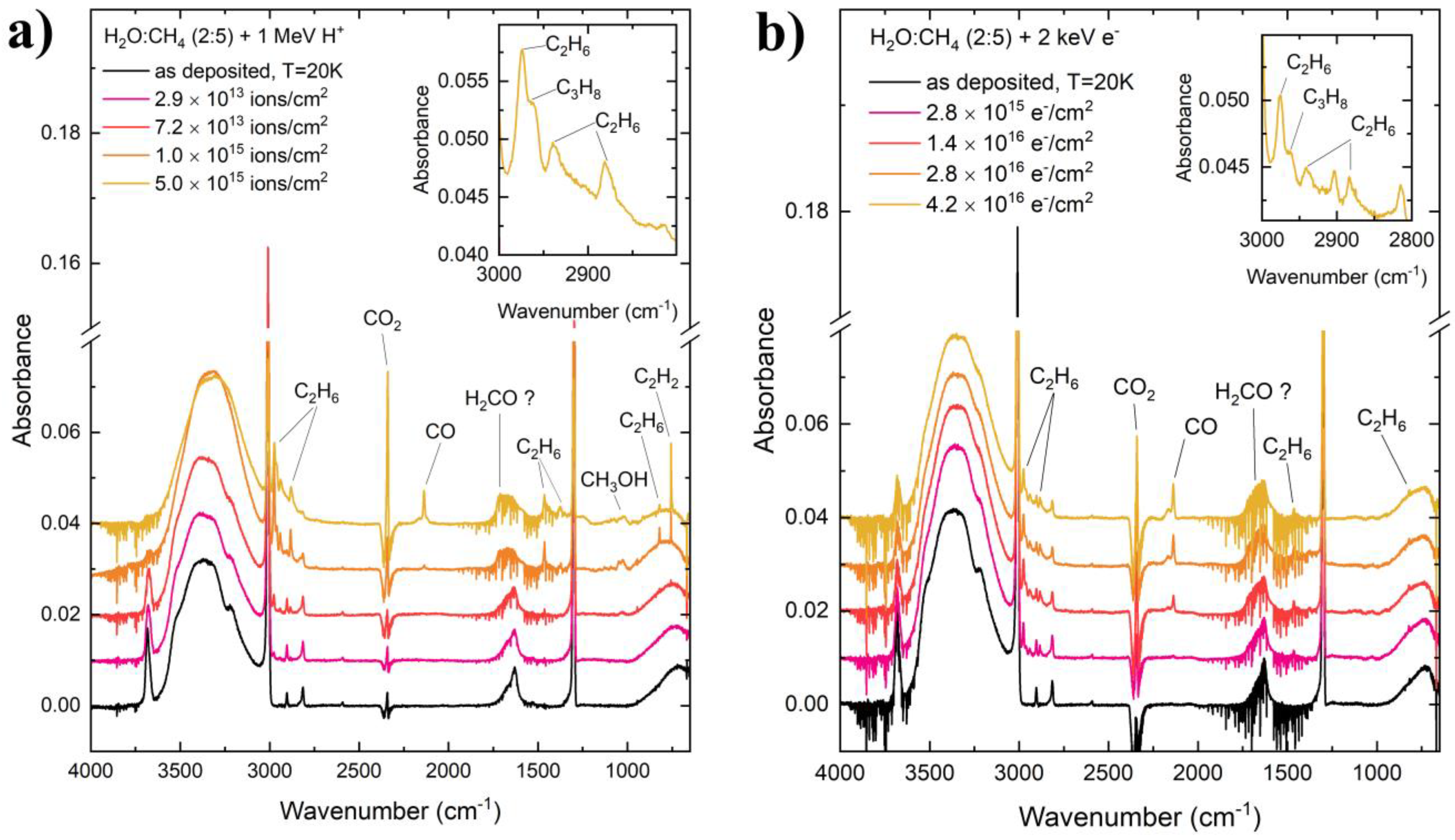
The mid-infrared spectra of the CH4:H2O ices after their preparation at 20 K, as well as during their processing by 1 MeV proton and 2 keV electron irradiation, are given in Figure 2. Upon preparation, various mid-infrared absorption features are immediately apparent, such as the ν4 (1300 cm−1), ν2 + ν4 (2816 cm−1), ν1 (2901 cm−1), and ν3 (3011 cm−1) modes of CH4 [16], as well as the νL (713 cm−1), ν2 (1634 cm−1), and νs (3370 cm−1) modes of H2O [33]. Of particular interest is the single, strong absorption band present at around 3616 cm−1, which is attributed to the hydroxyl dangling bond vibration of H2O [33,34,35,36]. In pure H2O ices, the dangling bond vibration typically presents as two weak absorption features; however, when mixed with CH4, these dangling bond absorption bands merge into one significantly stronger band, which is red-shifted to lower wavenumbers. This phenomenon has been studied in detail by Gálvez et al. [37] and Herrero et al. [38], who demonstrated that this could be ascribed to the interaction of CH4 with microporous H2O and that the intensity of the band increases with increasing CH4 content in the mixed ice. Given that our ice is rich in CH4 (CH4:H2O = 5:2), it is therefore not surprising that the hydroxyl dangling bond absorption band is one of the strongest features in the acquired mid-infrared spectra (Figure 2). The interaction of CH4 with microporous H2O is also responsible for the appearance of the symmetry-forbidden ν1 mode of CH4, which is present in our spectra due to the distortion of the cubic structure of small crystallites, resulting in the breakdown of the symmetry of the CH4 molecule [38].
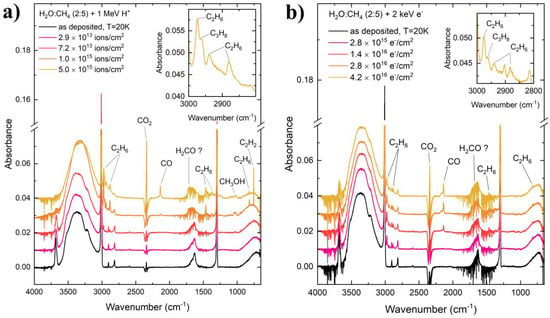
Figure 2.
Mid-infrared absorption spectra of CH4:H2O (= 5:2) mixed ices upon preparation at 20 K and during irradiative processing using (a) 1 MeV protons and (b) 2 keV electrons. The absorption bands of various daughter molecules have been labelled. Note that the fine structures and negative absorptions in the regions of the H2O and CO2 bands are due to variations in the concentrations of atmospheric gas-phase H2O and CO2 in the pathlength leading to the detector, which is not maintained under vacuum.
The processing of CH4:H2O mixed ices results in the appearance of a number of new absorption bands due to the formation of various daughter molecules as a result of the radiation chemistry occurring within the ices. The most likely first steps leading to the formation of daughter molecules is the radiation-induced dehydrogenation of CH4 and H2O to respectively yield CH3 and OH radicals [21,22,23,24].
CH4 → CH3 + H
H2O → OH + H
Hydrogen atoms and OH radicals are likely to be very mobile within the ice structure and may thus partake in various hydrogenation or hydrogen abstraction reactions, including the further dissociation of CH4 to CH3 radicals, or the combination reactions leading to the formation of molecular hydrogen, H2, and hydrogen peroxide, H2O2. Neither of these latter two species were detected in our experiments. The non-detection of H2 is ascribed to its lack of a permanent dipole moment, which makes it infrared-inactive, as well as its volatility, which allows it to efficiently escape from the ice via sublimation to the gas phase; meanwhile, the non-detection of H2O2 is likely due to its mid-infrared absorption bands being obscured by those of hydrocarbon species, which are more abundant within the ice.
H (or OH) + CH4 → H2 (or H2O) + CH3
H + H → H2
OH + OH → H2O2
Given the preponderance of CH4 within our ices, it is most likely that radicals derived from the radiolysis of this species would come into contact with one another. For instance, the combination of two CH3 radicals would yield C2H6 as a result of the formation of a single bond between the two carbon atoms. Indeed, mid-infrared absorption bands attributable to the ν12 (822 cm−1), ν6 (1375 cm−1), ν11 (1464 cm−1), ν5 (2883 cm−1), ν8 + ν11 (2941 cm−1), and ν10 (2975 cm−1) modes of C2H6 [16] were observed after both proton and electron irradiation of the ice mixtures (Figure 2).
CH3 + CH3 → C2H6
The formation of C2H6 is of inherent astrochemical interest since it may be classified as a complex organic molecule (the astrochemical definition of a complex molecule includes all those with six or more constituent atoms). To the best of our knowledge, C2H6 has yet to be detected in the interstellar medium [1], although it has been found in various icy outer Solar System objects. Solid C2H6 has been observed on the surface of Pluto [39], while liquid C2H6 is thought to be an important component of oceanic and lacustrine geochemical systems on Titan [40,41]. Our results conclusively show that C2H6 production from CH4 radiolysis is not inhibited by the presence of H2O in the ice mixture, and thus it may conceivably form by solid-phase radiation chemistry in realistic interstellar icy grain mantles rich in both species [3,4]. Indeed, observational studies of comet C/1996 B2 Hyakutake revealed an abundance of C2H6 comparable to that of CH4, implying that the comet did not originate in a thermochemically equilibrated astronomical environment [42]. Since the production of C2H6 by gas-phase ion–molecule reactions is energetically forbidden [43], this observation strongly points to a solid-phase production route towards C2H6 in dense interstellar cloud cores, which is consistent with our experimental results.
The presence of C2H6 within the ice opens further radiolytic channels towards the formation of higher hydrocarbons: C3H8 is another daughter species formed after the irradiation of our CH4:H2O mixed ices (Figure 2). The presence of C3H8 in the irradiated mixed ices is likely the result of a two-step process first involving the dehydrogenation of C2H6 to yield ethyl radicals, C2H5, followed by the addition of CH3 to yield the observed product.
C2H6 + H (or OH) → C2H5 + H2 (or H2O)
C2H5 + CH3 → C3H8
Furthermore, C2H6 may partake in successive dehydrogenation reactions leading to the formation of alkenes and alkynes: C2H2 was efficiently formed after the 1 MeV proton irradiation of our CH4:H2O mixed ice, as indicated by the intensity of its ν5 mode at 757 cm−1 [44] (Figure 2). The formation of C2H2 from C2H6 is a multi-step process involving four dehydrogenation reactions, with C2H4 being formed in the process after two hydrogen abstractions. Interestingly, no C2H2 was detected after the irradiation of our mixed ice using 2 keV electrons (Figure 2). This observation may be the result of the lower molecular destruction and formation cross-sections associated with projectile electrons compared with protons [45,46], and constitutes a major difference in the irradiation experiments considered in this paper. It is also worth noting that C2H4 was not detected in either the proton- or the electron-irradiated ice, although we speculate that this may have been due to the absorption bands of this molecule having been obscured by the stronger absorption bands of the parent CH4 and H2O species [16].
C2H6 + 2 H (or 2 OH) → C2H4 + 2 H2 (or 2 H2O)
C2H4 + 2 H (or 2 OH) → C2H2 + 2 H2 (or 2 H2O)
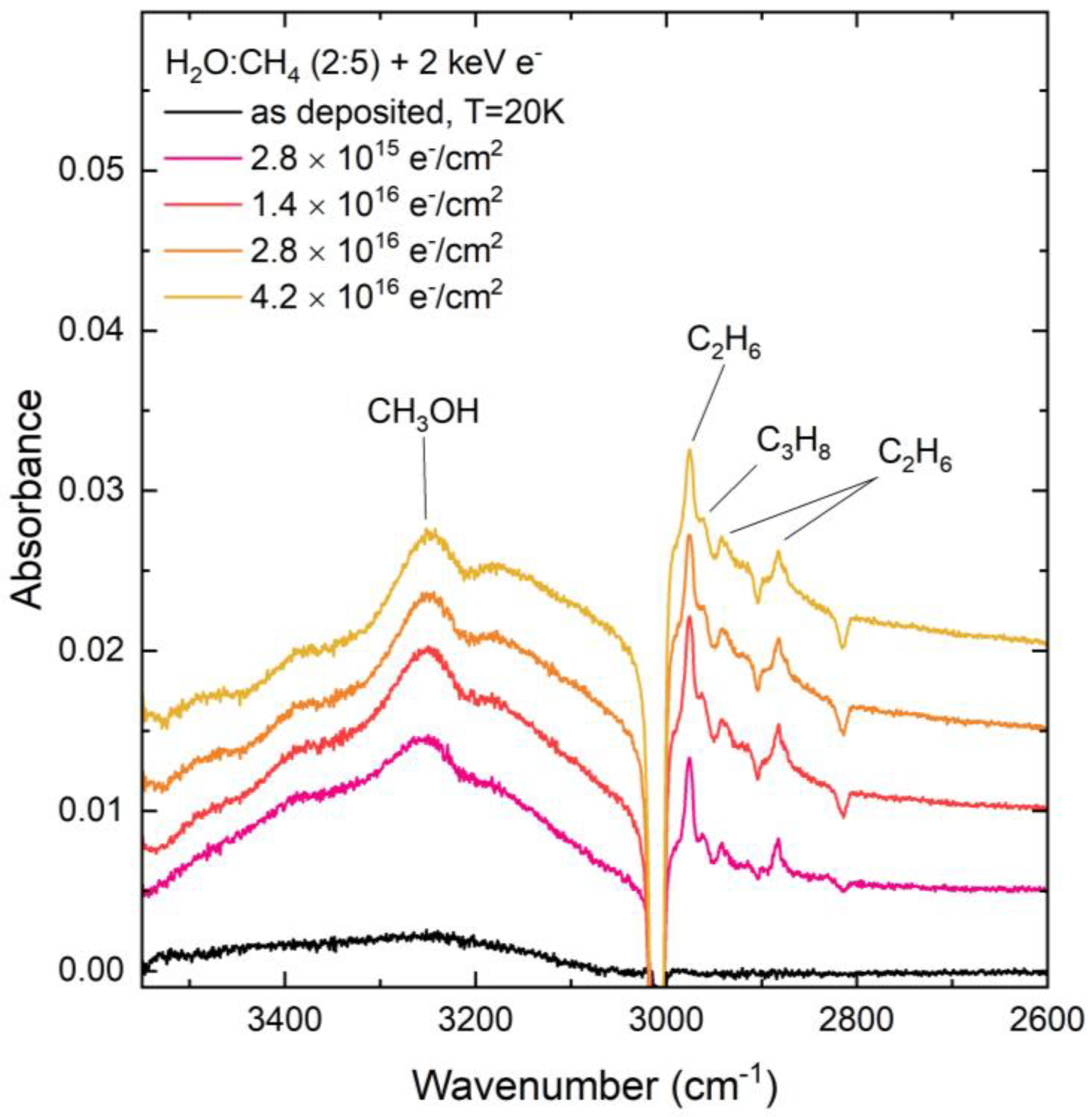
Another molecule for which there exists a significant discrepancy in the comparative abundance produced after proton and electron irradiation of the CH4:H2O ices is CH3OH, with more of this molecule being formed after proton irradiation compared to electron irradiation (Figure 2 and Figure 3). The formation of CH3OH may have occurred as a result of a number of different radiolytic pathways. One such pathway is the direct combination of CH3 and OH radicals [47].
OH + CH3 → CH3OH
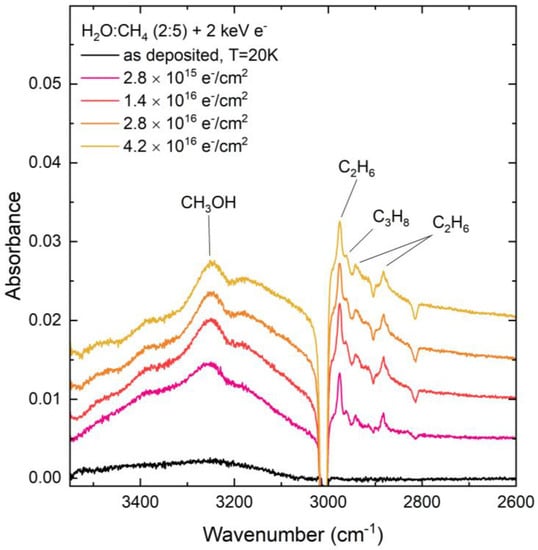
Figure 3.
Mid-infrared absorption spectra of a CH4:H2O (=5:2) mixed ice upon preparation at 20 K and during irradiative processing using 2 keV electrons. Note that spectra acquired during irradiation are so-called ‘difference spectra’ yielded after the subtraction of the ‘as-deposited’ spectrum. The spectra are focused on the 3500–2600 cm−1 region so as to highlight the formation of CH3OH as a result of the electron irradiation of the CH4:H2O mixed ice.
However, further dehydrogenation of the nascent CH3 radical may yield other reactive carbon-based radicals, including CH2 and CH radicals. The reaction between CH2 and OH, followed by the hydrogenation of the carbon atom, could also yield CH3OH.
CH2 + OH → CH2OH
CH2OH + H → CH3OH
Wada et al. [22] also provided evidence for the insertion of CH2 into the H2O molecular structure as an alternative route towards CH3OH formation.
CH2 + H2O → CH3OH
The necessary precursor radicals in the reactions leading to the formation of CH3OH highlighted above have all been observed in experimental studies [24], and thus all likely contribute to its formation in our work to at least some extent.
Another formation pathway for CH3OH in our irradiated ices is the step-wise hydrogenation of CO to sequentially yield HCO, H2CO, CH3O (or H2COH), and finally CH3OH [48,49,50]. The contribution of such a reaction sequence to CH3OH formation in our ices necessitates the presence of CO, which was observed in our irradiated ices. Although the specific mechanism for CO formation as a result of the irradiation of CH4:H2O mixed ices is not known for certain, it is possible to suggest that carbon-rich radicals such as CH or even possibly carbon atoms produced as a result of the repeated dehydrogenation of CH4 may have been able to react directly with H2O to yield CO. Although speculative, such a suggestion is in agreement with previous works that have demonstrated the formation of both CO and CO2 as a result of the irradiation of H2O ice deposited on top of carbonaceous residues and hydrogenated carbon grains [51,52,53].
It should be noted that the sequential hydrogenation of CO should also yield H2CO, another closed-shell daughter molecule. This species is usually detected by the observation of its intense ν2 mode at around 1725 cm−1 [31]. However, in our ices, this detection is made somewhat more complicated by two factors: firstly, this band would at least partially coincide with the broad ν2 mode of H2O. Secondly, the appearance of many fine absorption features in this region of the spectrum due to gas-phase H2O absorption in the pathlength near the spectroscopic detector makes the deconvolution of any possible composite absorption band to its individual contributors practically impossible. However, by looking more closely at Figure 2, it is possible to note that the broad band attributed to the ν2 mode of H2O in the pre-irradiation spectra appears to broaden further and blue-shift to higher wavenumbers during irradiation. This could be interpreted as being the result of the appearance of the H2CO ν2 mode. However, in the absence of confirmatory spectroscopic deconvolution analyses, we limit ourselves to only tentatively detecting H2CO in the ices (hence the ‘H2CO ?’ label in Figure 2).
The final molecule to be spectroscopically detected in our irradiation experiments is CO2 (Figure 2). The formation of CO2 likely proceeded via a combination of three radiation-driven processes. Firstly, CO2 formation may occur as a result of the reaction of carbon-rich radicals or carbon atoms with H2O ice, analogously to the process described previously for CO formation [51,52,53]. CO2 is also a known dissociation product of CH3OH [26,27,54,55,56,57,58], and so the radiolytic destruction of this latter species may contribute somewhat to the formation of CO2. Lastly, and possibly most significantly, the formation of CO2 may occur as a result of the reaction of CO with OH radicals via the production of the HO–CO intermediate [59,60]. Conversely, the reaction between CO and any oxygen atoms yielded from the radiolytic destruction of some oxygen-containing precursor species is not anticipated to contribute to the presence of CO2 in our ices due to the known high activation energy barriers associated with the reaction [61,62,63].
CO + OH → HO–CO → CO2 + H
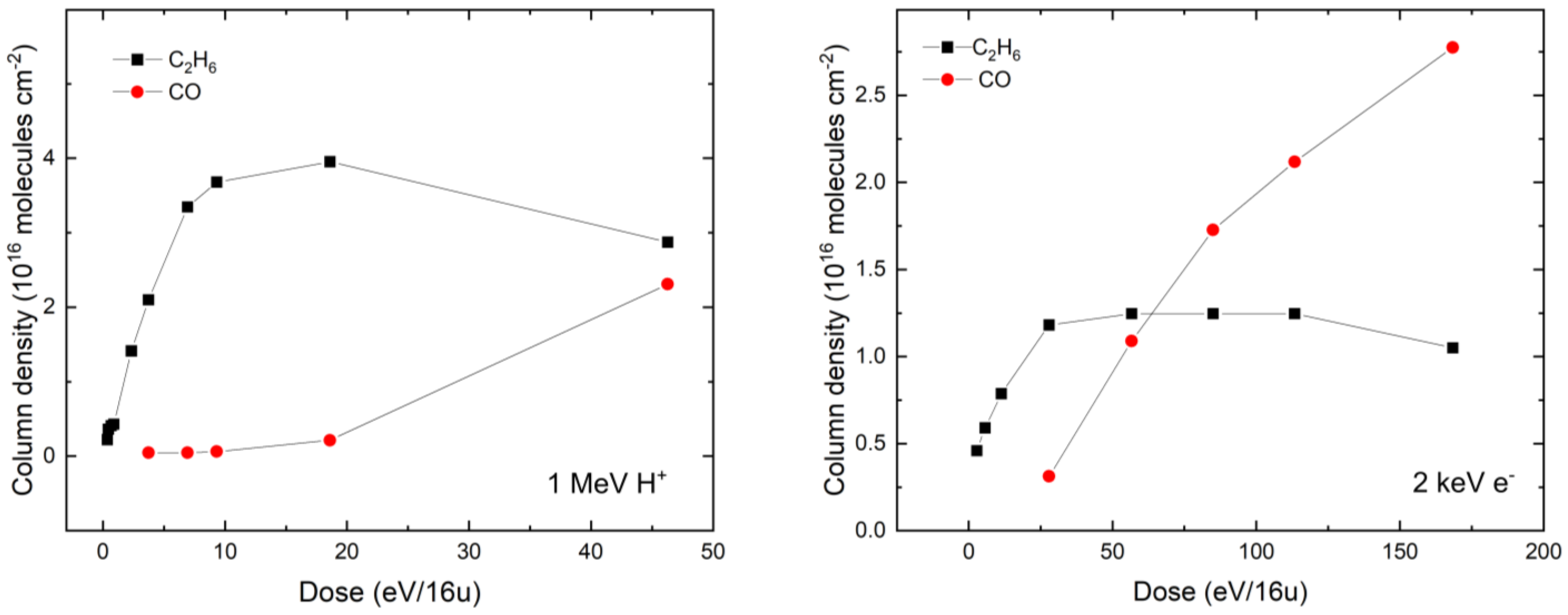
In order to better quantify the differences in the radiation chemistry of our CH4:H2O ices mediated by 1 MeV protons or 2 keV electrons, we have compared the column density evolution as a function of the radiation dose for two radiolytic daughter molecules: CO and C2H6 (Figure 4). By more closely examining the evolution of the column densities of these molecules in both the proton- and the electron-irradiated ices, it is possible to note that the overall qualitative trend is similar: the column density of C2H6 begins to increase until it peaks at a given dose (approximately 19 and 55 eV per 16 u in the case of proton and electron irradiation, respectively), after which it slowly decreases. Conversely, the column density of CO is observed to continually increase with higher radiation doses, with no evidence of peak abundance having yet been reached in either irradiated ice. Interestingly, extrapolation of the proton irradiation data plotted in Figure 4 suggests that the column densities of the C2H6 and CO daughter molecules are equal at approximately the same dose (around 63 eV per 16 u) in both the proton- and the electron-irradiated ice.
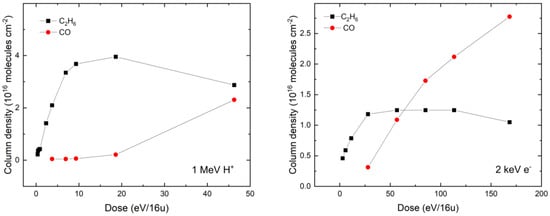
Figure 4.
Dose evolution of the column densities of CO and C2H6 radiolytic daughter molecules during 1 MeV proton irradiation (left panel) and 2 keV electron irradiation (right panel) of a CH4:H2O (= 5:2) mixed ice. Column densities were calculated using integrated band strength constants of 3.51 × 10−18 and 1.12 × 10−17 cm molecule−1 for the C2H6 absorption band at 2883 cm−1 and the CO absorption band at 2139 cm−1, respectively [31,64].
More generally, it is possible to note that the column densities of daughter molecules measured after proton irradiation are significantly greater than those measured after electron irradiation (Figure 4), despite similar initial (i.e., pre-irradiative) CH4 and H2O column densities in each case. Once again, we speculate that this is the result of the larger cross-sections associated with molecular formation and destruction induced by proton irradiation compared to electron irradiation [45,46]. We note that this reason was not invoked by Baratta et al. [12], who investigated the comparative processing of pure CH4 ice using 30 keV He+ and 10.2 eV ultraviolet photons. In their study, Baratta et al. [12] noted that, at low radiation doses of less than 20 eV per 16 u, the decay of CH4 and the concomitant production of C2H6 followed very similar trends in both the He+ and the photon irradiation experiments, both qualitatively and quantitatively. At higher doses, however, the decay of CH4 proved to be more rapid in the case of He+ irradiation. Furthermore, the column density of C2H6 eventually peaked and subsequently began to decay during He+ irradiation, whereas it eventually plateaued during ultraviolet photon irradiation.
The differences observed by Baratta et al. [12] were ascribed to the dependence of the photochemistry induced by the ultraviolet photons on the optical properties of the ice, which would have undoubtedly changed as a result of its changing chemical composition, thus attenuating many of the incident photons at the most heavily processed regions closest to the surface of the ice. This contrasts with our study, where the radiation chemistry induced by both the projectile protons and electrons was independent of the optical properties of the ice, and both projectile types were able to access practically the entirety of the deposited ice. As such, differences in the total cross-sections of each radiation process seem to be the most parsimonious explanation for the observed differences in the column densities of the radiolytic daughter molecules (Figure 4).
4. Conclusions
In this paper, we have reported the results of 1 MeV proton and 2 keV electron irradiations of CH4:H2O (= 5:2) mixed ices representative of those that may conceivably exist within icy grain mantles in dense interstellar clouds. Our results have shown that both irradiations result in the formation of many new molecules, some of which are considered to be complex organic molecules in the field of astrochemistry. Among these radiolytic daughter molecules were C2H6, C3H8, CH3OH, CO, CO2, and probably H2CO. Proton irradiation of the ice mixture additionally resulted in the production of C2H2, which was not observed after electron irradiation of the ice, despite a significantly higher radiation dose being administered during electron irradiation. Our results have further demonstrated that the abundances of these daughter species are greater after proton irradiation compared to electron irradiation, possibly due to the larger molecular formation and destruction cross-sections in the case of the former. Interestingly, the column densities of CO and C2H6 were found to be equal after an administered radiation dose of approximately 63 eV per 16 u in both the proton- and the electron-irradiated ices.
Our results are of inherent interest to the astrochemistry occurring within icy grain mantles in dense interstellar clouds as they effectively demonstrate the production of complex organic species such as hydrocarbons and alcohols after processing by galactic cosmic rays, for which we have used energetic protons and electrons as an analogue. Such molecules may serve as feedstocks for the production of even more complex molecules of inherent interest to biology and biochemistry. Many of the daughter molecules produced by the irradiation of the CH4:H2O ices may also be preserved and seeded to nascent planetary systems, as evidenced by their detections in comets and other Solar System objects. Our results therefore contribute to the better understanding and quantification of daughter molecules that can be produced as a result of different irradiation regimes that may dominate in different astronomical settings.
Author Contributions
Conceptualization, D.V.M.; Data curation, Z.K.; Formal analysis, Z.K.; Funding acquisition, N.J.M.; Investigation, D.V.M., P.H. and Z.K.; Methodology, D.V.M., P.H., B.S., Z.J. and Z.K.; Resources, I.V. and I.R.; Supervision, N.J.M.; Validation, G.S.; Visualization, S.I. and G.S.; Writing—original draft, D.V.M.; Writing—review and editing, D.V.M., P.H., B.S., Z.J., I.V., I.R., S.I., N.J.M., G.S. and Z.K. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge support from Europlanet 2024 RI, which has received funding from the European Union’s Horizon 2020 Research Innovation Program under grant agreement No. 871149. The main components of the ICA set-up were purchased using funds from the Royal Society obtained through grant Nos. UF130409, RGF/EA/180306, and URF/R/191018. Further developments of the installation were supported in part by the Eötvös Loránd Research Network through grants ELKH IF-2/2019 and ELKH IF-5/2020. This work has also received support from the European Union and the State of Hungary, co-financed by the European Regional Development Fund through grant No. GINOP-2.3.3-15-2016-00005. Support has also been received from the Research, Development, and Innovation Fund of Hungary through grant No. K128621. D.V.M. is the grateful recipient of a University of Kent Vice-Chancellor’s Research Scholarship. Z.J. acknowledges support from the Hungarian Academy of Sciences through the János Bolyai Research Scholarship. S.I. acknowledges support from the Danish National Research Foundation through the Centre of Excellence ‘InterCat’ (grant agreement No. DNRF150) and from the Royal Society. The research of Z.K. is supported by the Slovak Grant Agency for Science (grant No. 2/0059/22) and the Slovak Research and Development Agency (contract No. APVV-19-0072).
Data Availability Statement
Data will be made available to interested parties upon reasonable request to one of the corresponding authors.
Acknowledgments
The authors would like to express their gratitude to Béla Paripás (University of Miskolc, Hungary) for his continued support and assistance, and to Thomas A. Field (Queen’s University Belfast, UK) for his helpful comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- McGuire, B.A. 2021 census of interstellar, circumstellar, extragalactic, protoplanetary disk, and exoplanetary molecules. Astrophys. J. Suppl. Ser. 2022, 259, 30. [Google Scholar] [CrossRef]
- Lacy, J.H.; Carr, J.S.; Evans, N.J.; Baas, F.; Achtermann, J.M.; Arens, J.F. Discovery of interstellar methane: Observations of gaseous and solid CH4 absorption toward young stars in molecular clouds. Astrophys. J. 1991, 376, 556–560. [Google Scholar] [CrossRef]
- Qasim, D.; Fedoseev, G.; Chuang, K.-J.; He, J.; Ioppolo, S.; van Dishoeck, E.F.; Linnartz, H. An experimental study of the surface formation of methane in interstellar molecular clouds. Nat. Astron. 2020, 4, 781–785. [Google Scholar] [CrossRef]
- Öberg, K.I.; Boogert, A.C.A.; Pontoppidan, K.M.; Blake, G.A.; Evans, N.J.; Lahuis, F.; van Dishoeck, E.F. The c2d Spitzer spectroscopic survey of ices around low-mass young stellar objects. III. CH4. Astrophys. J. 2008, 678, 1032. [Google Scholar] [CrossRef]
- Kawara, K.; Gregory, B.; Yamamoto, T.; Shibai, H. Infrared spectroscopic observation of methane in comet P/Halley. Astron. Astrophys. 1988, 207, 174–181. [Google Scholar]
- Schuhmann, M.; Altwegg, K.; Balsiger, H.; Berthelier, J.-J.; De Keyser, J.; Fiethe, B.; Fuselier, S.A.; Gasc, S.; Gombosi, T.I.; Hänni, N.; et al. Aliphatic and aromatic hydrocarbons in comet 67P/Churyumov-Gerasimenko seen by ROSINA. Astron. Astrophys. 2019, 630, A31. [Google Scholar] [CrossRef]
- Rubin, M.; Bekaert, D.V.; Broadley, M.W.; Drozdovskaya, M.N.; Wampfler, S. Volatile species in comet 67P/Churyumov-Gerasimenko: Investigating the link from the ISM to the terrestrial planets. ACS Earth Space Chem. 2019, 3, 1792–1811. [Google Scholar] [CrossRef]
- Lunine, J.I.; Atreya, S.K. The methane cycle on Titan. Nat. Geosci. 2008, 1, 159–164. [Google Scholar] [CrossRef]
- Bertrand, T.; Forget, F.; Schmitt, B.; White, O.L.; Grundy, W.M. Equatorial mountains on Pluto are covered by methane frosts resulting from a unique atmospheric process. Nat. Commun. 2020, 11, 5056. [Google Scholar] [CrossRef]
- Mason, N.J.; Nair, B.; Jheeta, S.; Szymańska, E. Electron induced chemistry: A new frontier in astrochemistry. Faraday Discuss. 2014, 168, 235–247. [Google Scholar] [CrossRef]
- Boyer, M.C.; Rivas, N.; Tran, A.A.; Verish, C.A.; Arumainayagam, C.R. The role of low-energy (<20 eV) electrons in astrochemistry. Surf. Sci. 2016, 652, 26–32. [Google Scholar]
- Baratta, G.A.; Leto, G.; Palumbo, M.E. A comparison of ion irradiation and UV photolysis of CH4 and CH3OH. Astron. Astrophys. 2002, 384, 343–349. [Google Scholar] [CrossRef]
- Baratta, G.A.; Domingo, M.; Ferini, G.; Leto, G.; Palumbo, M.E.; Satorre, M.A.; Strazzulla, G. Ion irradiation of CH4-containing icy mixtures. Nucl. Instrum. Methods Phys. Res. B Beam Interact. Mater. Atom. 2003, 209, 283–287. [Google Scholar] [CrossRef]
- Bennett, C.J.; Jamieson, C.S.; Osamura, Y.; Kaiser, R.I. Laboratory studies on the irradiation of methane in interstellar, cometary, and Solar System ices. Astrophys. J. 2006, 653, 792–811. [Google Scholar] [CrossRef]
- Mejía, C.F.; de Barros, A.L.F.; Bordalo, V.; da Silveira, E.F.; Boduch, P.; Domaracka, A.; Rothard, H. Cosmic ray-ice interaction studied by radiolysis of 15 K methane ice with MeV O, Fe and Zn ions. Mon. Not. R. Astron. Soc. 2013, 433, 2368–2379. [Google Scholar] [CrossRef]
- Vasconcelos, F.A.; Pilling, S.; Rocha, W.R.M.; Rothard, H.; Boduch, P.; Ding, J.J. Ion irradiation of pure and amorphous CH4 ice relevant for astrophysical environments. Phys. Chem. Chem. Phys. 2017, 19, 12845–12856. [Google Scholar] [CrossRef]
- Turner, A.M.; Abplanalp, M.J.; Kaiser, R.I. Probing the carbon-phosphorus bond coupling in low-temperature phosphine (PH3)-methane (CH4) interstellar ice analogues. Astrophys. J. 2016, 819, 97. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Turner, A.M.; Abplanalp, M.J.; Kaiser, R.I. Formation and high-order carboxylic acids (RCOOH) in interstellar analogous ices of carbon dioxide (CO2) and methane (CH4). Astrophys. J. Suppl. Ser. 2018, 234, 15. [Google Scholar] [CrossRef]
- Esmaili, S.; Bass, A.D.; Cloutier, P.; Sanche, L.; Huels, M.A. Glycine formation in CO2:CH4:NH3 ices induced by 0–70 eV electrons. J. Chem. Phys. 2018, 148, 164702. [Google Scholar] [CrossRef]
- van Dishoeck, E.F.; Herbst, E.; Neufeld, D.A. Interstellar water chemistry: From laboratory to observations. Chem. Rev. 2013, 113, 9043–9085. [Google Scholar] [CrossRef]
- Moore, M.H.; Hudson, R.L. Infrared study of ion-irradiated water-ice mixtures with hydrocarbons relevant to comets. Icarus 1998, 135, 518–527. [Google Scholar] [CrossRef]
- Wada, A.; Mochizuki, N.; Hiraoka, K. Methanol formation from electron-irradiated mixed H2O/CH4 ice at 10 K. Astrophys. J. 2006, 644, 300–306. [Google Scholar] [CrossRef]
- Mejía, C.F.; de Barros, A.L.F.; Rothard, H.; Boduch, P.; da Silveira, E.F. Radiolysis of ices by cosmic rays: CH4 and H2O ices mixtures irradiated by 40 MeV 58Ni11+ ions. Astrophys. J. 2020, 894, 132. [Google Scholar] [CrossRef]
- Krim, L.; Jonusas, M. VUV photolysis of CH4-H2O mixture in methane-rich ices: Formation of large complex organic molecules in astronomical environments. Low Temp. Phys. 2019, 45, 606. [Google Scholar]
- Moore, M.H.; Hudson, R.L. Infrared study of ion-irradiated N2 dominated ices relevant to Triton and Pluto: Formation of HCN and HNC. Icarus 2003, 161, 486–500. [Google Scholar] [CrossRef]
- Herczku, P.; Mifsud, D.V.; Ioppolo, S.; Juhász, Z.; Kaňuchová, Z.; Kovács, S.T.S.; Traspas Muiña, A.; Hailey, P.A.; Rajta, I.; Vajda, I.; et al. The Ice Chamber for Astrophysics-Astrochemistry (ICA): A new experimental facility for ion impact studies of astrophysical ice analogues. Rev. Sci. Instrum. 2021, 92, 084501. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, D.V.; Juhász, Z.; Herczku, P.; Kovács, S.T.S.; Ioppolo, S.; Kaňuchová, Z.; Czentye, M.; Hailey, P.A.; Traspas Muiña, A.; Mason, N.J.; et al. Electron irradiation and thermal chemistry studies of interstellar and planetary ice analogues at the ICA astrochemistry facility. Eur. Phys. J. D 2021, 75, 182. [Google Scholar] [CrossRef]
- Rajta, I.; Vajda, I.; Gyürky, G.; Csedreki, L.; Kiss, Á.Z.; Biri, S.; van Oosterhout, H.A.P.; Podaru, N.C.; Mous, D.J.W. Accelerator characterization of the new ion beam facility at MTA Atomki in Debrecen, Hungary. Nucl. Instrum. Methods Phys. Res. A 2018, 880, 125–130. [Google Scholar] [CrossRef]
- Biri, S.; Vajda, I.; Hajdu, P.; Rácz, R.; Csik, A.; Kormány, Z.; Perduk, Z.; Kocsis, F.; Rajta, I. The Atomki accelerator center. Eur. Phys. J. Plus 2021, 136, 247. [Google Scholar] [CrossRef]
- Gerakines, P.A.; Hudson, R.L. A modified and open-source computational package for the determination of infrared optical constants relevant to astrophysics. Astrophys. J. 2020, 901, 52. [Google Scholar] [CrossRef]
- Bouilloud, M.; Fray, N.; Bénilan, Y.; Cottin, H.; Gazeau, M.-C.; Jolly, A. Bibliographic review and new measurements of the infrared band strengths of pure molecules at 25 K: H2O, CO2, CO, CH4, NH3, CH3OH, HCOOH, and H2CO. Mon. Not. R. Astron. Soc. 2015, 451, 2145–2160. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Ziegler, M.D.; Biersack, J.P. SRIM—The stopping and range of ions in matter (2010). Nucl. Instrum. Methods Phys. Res. B Beam Interact. Mater. Atom. 2010, 268, 1818–1823. [Google Scholar] [CrossRef]
- Mifsud, D.V.; Hailey, P.A.; Herczku, P.; Juhász, Z.; Kovács, S.T.S.; Sulik, B.; Ioppolo, B.; Kaňuchová, Z.; McCullough, R.W.; Paripás, B.; et al. Laboratory experiments on the radiation astrochemistry of water ice phases. Eur. Phys. J. D Atom. Mol. Opt. Plasma Phys. 2022, 76, 87. [Google Scholar] [CrossRef]
- Palumbo, M.E. Formation of compact solid water after ion irradiation at 15 K. Astron. Astrophys. 2006, 453, 903–909. [Google Scholar] [CrossRef]
- Raut, U.; Teolis, B.D.; Loeffler, M.J.; Vidal, R.A.; Famá, M.; Baragiola, R.A. Compaction of microporous amorphous solid water by ion irradiation. J. Chem. Phys. 2007, 126, 244511. [Google Scholar] [CrossRef]
- Dartois, E.; Ding, J.J.; de Barros, A.L.F.; Boduch, P.; Brunetto, R.; Chabot, M.; Domaracka, A.; Godard, M.; Lv, X.Y.; Mejía Guamán, C.F.; et al. Swift heavy ion irradiation of water ice from MeV to GeV energies: Approaching true cosmic ray compaction. Astron. Astrophys. 2013, 557, A97. [Google Scholar] [CrossRef]
- Gálvez, Ó.; Maté, B.; Herrero, V.J.; Escribano, R. Spectroscopic effects in CH4/H2O ices. Astrophys. J. 2009, 703, 2101–2107. [Google Scholar] [CrossRef]
- Herrero, V.J.; Gálvez, Ó.; Maté, B.; Escribano, R. Interaction of CH4 and H2O in ice mixtures. Phys. Chem. Chem. Phys. 2010, 12, 3164–3170. [Google Scholar] [CrossRef]
- DeMeo, F.; Dumas, C.; de Bergh, C.; Protopapa, S.; Cruikshank, D.P.; Geballe, T.R.; Alvarez-Candal, A.; Merlin, F.; Barucci, M.A. A search for ethane on Pluto and Triton. Icarus 2010, 208, 412–424. [Google Scholar] [CrossRef]
- Lunine, J.I.; Stevenson, D.J.; Yung, Y.L. Ethane ocean on Titan. Science 1983, 222, 1229–1230. [Google Scholar] [CrossRef]
- Glein, C.R.; Shock, E.L. A geochemical model of non-ideal solutions in the methane-ethane-propane-nitrogen-acetylene system on Titan. Geochim. Cosmochim. Acta 2013, 115, 217–240. [Google Scholar] [CrossRef]
- Mumma, M.J.; Disanti, M.A.; Dello Russo, N.; Fomenkova, M.; Magee-Sauer, K.; Kaminski, C.D.; Xie, D.X. Detection of abundant ethane and methane, along with carbon monoxide and water, in comet C/1996 B2 Hyakutake: Evidence for interstellar origin. Science 1996, 272, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Herbst, E.; Adams, N.G.; Smith, D. Laboratory measurements of ion-molecule reactions pertaining to interstellar hydrocarbon synthesis. Astrophys. J. 1983, 269, 329–333. [Google Scholar] [CrossRef]
- Knez, C.; Moore, M.H.; Ferrante, R.F.; Hudson, R.L. Laboratory IR studies and astrophysical implications of C2H2-containing binary ices. Astrophys. J. 2012, 748, 95. [Google Scholar] [CrossRef]
- Song, M.-Y.; Yoon, J.-S.; Cho, H.; Itikawa, Y.; Karwasz, G.P.; Kokoouline, V.; Nakamura, Y.; Tennyson, J. Cross sections for electron collisions with methane. J. Phys. Chem. Ref. Data 2015, 44, 023101. [Google Scholar] [CrossRef]
- Bug, M.U.; Gargioni, E.; Nettelbeck, H.; Baek, W.-Y.; Hilgers, G.; Rosenfeld, A.B.; Rabus, H. Ionization cross section data of nitrogen, methane, and propane for light ions and electrons and their suitability for use in track structure simulations. Phys. Rev. E 2013, 88, 043308. [Google Scholar] [CrossRef] [PubMed]
- Qasim, D.; Chuang, K.-J.; Fedoseev, G.; Ioppolo, S.; Boogert, A.C.A.; Linnartz, H. Formation of interstellar methanol ice prior to the heavy CO freeze-out stage. Astron. Astrophys. 2018, 612, A83. [Google Scholar] [CrossRef]
- Watanabe, N.; Kouchi, A. Efficient formation of formaldehyde and methanol by the addition of hydrogenation atoms to CO in H2O-CO ice at 10 K. Astrophys. J. 2002, 571, L173. [Google Scholar] [CrossRef]
- Watanabe, N.; Nagaoka, A.; Shiraki, T.; Kouchi, A. Hydrogenation of CO on pure solid CO and CO-H2O mixed ice. Astrophys. J. 2004, 616, 638–642. [Google Scholar] [CrossRef]
- Fuchs, G.W.; Cuppen, H.M.; Ioppolo, S.; Romanzin, C.; Bisschop, S.E.; Andersson, S.; van Dishoeck, E.F.; Linnartz, H. Hydrogenation reactions in interstellar CO ice analogues: A combined experimental/theoretical approach. Astron. Astrophys. 2009, 505, 629–639. [Google Scholar] [CrossRef]
- Gomis, O.; Strazzulla, G. CO2 production by ion irradiation of H2O ice on top of carbonaceous materials and its relevance to the Galilean satellites. Icarus 2005, 177, 570–576. [Google Scholar] [CrossRef]
- Mennella, V.; Palumbo, M.E.; Baratta, G.A. Formation of CO and CO2 molecules by ion irradiation of water ice-covered hydrogenated carbon grains. Astrophys. J. 2004, 615, 1073–1080. [Google Scholar] [CrossRef]
- Mennella, V.; Baratta, G.A.; Palumbo, M.E.; Bergin, E.A. Synthesis of CO and CO2 molecules by UV irradiation of water ice-covered hydrogenated carbon grains. Astrophys. J. 2006, 643, 923–931. [Google Scholar] [CrossRef]
- Palumbo, M.E.; Castorina, A.C.; Strazzulla, G. Ion irradiation effects on frozen methanol (CH3OH). Astron. Astrophys. 1999, 342, 551–562. [Google Scholar]
- de Barros, A.L.F.; Domaracka, A.; Andrade, D.P.P.; Boduch, P.; Rothard, H.; da Silveira, E.F. Radiolysis of frozen methanol by heavy cosmic ray and energetic solar particle analogues. Mon. Not. R. Astron. Soc. 2011, 418, 1363–1374. [Google Scholar] [CrossRef]
- Jheeta, S.; Domaracka, A.; Ptasinska, S.; Sivaraman, B.; Mason, N.J. The irradiation of pure CH3OH and 1:1 mixture of NH3:CH3OH ices at 30 K using low energy electrons. Chem. Phys. Lett. 2013, 556, 359–364. [Google Scholar] [CrossRef]
- Boamah, M.D.; Sullivan, K.K.; Shulenberger, K.E.; Soe, C.M.; Jacob, L.M.; Yhee, F.C.; Atkinson, K.E.; Boyer, M.C.; Haines, D.R.; Arumainayagam, C.R. Low-energy electron-induced chemistry of condensed methanol: Implications for the interstellar synthesis of prebiotic molecules. Faraday Discuss. 2014, 168, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.; Swiderek, P.; Bredehöft, J.H. Electron-induced processing of methanol ice. ACS Earth Space Chem. 2021, 5, 391–408. [Google Scholar] [CrossRef]
- Goumans, T.P.M.; Uppal, M.A.; Brown, W.A. Formation of CO2 on a carbonaceous surface: A quantum chemical study. Mon. Not. R. Astron. Soc. 2008, 384, 1158–1164. [Google Scholar] [CrossRef]
- Ioppolo, S.; van Boheemen, Y.; Cuppen, H.M.; van Dishoeck, E.F.; Linnartz, H. Surface formation of CO2 ice at low temperatures. Mon. Not. R. Astron. Soc. 2011, 413, 2281–2287. [Google Scholar] [CrossRef]
- Grim, R.J.A.; d’Hendecourt, L.B. Time-dependent chemistry in dense molecular clouds. IV. Interstellar grain surface reactions inferred from a matrix isolation study. Astron. Astrophys. 1986, 167, 161–165. [Google Scholar]
- Roser, J.E.; Vidali, G.; Manicò, G.; Pirronello, V. Formation of carbon dioxide by surface reactions on ices in the interstellar medium. Astrophys. J. 2001, 555, L61. [Google Scholar] [CrossRef]
- Madzunkov, S.; Shortt, B.J.; MacAskill, J.A.; Darrach, M.R.; Chutjian, A. Measurements of polyatomic molecule formation on an icy grain analog using fast atoms. Phys. Rev. A 2006, 73, 020901(R). [Google Scholar] [CrossRef]
- Hudson, R.L.; Gerakines, P.A.; Moore, M.H. Infrared spectra and optical constants of astronomical ices: II. Ethane and ethylene. Icarus 2014, 243, 148–157. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).