Geographic Distribution of Lung and Bronchus Cancer Mortality and Elevation in the United States: Exploratory Spatial Data Analysis and Spatial Statistics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lung-Bronchus Cancer Mortality Data
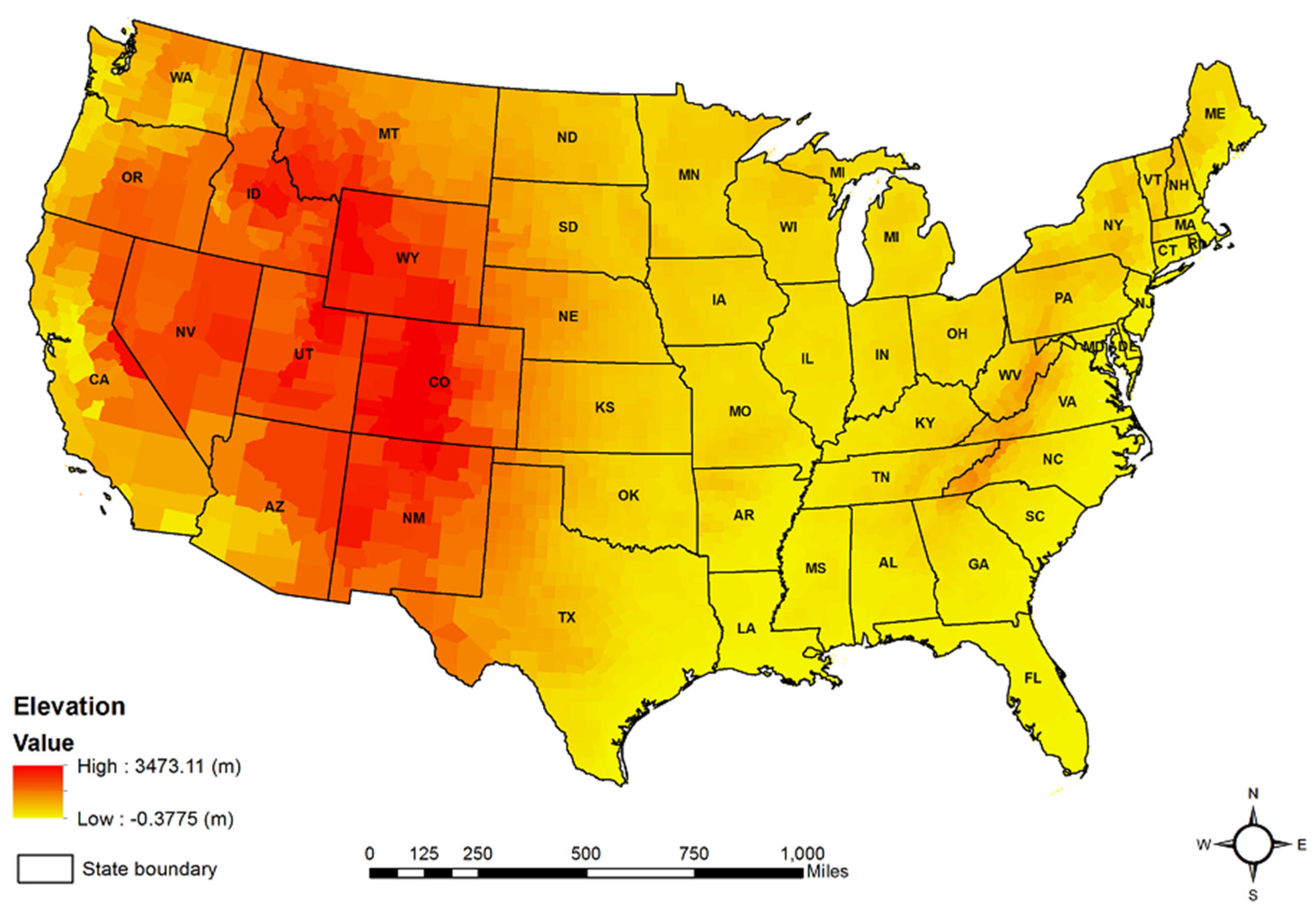
2.2. Mean County Elevation Data
2.3. Potential Confounders
2.4. Model Analysis
2.5. Local Mortan’s I Analysis
3. Results
3.1. Descriptive and Bivariate Statistics
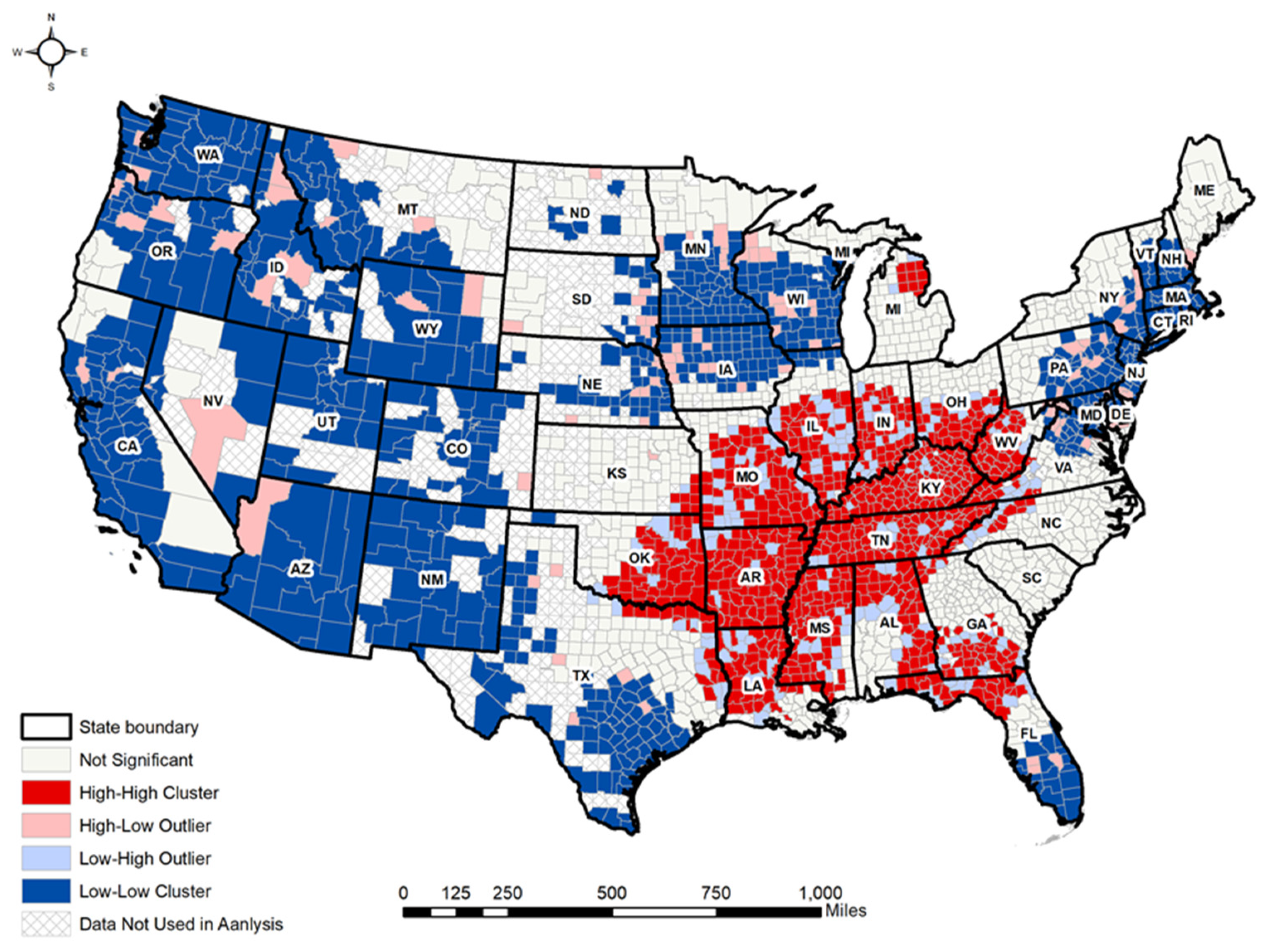
3.2. Local Moran’s I Analyses
3.3. Hierarchical Regression Analyses
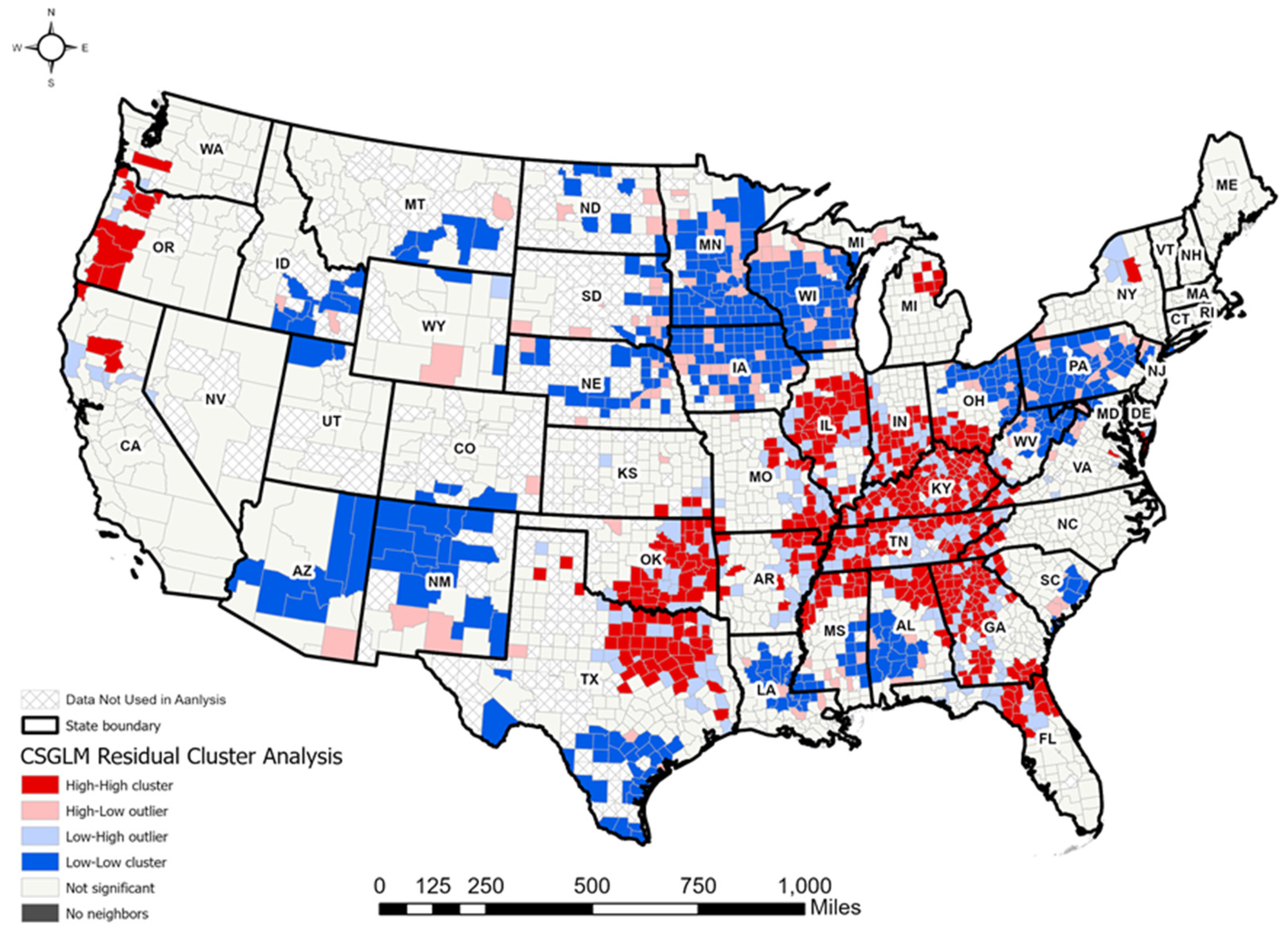
3.4. CSGLM Analyses
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Cancer. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 11 June 2023).
- American Cancer Society. Cancer Facts and Figures 2023; American Cancer Society: Atlanta, GA, USA, 2023. [Google Scholar]
- NIH. Cancer Stat Facts: Lung and Bronchus Cancer. 2023. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 11 June 2023).
- American Cancer Society. Lung Cancer Risk Factors. 2021. Available online: https://www.cancer.org/cancer/lung-cancer/causes-risks-prevention/risk-factors.html (accessed on 11 June 2023).
- Samet, J.M.; Avila-Tang, E.; Boffetta, P.; Hannan, L.M.; Olivo-Marston, S.; Thun, M.J.; Rudin, C.M. Lung cancer in never smokers: Clinical epidemiology and environmental risk factors. Clin. Cancer Res. 2009, 15, 5626–5645. [Google Scholar] [CrossRef] [PubMed]
- Kuśnierczyk, P. Genetic differences between smokers and never-smokers with lung cancer. Front. Immunol. 2023, 14, 1063716. [Google Scholar] [CrossRef]
- Zhu, X.; Lu, Y.; Shen, T.; Dong, W. Socioeconomic status and lung cancer risk: A meta-analysis. Transl. Lung Cancer Res. 2019, 8, 412–428. [Google Scholar]
- Beall, C.M. Adaptation to High Altitude: Phenotypes and Genotypes. Annu. Rev. Anthropol. 2014, 43, 251–272. [Google Scholar]
- Grant, W.B. Role of solar UVB and vitamin D in reducing cancer risk and increasing survival. Anticancer Res. 2016, 36, 1357–1370. [Google Scholar]
- Ward, M.P.; Milledge, J.S.; West, J.B. High Altitude Medicine and Physiology; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Beall, C.M. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc. Natl. Acad. Sci. USA 2007, 104, 8655–8660. [Google Scholar]
- Turner, M.C.; Krewski, D.; Pope, C.A.; Chen, Y.; Gapstur, S.M.; Thun, M.J. Long-term ambient fine particulate matter air pollution and lung cancer in a large cohort of never-smokers. Am. J. Respir. Crit. Care Med. 2011, 184, 1374–1381. [Google Scholar]
- Baciu, A.; Negussie, Y.; Geller, A. The State of Health Disparities in the United States; National Academies Press (US): Washington, DC, USA, 2017. [Google Scholar]
- Ha, H. Spatial variations in the associations of mental distress with sleep insufficiency in the United States: A county-level spatial analysis. Int. J. Environ. Health Res. 2023, 34, 911–922. [Google Scholar] [CrossRef]
- Ha, H.; Tu, W. An ecological study on the spatially varying relationship between county-level suicide rates and altitude in the United States. Int. J. Environ. Res. Public Health 2018, 15, 671. [Google Scholar] [CrossRef]
- Bethlehem, J. Applied Survey Methods: A Statistical Perspective; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Heeringa, S.G.; West, B.T.; Berglund, P.A. Applied Survey Data Analysis; Chapman and Hall: London, UK; CRC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Lohr, S.L. Sampling: Design and Analysis; Chapman and Hall: London, UK; CRC: Boca Raton, FL, USA, 2019. [Google Scholar]
- Campbell, R.T.; Berbaum, M.L. Analysis of Data from Complex Survey in a Handbook of Survey Research; Marsden, P.V., Ed.; Emerald: Leeds, UK, 2010; pp. 221–262. [Google Scholar]
- Sturgis, P. Analyzing Complex Survey Data: Clustering, Stratification and Weights. In Social Research Update; 43 Autumn Issue; University of Surrey: Surrey, UK, 2004. [Google Scholar]
- Anselin, L. Spatial Econometrics: Methods and Models; Kluwer Academic Publishers: Norwell, MA, USA, 1988. [Google Scholar]
- Cromley, E.K.; McLafferty, S.L. GIS and Public Health; Guilford Press: New York, NY, USA, 2011. [Google Scholar]
- Pfeiffer, D.U.; Robinson, T.P.; Stevenson, M.; Stevens, K.B.; Rogers, D.J.; Clements, A.C.A. Spatial Analysis in Epidemiology; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- U.S. Geological Survey. 100-Meter Resolution Elevation of the Conterminous United States. 2012. Available online: https://apps.nationalmap.gov/downloader/ (accessed on 10 September 2017).
- Huber, R.S.; Kim, T.; Kim, N.; Kuykendall, M.D.; Sherwood, S.N.; Renshaw, P.F.; Kondo, D.G. Association between altitude and regional variation of ADHD in youth. J. Atten. Disord. 2015, 22, 1299–1306. [Google Scholar] [CrossRef]
- Brenner, B.; Cheng, D.; Clark, S.; Camargo, C.A., Jr. Positive association between altitude and suicide in 2584 U.S. counties. High Alt. Med. Biol. 2011, 12, 31–35. [Google Scholar] [PubMed]
- de Groot, P.M.; Wu, C.C.; Carter, B.W.; Munden, R.F. The epidemiology of lung cancer. Transl. Lung Cancer Res. 2018, 7, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.C.; Andersen, Z.J.; Baccarelli, A.; Diver, W.R.; Gapstur, S.M.; Pope, C.A.; Prada, D.; Samet, J.M.; Thurston, G.D.; Cohen, A. Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA Cancer J. Clin. 2020, 70, 460–479. [Google Scholar] [CrossRef]
- Ha, H. Using geographically weighted regression for social inequality analysis: Association between mentally unhealthy days (MUDs) and socioeconomic status (SES) in U.S. counties. Int. J. Environ. Health Res. 2018, 29, 140–153. [Google Scholar] [CrossRef]
- Rogerson, P.A. Statistical Methods for Geography, 2nd ed.; Sage: London, UK, 2006. [Google Scholar]
- Anselin, L. Local Indicators of Spatial Association—LISA. Geogr. Anal. 1995, 27, 93–115. [Google Scholar]
- Ord, J.K.; Getis, A. Local Spatial Autocorrelation Statistics: Distributional Issues and an Application. Geogr. Anal. 1995, 27, 286–306. [Google Scholar]
- Burtscher, J.; Mallet, R.T.; Burtscher, M.; Millet, G.P. Hypoxia and brain aging: Neurodegeneration or neuroprotection? Ageing Res. Rev. 2021, 68, 101343. [Google Scholar]
- Moore, L.G.; Niermeyer, S.; Zamudio, S. Human adaptation to high altitude: Regional and life-cycle perspectives. Am. J. Biol. Anthropol. 1998, 41, 25–64. [Google Scholar] [CrossRef]
- West, J.B. High-Altitude Medicine and Physiology; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Akil, L.; Ahmad, H.A. Relationships between Obesity and Cardiovascular Diseases in Four Southern States and Colorado. J. Health Care Poor Underserved 2011, 22, 61–72. [Google Scholar] [CrossRef]
- CDC. Adult Obesity Prevalence Maps. 2024. Available online: https://www.cdc.gov/obesity/data-and-statistics/adult-obesity-prevalence-maps.html (accessed on 20 August 2024).
- Casper, M.L.; Barnett, E.; Williams, G.I.; Halverson, J.A.; Braham, V.E.; Greenlund, K.J. Atlas of Stroke Mortality: Racial, Ethnic, and Geographic Disparities in the United States; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2003. [Google Scholar]
- Barker, L.E.; Kirtland, K.A.; Gregg, E.W.; Geiss, L.S.; Thompson, T.J. Geographic distribution of diagnosed diabetes in the U.S.: A diabetes belt. Am. J. Prev. Med. 2011, 40, 434–439. [Google Scholar] [PubMed]
- Adler, N.E.; Newman, K. Socioeconomic disparities in health: Pathways and policies. Health Aff. 2002, 21, 60–76. [Google Scholar] [CrossRef]
- Williams, D.R.; Jackson, P.B. Social sources of racial disparities in health. Health Aff. 2005, 24, 325–334. [Google Scholar] [CrossRef]
- Thiersch, M.; Swenson, E.R. High altitude and cancer mortality. High Alt. Med. Biol. 2018, 19, 116–123. [Google Scholar]
- Yousefi, J. Geographical disparities in lung cancer in Canada: A Review. Curr. Oncol. Rep. 2024, 26, 221–235. [Google Scholar]
- Zhu, Y.; McKeon, T.P.; Tam, V.; Vachani, A.; Penning, T.M.; Hwang, T. Geographic differences in lung cancer incidence: A study of a major metropolitan area within southeastern Pennsylvania. Int. J. Environ. Res. Public Health 2020, 17, 9498. [Google Scholar] [CrossRef]
- Ullah, E.I.; Khan, S.; Baig, S.U.; Khan, S.; Hronec, M.; Waheed, F. Effects of altitude on socio-economic conditions and environmental sustainability of farm households in North Pakistan (A Case Study of Namli Maira, Hazara Division). Glob. Bus. Rev. 2024. published online. [Google Scholar] [CrossRef]
- Ye, V.Y.; Becker, C.M. The Z-axis: Elevation gradient effects in Urban America. Reg. Sci. Urban Econ. 2018, 70, 312–329. [Google Scholar] [CrossRef]


| N | Mean | SD | Bivariate | |
|---|---|---|---|---|
| Dependent variable: | ||||
| Lung-Bronchus cancer death rate | 2662 | 43.095 | 12.320 | 1.000 ** |
| Independent variables: | ||||
| Health behaviors: | ||||
| % adult smoking | 2662 | 18.601 | 3.645 | 0.647 ** |
| % insufficient sleep | 2662 | 33.460 | 3.962 | 0.439 ** |
| % adult obesity | 2662 | 31.181 | 4.469 | 0.566 ** |
| Food environment index | 2662 | 7.052 | 1.098 | −0.290 ** |
| % physically inactive | 2662 | 27.403 | 5.490 | 0.637 ** |
| % alcohol impaired | 30.827 | 12.965 | −0.018 | |
| Clinical care: | ||||
| % uninsured | 2662 | 16.955 | 5.103 | 0.110 ** |
| Primary care physician ratio | 2662 | 56.298 | 32.420 | −0.300 ** |
| Preventable hospital stays | 2662 | 63.482 | 24.646 | 0.573 ** |
| Social economic environment: | ||||
| % of college education | 2662 | 56.034 | 11.167 | −0.469 ** |
| % unemployment | 2662 | 6.460 | 2.121 | 0.355 ** |
| 80th percentile income | 2662 | 88,987.691 | 20,137.157 | −0.504 ** |
| Association rate | 2662 | 13.094 | 5.291 | 0.019 |
| Physical environment: | ||||
| Average daily PM2.5 | 2662 | 11.700 | 1.524 | 0.297 ** |
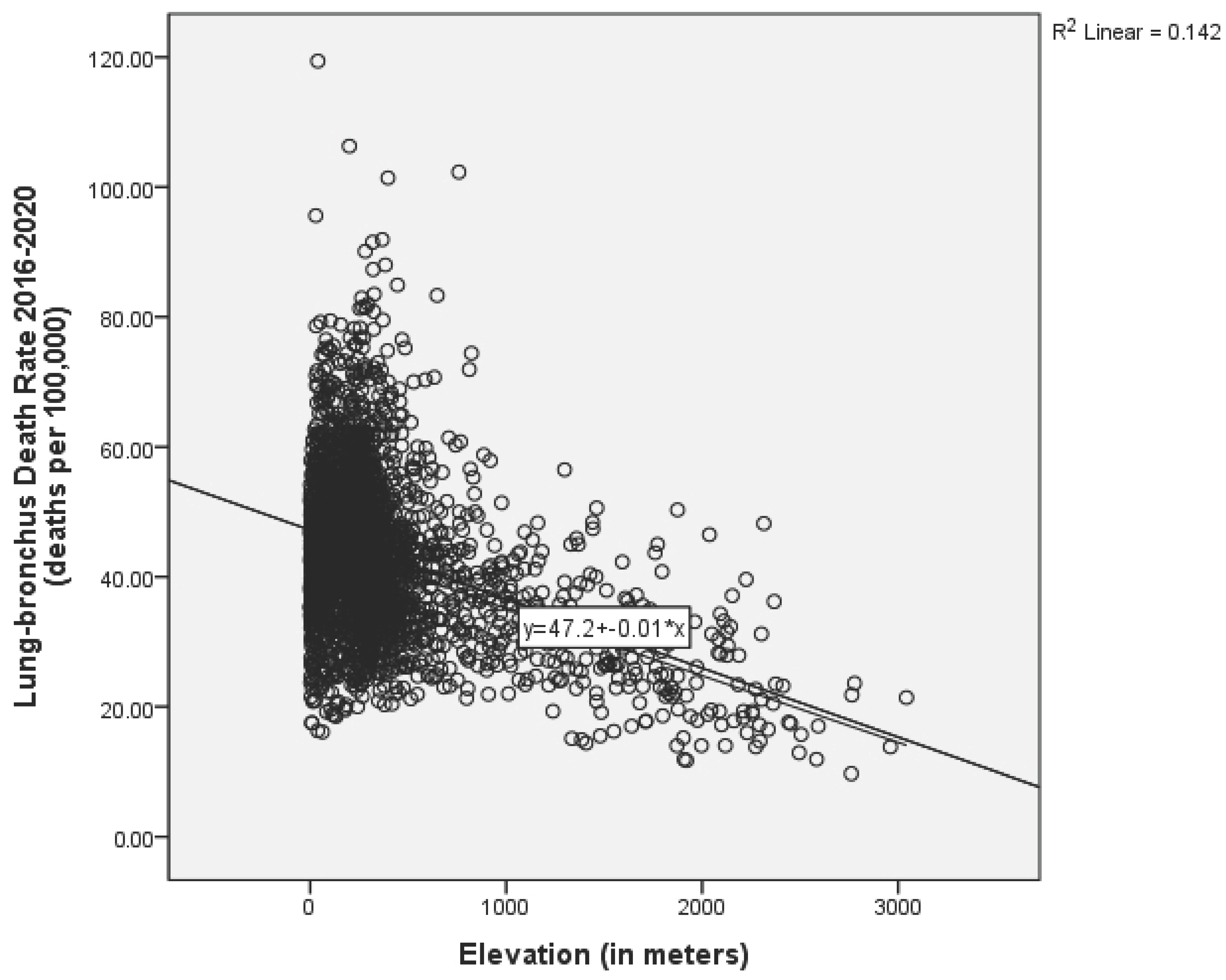
| Elevation | 2662 | 373.160 | 439.119 | −0.382 ** |
| Demographics: | ||||
| % 65 and over | 2662 | 17.248 | 4.109 | 0.108 ** |
| % African American | 2662 | 9.874 | 14.725 | 0.136 ** |
| % Female | 2662 | 50.133 | 1.980 | 0.006 |
| % Rural | 2662 | 53.824 | 29.558 | 0.368 ** |
| Coefficient | S.E | t-Value | p-Value | 95% C.I | VIF | ||
|---|---|---|---|---|---|---|---|
| Model 1—AIC: 13,077.853 | Lower Bound | Upper Bound | |||||
| Constant | 47.093 | 0.290 | 162.550 | 0.000 | 46.525 | 47.661 | |
| Elevation | −0.011 | 0.001 | −21.303 | 0.000 ** | −0.012 | −0.010 | 1.000 |
| Model 2—AIC: 10,962.824 | |||||||
| Constant | 21.553 | 5.634 | 3.822 | 0.000 | 10.485 | 32.580 | |
| Health behaviors: | |||||||
| % adult smoking | 0.793 | 0.077 | 10.284 | 0.000 ** | 0.642 | 0.944 | 3.444 |
| % insufficient sleep | 0.190 | 0.074 | 2.571 | 0.010 ** | 0.045 | 0.335 | 3.742 |
| % adult obesity | 0.087 | 0.060 | 1.457 | 0.145 | −0.030 | 0.205 | 3.131 |
| Food environment index | −1.209 | 0.245 | −4.937 | 0.000 ** | −1.689 | −0.729 | 3.152 |
| % physically inactive | 0.389 | 0.052 | 7.554 | 0.000 ** | 0.288 | 0.490 | 3.486 |
| % alcohol impaired | −0.014 | 0.012 | −0.015 | 0.239 | −0.037 | 0.009 | 1.036 |
| Clinical care: | |||||||
| % uninsured | −0.310 | 0.045 | −6.879 | 0.000 ** | −0.399 | −0.222 | 2.312 |
| Primary care physician ratio | 0.000 | 0.006 | 0.038 | 0.970 | −0.011 | 0.012 | 1.625 |
| Preventable hospital stays | 0.077 | 0.008 | 9.182 | 0.000 ** | 0.061 | 0.094 | 1.879 |
| Social economic environment: | |||||||
| % of college education | −0.046 | 0.026 | 1.791 | 0.073 | −0.096 | 0.004 | 3.539 |
| % unemployment | 0.229 | 0.105 | 0.039 | 0.030 ** | 0.022 | 0.425 | 2.174 |
| 80th percentile income | −6.504 × 10−5 | 0.000 | −4.467 | 0.000 ** | 0.000 | 0.000 | 3.747 |
| Association rate | −0.096 | 0.058 | −2.542 | 0.011 ** | −0.170 | −0.022 | 1.744 |
| Physical environment: | |||||||
| Average daily PM2.5 | 0.503 | 0.127 | 3.945 | 0.000 ** | 0.253 | 0.752 | 1.644 |
| Elevation | −0.006 | 0.000 | −14.398 | 0.000 ** | −0.007 | −0.005 | 1.622 |
| Demographics: | |||||||
| % 65 and over | 0.119 | 0.053 | 2.232 | 0.026 ** | 0.014 | 0.224 | 2.096 |
| % African American | −0.168 | 0.017 | 10.004 | 0.000 ** | −0.201 | −0.135 | 2.669 |
| % Female | −0.013 | 0.088 | −0.145 | 0.885 | −0.185 | 0.159 | 1.312 |
| % Rural | 0.023 | 0.008 | 2.986 | 0.003 ** | 0.008 | 0.037 | 2.172 |
| Coefficient | S.E | t-Value | p-Value | 95% C.I | ||
|---|---|---|---|---|---|---|
| Model 3—AIC: 11,225.379 | Lower Bound | Upper Bound | ||||
| Constant | 35.512 | 9.082 | 3.577 | 0.000 | 16.045 | 54.980 |
| Health behaviors: | ||||||
| % adult smoking | 0.952 | 0.098 | 9.731 | 0.000 ** | 0.760 | 1.144 |
| % insufficient sleep | −0.142 | 0.082 | −1.739 | 0.082 | −0.302 | 0.018 |
| % adult obesity | 0.297 | 0.118 | 2.507 | 0.012 ** | 0.065 | 0.529 |
| Food environment index | −1.287 | 0.405 | −3.175 | 0.002 ** | −2.082 | −0.492 |
| % physically inactive | 0.216 | 0.080 | 2.714 | 0.007 ** | 0.060 | 0.372 |
| % alcohol impaired | 0.060 | 0.019 | 3.209 | 0.001 ** | 0.024 | 0.097 |
| Clinical care: | ||||||
| % uninsured | −0.340 | 0.088 | −3.841 | 0.001 ** | −0.513 | −0.166 |
| Primary care physician ratio | −1.099 × 10−5 | 0.007 | −0.002 | 0.999 | −0.013 | 0.013 |
| Preventable hospital stays | 0.092 | 0.014 | 6.769 | 0.000 ** | 0.065 | 0.118 |
| Social economic environment: | ||||||
| % of college education | −0.015 | 0.038 | −0.386 | 0.699 | −0.089 | 0.060 |
| % unemployment | 0.058 | 0.165 | 0.350 | 0.726 | −0.266 | 0.381 |
| 80th percentile income | −2.010 × 10−5 | 1.793 × 10−5 | −1.121 | 0.262 | −5.526 × 10−5 | 1.506 × 10−5 |
| Association rate | −0.055 | 0.063 | −0.872 | 0.384 | −0.178 | 0.068 |
| Physical environment: | ||||||
| Average daily PM2.5 | 0.450 | 0.147 | 3.055 | 0.002 ** | 0.161 | 0.738 |
| Elevation | −0.004 | 0.001 | −6.106 | 0.000 ** | −0.005 | −0.002 |
| Demographics: | ||||||
| % 65 and over | 0.213 | 0.065 | 3.288 | 0.001 ** | 0.086 | 0.340 |
| % African American | −0.060 | 0.029 | −2.090 | 0.037 ** | −0.117 | −0.004 |
| % Female | −0.393 | 0.208 | −1.890 | 0.059 | −0.800 | 0.015 |
| % Rural | 0.045 | 0.009 | 5.177 | 0.000 ** | 0.028 | 0.062 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Published by MDPI on behalf of the International Society for Photogrammetry and Remote Sensing. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, H. Geographic Distribution of Lung and Bronchus Cancer Mortality and Elevation in the United States: Exploratory Spatial Data Analysis and Spatial Statistics. ISPRS Int. J. Geo-Inf. 2025, 14, 141. https://doi.org/10.3390/ijgi14040141
Ha H. Geographic Distribution of Lung and Bronchus Cancer Mortality and Elevation in the United States: Exploratory Spatial Data Analysis and Spatial Statistics. ISPRS International Journal of Geo-Information. 2025; 14(4):141. https://doi.org/10.3390/ijgi14040141
Chicago/Turabian StyleHa, Hoehun. 2025. "Geographic Distribution of Lung and Bronchus Cancer Mortality and Elevation in the United States: Exploratory Spatial Data Analysis and Spatial Statistics" ISPRS International Journal of Geo-Information 14, no. 4: 141. https://doi.org/10.3390/ijgi14040141
APA StyleHa, H. (2025). Geographic Distribution of Lung and Bronchus Cancer Mortality and Elevation in the United States: Exploratory Spatial Data Analysis and Spatial Statistics. ISPRS International Journal of Geo-Information, 14(4), 141. https://doi.org/10.3390/ijgi14040141




