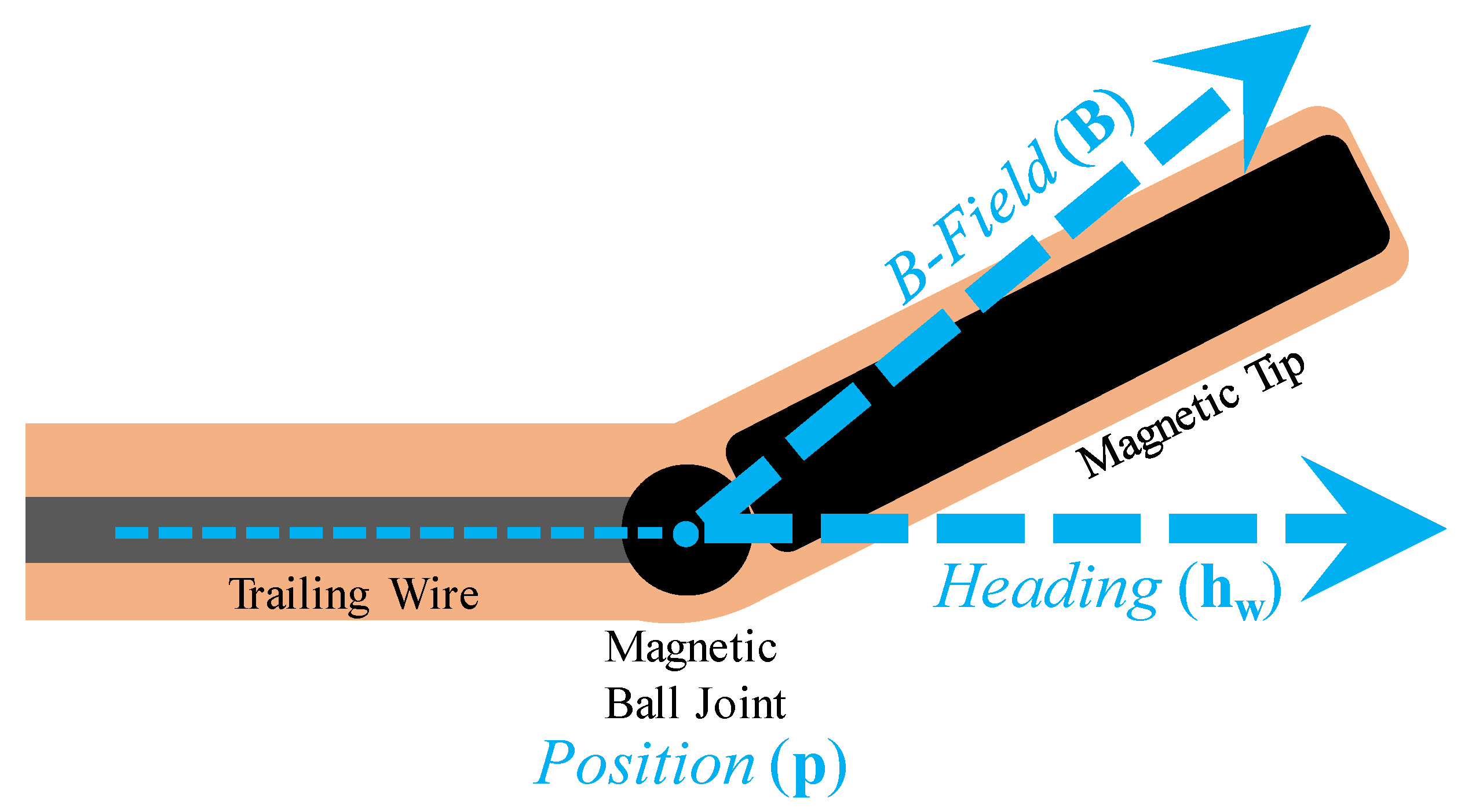
1. Introduction
Needles are a longstanding and widespread tool used by surgeons as they are minimally invasive and versatile in a wide range of clinical applications. In recent years, robotically guided needles have been investigated, and one area with potential benefits is needle insertions into the brain. One such surgical intervention is Deep Brain Stimulation (DBS), which is applied to a wide range of central nervous system pathologies such as Parkinson’s disease and Alzheimer’s disease [
1,
2]. DBS surgeries place an electrode in the brain by inserting it through the skull along a straight path [
3]. The required precision for an effective DBS electrode placement is estimated at 2 mm [
4], and current surgical procedures achieve accuracies of about 2–3 mm [
5], which means this technique can unfortunately lead to inaccurate electrode placement. This results in the need for additional electrode insertions, which reduce the likelihood of a positive surgical outcome [
6]. Reducing or correcting trajectory error can reduce the number of penetrations into the brain for this procedure [
7]. As most are bilateral, DBS surgeries typically require two entry points through the skull [
8]. Fewer entry points would allow safer, faster, and less traumatic procedures and may be achieved by using curved trajectories to perform multiple electrode placements during a single insertion. Entry points must also be chosen to provide a straight path to the electrode placement target without penetrating sensitive areas of the brain and compromising safety. This can be difficult if not impossible depending on patient anatomy [
9], whereas a curved trajectory could allow for an optimal insertion point while still allowing a safe trajectory by circumventing sensitive areas.
Another clinical application that could benefit from needle navigation around obstacles specifically in brain tissue is the treatment of cancerous brain tumors, such as glioblastoma. These tumors can develop near sensitive tissues such as venous sinuses, the brain stem, or deep cerebellar nuclei [
10]. Convection-Enhanced Delivery (CED) is a targeted drug delivery technique used to treat cancerous brain tumors, as well as Parkinson’s disease and Alzheimer’s disease, which, similarly to DBS, requires one or more insertions through burr holes in the skull and accurate positioning of the needle at the injection site [
11].
Expanding beyond in-brain applications, there are numerous medical procedures that can benefit from steerable needle technology. A study conducted at the Annual Meeting of Cardiovascular and Interventional Radiology Society of Europe in 2016 supports the desire for steerable needles by practitioners in the field [
12]. Respondents consisted primarily of interventional radiologists with experience in needle placement. The study found that added value for steerable needles in current interventions was seen by 93% of the respondents, while 85% of the respondents found needle steering to be a useful tool for steering around anatomical obstacles.
Both passive and active steering approaches to provide tip control to the surgeon have been investigated, and both present their own challenges. Passive bevel-tip needles are typically less complex, but manipulability is limited [
13,
14]. Active methods are typically more capable, but achieve this through sacrificing the simplicity and flexibility of the needle [
15]. Magnetically steered needles may provide an ideal solution as the only added complexity is a permanent magnet in the needle tip, yet they are highly steerable by virtue of an external magnetic field. The design and actuation of a magnetic-tip steerable needle for guiding a DBS electrode insertion was presented in [
16], and its functionality was demonstrated in an agar brain tissue phantom. The design followed curved trajectories under direct human operator control and executed trajectories with multiple targets.
In their 2006 seminal paper, Webster et al. showed that a bevel-tip needle could be represented with a bicycle model with a fixed steering angle [
17]. Slight variations and modifications to the needle model and design have been investigated, including augmenting the model with a steerable angle [
18] and, recently, magnetic control of a flexible needle using the bicycle model modified to work with magnetic inputs [
19]. However, the bicycle model does not necessarily represent the physics of a magnetic-tip needle in soft tissue. It is subject to a no-slip condition that constrains the velocity of the needle wire and tip in the direction perpendicular to their orientation. This can be an effective constraint on needle tip steering in stiff tissue, but has been found to be violated for magnetic needle insertion in the softer brain tissue phantom [
16]. Thus, despite its use in previous applications, the scope of the bicycle model may be limited when applied to magnetic needle steering, particularly in the brain.
Steerable needle models typically include parameters containing the physical properties of the system that dictate needle–tissue interaction. In order to implement a model, these parameters must be identified. Many models directly parameterize tissue and needle properties such as stiffness, elastic modulus, and cutting and friction forces [
20,
21]. This often requires a specific setup and sensors to explore the tissue–needle interaction. Other models use more abstract parameters that indirectly encode tissue parameters, which are often identified by performing a calibration-specific testing protocol using a theoretically derived best-fit optimization on the calibration data [
16,
17,
22]. These needle model parameterization approaches do not incorporate the identification of measurement or process noise covariances, which precludes optimal model implementation.
Recently, the authors presented a physically motivated magnetic needle model (MNM) specifically for magnetic needle steering in soft tissue and an algorithmic parameter identification methodology for steerable needle models in general [
23]. The experimental validity of the model was left undetermined, while the parameter identification methodology was validated in simulation. Building from this prior work, the three primary research contributions of this paper are: (1) algorithmically parameterizing two steerable needle models using the framework from [
23] with empirical data, (2) presenting a generalizable method to use the parameterization results to assess which model better fits the empirical data, and (3) demonstrating that the MNM provides a simplified and more physically correct model than the state-of-the-art bicycle needle model (BiNM) in artificial soft tissue.
The remainder of this paper is structured as follows: In
Section 2.1, the needle models MNM and BiNM used for parameterization are presented. In
Section 2.2, the expectation-maximization (EM) technique presented in [
23] to identify the unknown model parameters, as well as process and measurement noise covariances is summarized and updated.
Section 2.3 describes the experimental methods for collecting data ex vivo in agar, while
Section 3 provides the results of running the EM algorithm with both the MNM and BiNM to parameterize the models on the experimental data.
Section 4 discusses the parameterization results and directly compares the two models’ results.
3. Results
Four trials experienced experimental error, and the data were discarded, resulting in a total of 44 successful individual trials. EM as described in Algorithm 1 was run on each trial of experimental data for both the MNM and BiNM with a limit of 100 iterations. An example trajectory result is shown in
Figure 5. EM converged on all trials with the MNM, but diverged on three trials when using the BiNM due to breaching the singularity. Those three trials were excluded from results of both models for consistency.
The EM algorithm was parameterized
c with an average of 5.3, 9.5, and 11.9 m
−1 for the three different field strengths tested and standard deviations (SDs) of 3.2, 4.8, and 5.6 m
−1, respectively, as shown in the first column of
Table 1. Similarly,
was parameterized with an average of 1.6, 3.5, and 4.9 m
−1 for the three different field strengths tested and standard deviations of 1.6, 3.0, and 3.4 m
−1, respectively, as shown in the first column of
Table 2.
Since Q and R are matrices, we reduced them to single average value representations by dividing their trace by the measurement dimension to facilitate our analysis and comparison. As the trace is the sum of the eigenvalues, it provides a measure of the total variation, and dividing provides an average variation per 3D dimension. and are each reduced using their trace to a single representative value because each measures only a single unit: position variance (m2) and B-field direction variance (unit vector2), respectively. Note from the squared units that the Q and R values are reported as variances in all tables and figures, so to interpret variation with respect to states, the square root must be taken.
Q contains three 3 × 3 sub-matrices corresponding to the three 3D states: position, heading, and B-field direction. Results represent each sub-matrix as a singular value using its trace:
,
, and
, respectively. Complete
Q and
R matrices’ cross-terms were found to be at least one order of magnitude lower than the diagonals, while the diagonals within each
Q block and
R were found to be within an order of magnitude, so the information lost by this reduced representation should not significantly impact the interpretation. These average parameterized values along with their standard deviations are reported in
Table 1 and
Table 2 for the MNM and BiNM, respectively. These results show that, on the order of a
variance of a unit vector,
is minuscule, as predicted in
Section 2.3.9, supporting its omission from further analysis.
The spread of the
,
,
, and
data are shown with boxplots in
Figure 6. The tight one-sided distribution of
was caused by the minimum noise threshold set at
m
2. Parameterization of
,
, and
was nearly identical for MNM and BiNM. In contrast, parameterization of
did not match between models and on average was an order of magnitude higher in the BiNM than in the MNM. Additionally, three extreme high-side outliers occurred in BiNM at
= 0.12, 0.036, and 0.026 (not pictured in
Figure 6 for clarity).
Ultimately, parameterization was used to improve the predictive accuracy of the models. Thus, positional trajectory sensitivity to the parameters was explored. The last row of
Table 1 and
Table 2 presents a difference in the RMS distance trajectory error that could be expected at one standard deviation from the mean for each parameter. To calculate these values, both T1 and T2 were first simulated nominally using the average parameters from
Table 1 and
Table 2. Then, each parameter was separately inflated and deflated by the ratio of its mean to the standard deviation. The average trajectory error from this nominal trajectory was calculated, and this process was repeated 100 times and averaged. This resulting measure of error provides a physical interpretation of the parameter variation.
4. Discussion
The experiments in this paper serve two goals: to use the EM algorithm to identify needle model parameters and to examine differences between the MNM and the BiNM to determine if and when the MNM provides superior performance to the BiNM. Having better model state estimation will ultimately result in improved control of the needle and, thus, more accurate electrode placement in surgery.
4.1. Parameter Identification
The EM algorithm converged to a solution for every single trial using the MNM. With the BiNM, a solution was found for all but three trials, and these failed due to the known model issue of approaching the singularity. Additionally, parameterization spread results expressed by the standard deviations in
Table 1 and
Table 2 demonstrate a level of consistency between the solutions.
These variation measures of the
c,
Q, and
R parameters help to understand the precision of the EM algorithm applied to these needle steering models. However, to practically evaluate the parameterization, we considered the sensitivity of the trajectory error to this variation. A higher trajectory error might indicate that the variance in parameterization was too large, and the model did not capture all significant physical effects, while a lower trajectory error indicates that average parameterization results can be generally applied. As seen in
Table 1 and
Table 2, the trajectory error produced by both models’ parameter spread at one standard deviation was submillimeter across all parameters, with the largest being 0.360 mm from
of the BiNM. This is very promising compared to the 2 mm surgical accuracy target for electrodes in Deep Brain Stimulation [
4] and supports using the resulting parameterized model for general needle steering application.
The mean
for MNM as reported in
Table 1 was 2.70 × 10
m
2, so the error in the position measurement (expressed as a standard deviation) was 0.05 mm. This is comparable to the camera pixel resolution due to the
distribution falling very near the minimum limit established at the camera resolution. Because both models use the same measurements, the measurement noise should be the same. This is supported in the results, as
closely matches between the models.
In these results, Q is expressed as a continuous variance per second, while R is a per update value that applies to each measurement. Therefore, on average, to prevent noise from accumulating larger than , position measurements would need to occur at a frequency of the ratio of the two: 5.2 Hz. Experimental measurements occurred at 6 Hz, which is not coincidentally very similar. This is because the EM algorithm matches model positional error to measurement error by pushing uncertainty into the heading. Since the heading is estimated, it has no measurement to reduce or correct its uncertainty like the position and B-field direction. from both models matched, which is reasonable, because both models use position in the same way.
Similarly, also matched between the models. was extremely small, at 7 × 10, which correlates with the 0.008%/s error of a unit vector, or equivalently, a 0.005°/s error in direction. is not expected to experience significant error because no dynamics affect the B-field direction, so it is free to follow the control input precisely.
is the pivotal element of process noise for two reasons. First, because the heading is estimated, the uncertainty of the models collects in
. This makes it a strong indicator of model accuracy. Second, the primary difference between models is how the heading state interacts, which will make it an important aspect of the model comparison in
Section 4.2. The average
for the MNM is 6.65 × 10
, which correlates with the 2.6%/s error of a unit vector, or equivalently, a 1.5
/s error. The average
for the BiNM was 6.40 × 10
, which correlates with the 8.0%/s error of a unit vector, or a 4.6
/s error, which is triple that of the MNM.
4.2. Model Comparison
The MNM is presented as an alternative to the current de facto needle steering model, the BiNM. Here, we compare the results from the two models, focusing on areas where they differ, and present why the MNM is the preferred model to use for magnetic needle steering.
4.2.1. Comparing Process Noise
Process noise Q is a measure of error accumulation per time of the difference between the true physical system and the model. When comparing Q between models, the model with a lower Q can be interpreted as more representative of the truth, and thus the better model.
The percent difference of BiNM to MNM noise traces is shown in the first row of
Table 3. To ensure no significant differences exist in any term, as was done in calculating the simulation results, full
Q and
R matrix blocks were compared between models by finding a percent difference between median data matrices using the matrix norm. Results are shown in the second row of
Table 3.
Both comparisons resulted in the same trends. , , and showed negligible differences, while was much larger in the BiNM than the MNM. This 863% larger in the BiNM demonstrates a significant disparity between the models and supports the MNM being a more physically representative model of the data than the BiNM.
To understand the practical effect of this difference in
Q,
Figure 7 presents data boxplots of the trajectory effect as both the average position RMS difference and the final position RMS difference between the two models. All measures of the difference in trajectory as a result of the difference in
Q between the models were negligible, with the largest being 0.04 mm. It is possible that with fewer or less accurate measurements where the model would play a greater role, there could be a larger distinction, but in this experimental data, there was no practical difference.
4.2.2. Bicycle Model Singularity Problem
To test the difference between models, we designed an experimental trajectory scenario where they diverged significantly. The model that more closely matches the data for this scenario will be the more physically realistic model. This trajectory scenario, T2
, occurred as the BiNM approaches its singularity and is shown in
Figure 8.
If the data followed the BiNM, the curvature would increase when approaching the singularity, effectively causing a “pulling” effect of the BiNM that keeps the model out of the singularity. On the other hand, the MNM allows crossing without issue, and so predicts a straighter trajectory. This effect becomes more pronounced at lower c and values, as c allows an even less capable turning rate, thus reaching earlier, while the BiNM forces the heading to stay out of the singularity, regardless of .
Preliminary data were collected using T2
and found to most closely match the MNM. As shown in
Figure 9, the measurements better followed the predicted open-loop MNM trajectory, thus violating the singularity in the BiNM, and diverged from the predicted open-loop BiNM trajectory.
An EKF forward pass of the BiNM is overlaid, which shows its heading diverging from the trajectory in a nonsensical way so that the model does not enter the singularity. This can be seen more starkly by plotting
directly (
Figure 10). The EM MNM estimate is shown crossing the singularity (
) in a continuous smooth line, while the forward pass BiNM estimate follows a similar trajectory until the singularity. There, it has a sharp slope discontinuity and remains just outside the singularity, predicting exceedingly unreasonable headings, as was seen in
Figure 9a, and diverging from the measurements, as seen in
Figure 9b. When attempting to refine the BiNM estimate using multiple EM passes, the algorithm became unstable because it cannot reconcile the position measurements with the BiNM while avoiding the singularity.
Because these data were unusable for the parameterization of the BiNM using the EM algorithm, T2
had to be modified to avoid the BiNM singularity. This resulted in the experimental T2, which is shown in
Figure 11. However, avoiding the BiNM singularity necessarily resulted in model trajectories that did not diverge, because divergence occurred near and as a result of the singularity. Thus, in comparing parameterization results for the purpose of identifying the more physically accurate model, as in
Section 4.2.1, the models predicted similar results, and only small differences were found, which did not significantly affect the resulting estimated trajectories. However, it must be recognized that while in this limited region, the models were similar, it is more important that, where they would not be similar, the BiNM cannot be used at all, because it failed due to its singularity.
5. Conclusions
We derived a physically motivated needle steering model and demonstrated the use of an EM algorithm with IEKS to identify the unknown model curvature parameters and process and measurement noise covariances, both in simulation and experimentally. The simulation analyzed the convergence envelope and accuracy of the model parameters based on the variables themselves, as well as their initial guesses. The parameters consistently converged, and under expected conditions, parameterization reduced the RMS position trajectory error to 0.81 mm, which compares favorably to the approximately 2 mm accuracy that is required in the final placement of electrodes in DBS [
4]. These simulation results support the application of this EM algorithm in identifying
c,
Q, and
R experimentally.
We collected experimental data in an agar brain tissue phantom using open-loop linear needle insertion and B-field steering inputs. These data were used to parameterize both our derived magnetic needle model and the commonly used bicycle needle model. Parameterization converged for all trials using the MNM, but the BiNM failed to converge with data near its singularity. Variation in c and curvature parameterization, when averaged for each field strength tested, did not express a significant effect on positional trajectory error. Variation in Q and R parameterization also did not manifest a significant effect on positional trajectory error, suggesting the experimental results were sufficiently accurate to use the parameterized models in future applications, such as prediction and control. The next step towards clinical application is developing a controller for this system. The physically determined c, Q, and R parameters will be needed to apply and demonstrate needle steering control in a physical system.
The MNM parameterized a lower Q than the BiNM in the experimental results, indicating that it more accurately models needle steering in soft brain tissue. For data not near the singularity however, there was no practical difference between the models, as the difference in the position trajectories was negligible. Therefore, if care is taken to avoid the singularity, either model can be used with similar success. However, being near the singularity is not only a likely scenario: it is desirable due to its strong influence on steering. In this case, the BiNM model breaks down and cannot be algorithmically parameterized, because it fails to represent reality. Given that no such limitations exist in the MNM and that it better models needle steering in all cases tested, our results support the use of the MNM over the BiNM in all magnetic needle steering applications.