Abstract
A major goal of green infrastructure (GI) is to provide functional networks of habitats and ecosystems to maintain biodiversity long-term, while at the same time optimizing landscape and ecosystem functions and services to meet human needs. Traditionally, connectivity studies are informed by movement ecology with species-specific attributes of the type and timing of movement (e.g., dispersal, foraging, mating) and movement distances, while spatial environmental data help delineate movement pathways across landscapes. To date, a range of methods and approaches are available that (a) are relevant across any organism and movement type independent of time and space scales, (b) are ready-to-use as standalone freeware or custom GIS implementation, and (c) produce appealing visual outputs that facilitate communication with land managers. However, to enhance the robustness of connectivity assessments and ensure that current trends in connectivity modeling contribute to GI with their full potential, common denominators on which to ground planning and design strategies are required. Likewise, comparable, repeatable connectivity assessments will be needed to put results of these scientific tools into practice for multi-functional GI plans and implementation. In this paper, we discuss use and limitations of state-of-the-art connectivity methods in contributing to GI implementation.
1. Introduction
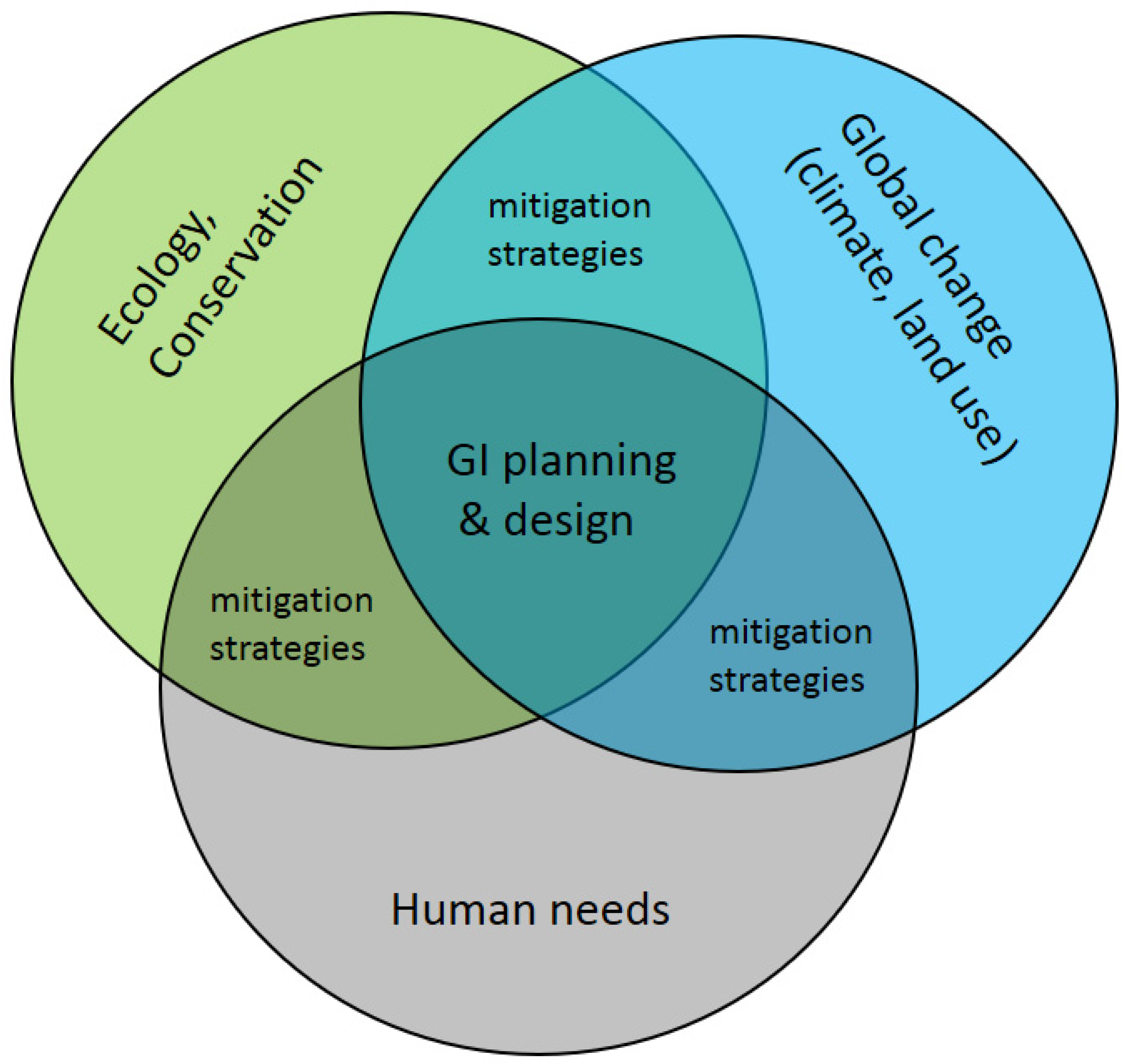
Environments have been reshaped by humans globally and at unprecedented rates during the Anthropocene [1,2,3]. Ecosystems and organisms are increasingly under stress through loss of quality, size and connectivity of habitats. Reduced habitat area is known to limit species biodiversity [4,5,6] and ecosystem services [7,8], while habitat fragmentation limits movement success [9,10], and thus reduces gene flow ([11], but see Luqman et al. [12]). Protected areas alone offer only limited solutions for such risks because, on the one hand, they are not exempt from global change and, on the other hand, protected areas alone may not retain sufficient habitat area or form connected networks sufficient to maintain biodiversity goals in the long term [13,14]. Therefore, integrative approaches of balancing growing conflicts between natural ecosystems and human ecosystems as intended by the conceptual framework of green infrastructure (GI) offer promising directions [15,16] (Figure 1). While GI is not a strictly defined term and has many facets [15,16], it is mainly based on the principle that “protecting and enhancing nature and natural processes […] are consciously integrated into spatial planning and territorial development” [17,18].
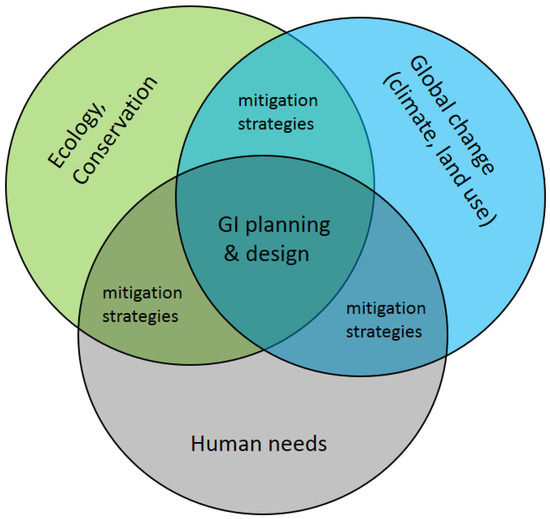
Figure 1.
Global environmental change and human pressure require planning and design of green infrastructure (GI) to ensure long-term persistence of functional connectivity at the landscape scale in human-dominated areas.
Accordingly, GI is defined as a “strategically planned network of natural and semi-natural areas with other environmental features designed and managed to deliver a wide range of ecosystem services” in both rural and urban settings [17]. Strong emphasis is given on ecosystem services with the scope of delivering a range of environmental benefits, including maintenance and improvement of ecological functions. GI elements are important in the context of nature-based solutions, for example to supply ecosystem services that mitigate vulnerability of systems [19,20], or plan for adaptation to extreme weather and a changing climate [21,22,23].
In the context of ecological connectivity, GI provides functional networks of spatially distributed habitats to maintain biodiversity and to optimize ecological functions and services of ecosystems [24] (Figure 1). Successful GIs are thus multi-faceted and spatial to facilitate ecological interactions in human-dominated landscapes [15] by accounting for the viability and life-history attributes of individual species such as their movement potentials. GI inherently relies on spatio-temporal data to understand landscape configuration and dynamics (for example Bartesaghi-Koc et al. [25] considered spatio-temporal patterns in green infrastructure as a driver of land surface temperature variability, and Liu, XL, et al. [26] used spatio-temporal data to assess urbanization processes). As species differ in their requirements and range of movement, corridors for some species may represent barriers for others. While structural connectivity refers to landscape elements that potentially connect habitats based on a landscape’s configuration and compositions, functional connectivity refers to the biotic processes of migration, dispersal, and gene flow, i.e., the ability to exchange individuals or genes across a landscape [27,28]. Thus, functional connectivity is the effective connectivity from a species perspective. Therefore, research on the role of landscape structure in driving functional connectivity under changing pressures such as land use and socio-economic contexts as well as climate change is required [29]. As such, it is key that GI is actively integrated into spatial planning to ensure the long-term persistence of functional connectivity [17]. Furthermore, geospatial approaches can be implemented in decision support tools for GI and spatial planning (as accomplished by Manna et al. [30] in olive landscapes, and by Stessens et al. [31] for urban ecosystem services).
In this paper, we identify opportunities and challenges of state-of-the-art connectivity assessments for GI from the point of view of data, concepts and methodological developments. We do so by first detailing the ways in which connectivity assessments characterize movement. Next, we identify and elaborate trends in quantifying connectivity. From there we discuss planning and design strategies for GI, proposing how connectivity assessments can contribute. That lastly leads to projecting avenues for sustainable GI to ensure persistence of long-term connectivity at the landscape scale to maintain and enhance biodiversity.
2. Characterizing Functional Connectivity
2.1. Movement
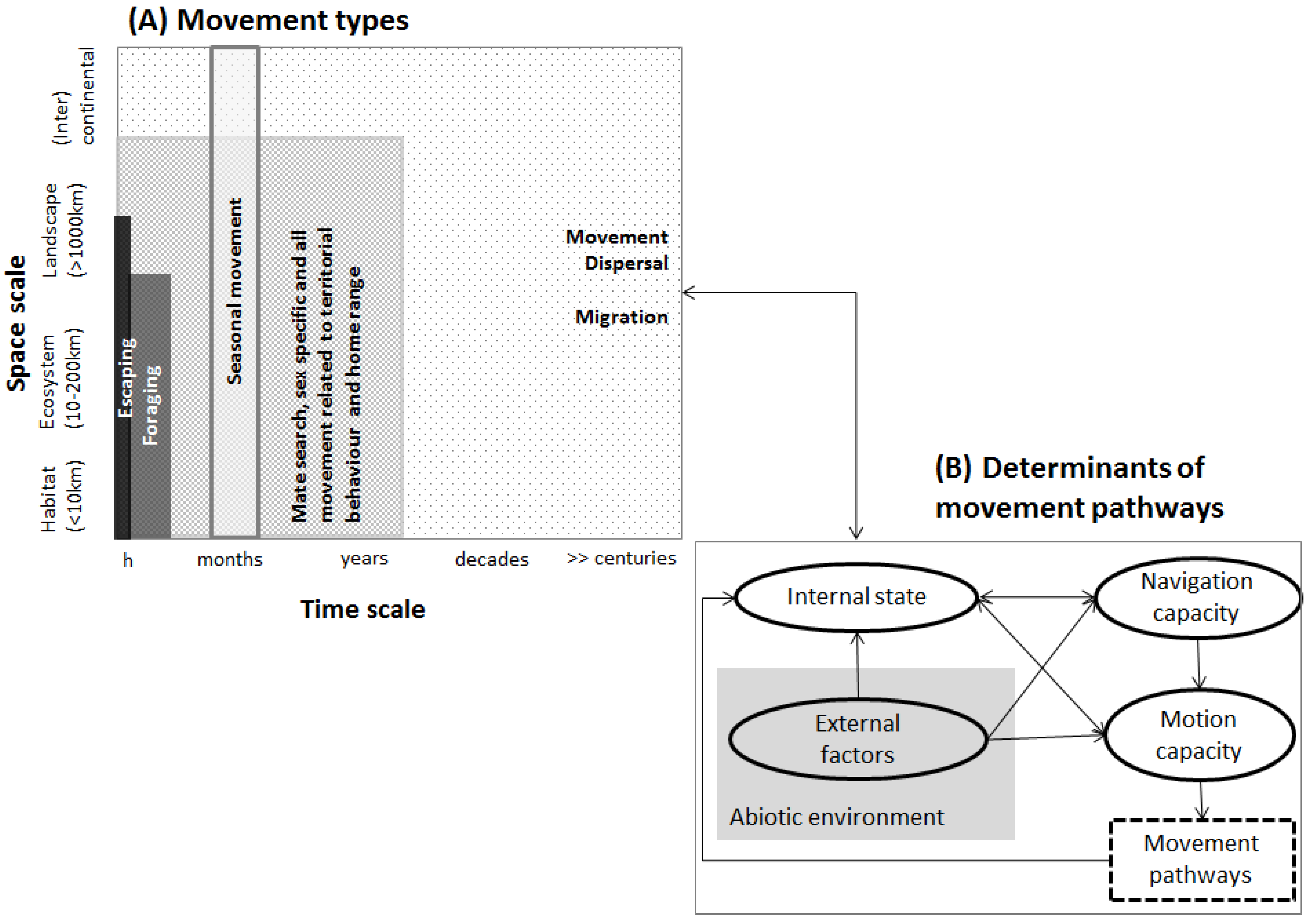
Movement of organisms is the fundamental assumption in connectivity assessments. Movement is common to all animal life and affects a broad range of biological processes, with many consequences ranging from individual organisms to populations or to entire ecosystems. Any movement is the result of a set of complex causes rooted in inter-species (e.g., social behavior, Williams et al., [32]) or in species–environment contexts, or both. Movement can be observed at all temporal scales affecting one or many generations or spatial scales involving single individuals, a range of populations or the spatial distribution of a species (Figure 2A). For example, within-population and shorter-term movement may refer to feeding or marking territories, while between-population movement at broad spatial and long temporal scales ultimately determines genetic exchange (Figure 2A). Clearly, the definition of the different movement types remains a challenge [33], and movement may not always be neatly categorized into one or another movement type (but see LaPoint et al. [34], and McClure et al., [35]). To obtain sound ecological inference of movement, it is therefore key to analyze use and limitations of the underlying assumptions and concepts used as substitutes for undefined movement. While the role of the environment in shaping movement is among the most profoundly researched, e.g., [36,37,38], there is compelling evidence that additional biological information such as population density, local carrying capacity, or endogenous processes such as species-specific life-history attributes (e.g., mating systems, generation time) are important in driving movement pathways [39,40,41]. Connectivity studies therefore (implicitly) challenge questions such as why move? How, where and when to move? What are likely triggers of movement? A framework by Nathan et al. [42] centers around movement itself and refers to three basic components related to the individual (internal state, motion and navigation capacity) and a fourth component referring to environmental factors determining movement pathways (Figure 2B). The organism’s internal state defines the motivation to move (e.g., behavior [43]), while motion capacity mirrors the organism’s overall capacity to move from a biomechanical perspective, and navigation capacities of the organism (e.g., cognitive and sensory capabilities) indicate where to move. From an animal’s point of view, the relative importance of these four components that determine movement varies between organisms but also within an organism’s lifespan, as e.g., changes in fitness, or the environment, predators or competitor abundance. Therefore, “movement” is a complex process whose parameterization in connectivity approaches account for exogenous and endogenous factors that drive movement patterns to various degrees depending on the species and the type of movement considered in the analyses.
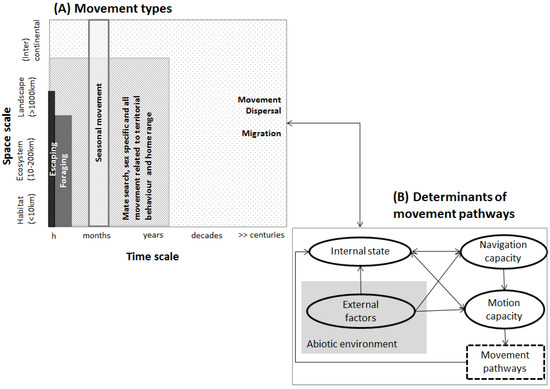
Figure 2.
(A) Movement types and (B) determinants of movement pathways (simplified from Nathan et al. [42]).
2.2. Data, Space and Time Scale for Movement
Connectivity assessments rely on different data sources to assess movement (Table 1). In our exploratory analysis we categorized three data geometries: point, polygon and polyline, that may, depending on the analysis, also be converted to raster data. Points or polygon of species presence and habitat obtained from monitoring programs or surveys are perhaps the simplest type of data used in connectivity studies. Species presence (point) data are traditionally the core of habitat distribution assessments.

Table 1.
Types of descriptor data for movement used in connectivity assessments.
Clearly, suitable habitat (either formatted as continuous potential maps (raster) or polygons or polylines of similar suitability) may play a key role in movement patterns of species as it may attract migrants while at the same time act as a source of outmigration if the habitat becomes densely populated [44]. While Fattebert et al. [45] suggest that habitat suitability may be an appropriate proxy for movement preference during dispersal for some species, there are many pitfalls to using proxies of movement that do not rely on actually measured movement [46]. For example, habitat suitability typically captures just one or two static dimensions of an organism’s ecology (e.g. breeding, roosting) and may therefore not reflect broader-scale or behavioral processes which drive movement [47,48]. Also, dispersing individuals may not require habitat that supports permanent residents [49]. Therefore, the question remains whether or to what degree suitable habitat (or unsuitable habitat [50,51]) may be a determinant of movement. Thus it is prerequisite to place movement results originating from simple presence data or modeled suitable habitat within a behavioral context to ensure the effectiveness and applicability of the analysis results [34,52]. Expert opinions are another data source, generally in the form of polygons, on which to ground movement assessments (Table 1). Experts aggregate locally detailed, often long-term but rather subjective observations on e.g., wildlife corridors or habitat. Also, local knowledge is often difficult for up-scaling to a landscape scale. While some connectivity studies are framed exclusively around expert opinions [53], others apply expert opinions as important supplemental information on movement [11,54], yet others claim that statistical approaches clearly outperform expert knowledge [55,56]. Expert opinions are also used to determine the resistance of land-cover types [57] or to define relations and transition probabilities between habitats [58]. Long-term local knowledge provided by experts are an irreplaceable resource for insight on relevant questions that contribute to solving real-world conservation challenges [59,60], as well as important feedback on the applicability and validity of large-scale connectivity assessments locally [11].
Another type of movement data refers to polyline data as assessed by GPS-tracking or telemetry. Particularly GPS tracking allows for detailed spatial and temporal information on the use of the landscape by individuals in the form of polylines and may add information on the behavior of an animal (Table 1). At the same time, telemetric or GPS-tracking data do not inform on the effects of the observed connectivity for population persistence. The latter is warranted by genetic data, as inferred polylines and spatial clustering (Table 1) that allow direct determination of whether and to which degree gene flow has been realized and how movement has impacted genetic differentiation and contributed to genetic diversity. Gene flow can be analyzed at different temporal depths. First-generation migrants may indicate short-term movement during the most recent season including the direction of exchange [62,63], while genetic assignment tests are indicative of movements in the recent past [64,65]. Genetic differentiation fst, considered a genetic measure indicative of historic processes (i.e., >20 generations), can be used to identify movement paths at greater temporal depths [66]. No temporal depth, but spatially explicit clustering of genetic similarity can be identified [67]. Applied to a connectivity assessment, the information drawn from such clustering algorithms indicates how the genetic composition may vary across a landscape [68]. In addition, many genetic studies normally account for more detailed population metrics such as genetic diversity or population size [63,69,70].
We conclude that there are trade-offs between movement data regarding the information on the movement processes (reproduction versus details on the realized movement pathways across the landscape versus information on the habitat distribution). While genetic data identify whether movement has an effect on reproduction or genetic diversity, and allow temporal differentiation of movement, the data type does not directly inform on the realized movement pathways (polylines) across landscapes. In contrast, GPS tracking or telemetry offer insights into detailed movement pathways of individuals across the landscape with high spatial and temporal resolution. However, the data often represents detailed movement patterns of only a few individuals between populations or individuals without information on reproductive success and gene flow. Observations from monitoring or surveys merely indicate that the species was present at the time of the survey (points) yet can represent a larger sample of the population over a given area and is more frequently collected. Yet this data does not directly reflect on the movement pathways and directions nor on whether the movement leads to reproductive success and is likely the weakest type of data used in connectivity assessments.
For example, Frei et al. [71] showed that monitoring data detected only negligible movements in Natterjack toads, while a combination of tracking, genetic and monitoring data significantly increased the detection rate of movement across the landscape. This shows that the type of movement data and geometry used to assess connectivity dictates the type of movement which can be detected in the analysis. We therefore advocate that, first, movement assessments apply a combination of complementary data sources to provide the most functionally interpretable connectivity assessments. Second, we suggest that the obtained connectivity corridors should be discussed with respect to movement type and geometry [71].
3. Trends in Quantifying Connectivity
Connectivity assessments represent a modeling framework well suited to assess the role of landscape structure in contributing to movement success of organisms and in realizing the goals of GI [72]. Connectivity studies have been applied to a broad range of species and environments ranging from e.g., conservation for individual species [73,74,75], including aquatic invertebrates [76], to multi-species corridors [50,57,77] to socio-ecological studies in the context of GI [78].
The typical workflow of an ecological connectivity assessment uses biotic input data originating either from monitoring programs, experiments, expert knowledge or from tracking approaches. This biotic information is then related to the environment. The environment is represented by spatial data layers whose properties (landscape elements) are assigned values indicating a landscape cost gradient from barrier to conducive corridor for the organisms under study, resulting in a resistance or cost surface. Among approaches, least-cost paths [79] or least-cost surfaces [38,80] can then be calculated to identify potential movement paths for animals across the landscape. Many connectivity assessments assume, but do not necessarily state, that an animal has either complete knowledge of the landscape [81,82] or no spatial memory at all (random walk, McRae et al. [80]).
Clearly, there is evidence that structural landscape elements are a proxy for functional connectivity [45,83]. However, as already highlighted in Section 2, using the landscape as primary driver of the environment may be an oversimplification of the complexity of connectivity. For example, as shown by several examples, landscapes may be intrinsically more permeable than expected for species movement and gene flow [12,41,60,62,71]. Therefore, endogenous processes (physiology, behavior) driving movement could be considered more comprehensively in connectivity studies [84].
There are many methods which can be used to calculate connectivity, ranging from simple pairwise distance calculations, e.g., using Euclidean distance to least-cost paths (LCP) in ArcGIS or Circuitscape [80], a software free to download. Both LCP and Circuitscape assume different resistances of individual landscape elements that facilitate or hinder movement. In spite of the various reviews stressing the fact that resistance surfaces are sensitive to the assigned resistance or cost values [85] and suggestions on how to improve the identification of resistance values [65,86], some studies still rely on arbitrary assignment of resistance values, e.g. [87], while others base resistance values on stakeholder or expert knowledge [68,88]. A few studies assessed resistance values based on (the inverse of) suitable habitat. For example, Bani et al. [89], relied on quantitative assessments, e.g., optimization of resistance surfaces using a nonlinear optimization algorithm to minimize model AIC (Akaike Information Criterion) [48], sensitivity analysis of different competing resistance-value scenarios [90], or experiments to assign resistance values [91]. Due to the general lack of empirical data on the movement of organisms, the calibration of resistance surfaces and the evaluation of connectivity models, or validity, remains a challenge. However, in line with other authors [92], we see two areas that could strengthen connectivity models and better link them to movement ecology and functional connectivity: (1) the calibration of resistance values should rely on different scenarios of conceptually sound criteria and should be subject to sensitivity analyses [65,85,93,94] and (2) connectivity-model output could be subject to an evaluation or validation, (e.g., compare model outputs against empirical data or compare different connectivity-modeling approaches) to increase confidence in model performance [49,51,95]. In addition, performing sensitivity analyses or incorporating uncertainty in parameter estimates are especially important for research that will result in conservation recommendations or conservation action. Presumably, much of the research that seeks to estimate resistance will use the resultant resistance surfaces in connectivity modeling and these connections or corridors will be promoted to planners and land managers for implementation. Presenting the full range of possibilities for proposed actions adds transparency to the process and increases the likelihood of buy-in from land managers and the public alike. However, few papers compare the effectiveness of connectivity methods among each other (e.g. LCP and Circuitscape, [96,97]), or compare modeled corridors to observations [87].
Along with technically improved resistance surfaces, significant conceptual and methodological progress, e.g., causal modeling [75,98], has increased the robustness and reliability of the analyses in various ways e.g., calibrating resistance surfaces [65,85,93,94] or comparing the performance of different connectivity methods and data types [49,51,95]. Advanced modeling methods apply a factorial, multi-model approach to evaluate alternative hypotheses of e.g., resistance surfaces and identifying the combination of environmental factors that statistically best explain, e.g., gene flow in a given landscape [50,73,98,99,100]. Another development includes connectivity simulators which overcome technical challenges such as long computational times or poor algorithmic efficiency [101,102].
4. Planning and Design Strategies for Green Infrastructure: Approaches, Considerations, Tools
Connectivity studies provide important information for protecting or planning conservation areas in spatially dynamic landscape contexts and are logical tools to contribute effectively to GI. First, connectivity research is inherently interdisciplinary or even transdisciplinary. Interdisciplinary approaches combine aspects of e.g., population and movement ecology, landscape genetics, geography and landscape ecology. While population and movement ecology informs the analysis with species-specific attributes of traits and behavior, geography and landscape ecology provide spatial theory and data to derive suitable movement pathways across the landscape (Leibovici and Claramunt [103] generalize landscape ecology theory to an entropy framework, while Perkl et al. [104] use spatial theory and data to assess urban growth and landscape connectivity threats). Some assessments add transdisciplinary approaches to the connectivity analysis by including experts who either calibrate [88,105] or evaluate the modeled inputs or outputs [106,107,108]. Second, outputs of connectivity studies are often visually appealing products which improve communication between scientists and practitioners [109]. For instance the green infrastructure model for the U.S., built by Esri, a GIS software company, in cooperation with the Green Infrastructure Center is accessible to communities and local planning efforts (https://www.esri.com/en-us/industries/green-infrastructure/overview). The output maps of connectivity models allow easy transfer of the scientific results to a non-scientific audience [38,59,110]. Therefore, robust connectivity assessments can have an important role in GI plans. There are divergent perspectives and approaches for considering GI in planning and design. Different regional and cultural contexts as well as disciplinary needs seem to have contributed to the varying conceptualizations of GI implementation. From the U.S. for example, stem two different branches of thinking. On the one hand, there is a broad scale mapping and modeling effort rooted in landscape and conservation ecology, applying landscape fragmentation and biodiversity metrics as well as connectivity models to design, evaluate, and prioritize GI plans [20,111,112]. This conceptualization emphasizes mapping green corridors, habitat cores, and biodiversity indices, and is supported in part by Esri and the Green Infrastructure Center [113]; http://www.gicinc.org/index.htm). The maps bring together regional to national datasets compiled from a multi-agency approach, while the available web-based GIS tools allow regional planners and stakeholders to prioritize areas for GI (https://www.esri.com/en-us/industries/green-infrastructure/overview). On the other hand, municipalities and urban planners consider GI in plans to build resiliency, particularly in terms of storm water and flood mitigation. In this case, GI is thought of less in terms of habitat and connectivity, and more as an alternative or supplement to grey infrastructure, with parcel level accountability tools and assessments for local governments [114,115,116]. Some have started to compare these divergent considerations and the feasibility for a GI plan to meet both goals [117]. In an application of GI planning for the city of Detroit, for example, Meerow and Newell [118] concluded that trade-offs will have to be made between siting to maximize stormwater management versus landscape connectivity goals. Zhang et al. [111] explicitly applied three site level design typologies to the same study.
Likewise, European models struggle with multifunctionality of GI. Szulczewska et al. [117], in fact, describe these varying conceptualizations as (1) structural, (2) hydrological, and (3) integrated. The European Union Biodiversity Strategy (GI, Nature 2000) emphasizes ecological networks, ecosystem services, and the need for spatial planning [119,120]. Yet many argue for approaches to better reconcile regional vision with local implementation [121]. In a pan-European study, Liquete et al. [122] applied an approach to (1) quantify and map natural capacity for ecosystem services, and (2) identify core habitats and wildlife corridors. Several authors propose conceptual approaches or frameworks to consider multifunctional criteria in GI [29,120,123]. Typically these include criteria such as: conservation value, natural value, recreation value, culture, and/or some aspect of human well-being [119], and also recognize urban needs [123].
A few authors explicitly address connectivity modeling and design for GI. For instance, Liu et al. [124] used circuit theory to map the landscape connectivity of urban GI in the city of Nanjing, China. Likewise, Perkl [112] integrated landscape linkages into a geodesign process for wildlife corridors, and Perkl et al. [104] assessed the threat of urban growth to landscape connectivity. While in Detroit, U.S., Zhang et al. [111] applied a LCP approach to map potential green corridors linking city parks through vacant parcels and small green spaces as a way of planning urban GI networks.
5. Avenues Towards Connectivity Assessments for Sustainable Future Green Infrastructures
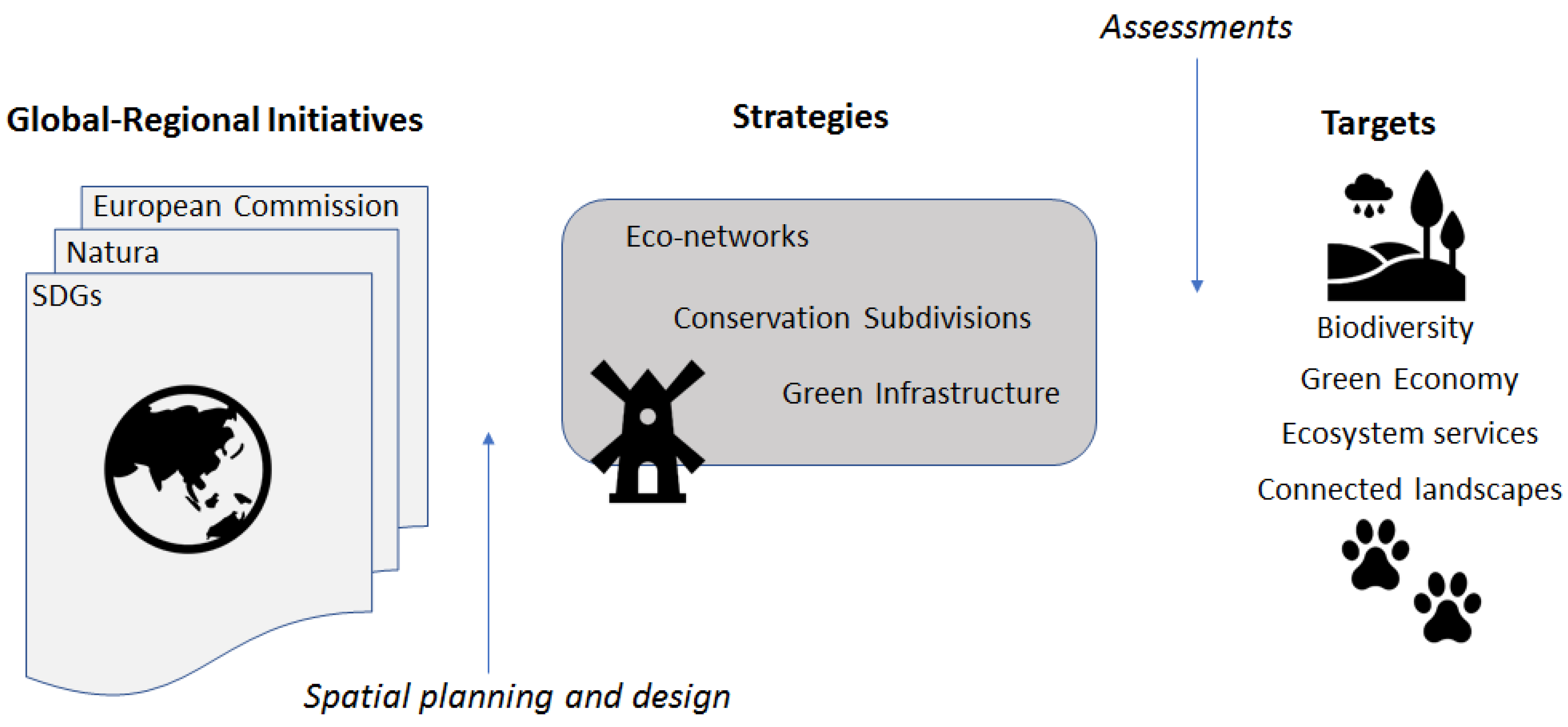
In 2012, the United Nations Conference on Sustainable Development Rio+20 came up with 17 sustainable development goals (SDGs) that were defined in the 2030 Agenda for Sustainable Development [125]. Biodiversity and ecosystems, as well as the green economy are key topics of the global SDGs. The goals address environmental challenges and opportunities in a world dominated by humans in reference to ecology, poverty, education, gender equality and city development. The sustainability debate encourages regions to develop and implement scenarios for desirable futures that are actively shaped by plans and policy [125,126,127]. A desirable future for GI thus not only focuses on the persistence of biodiversity, but also on how functional GI can support the environmental, socio-cultural and economic SDG (Figure 3).
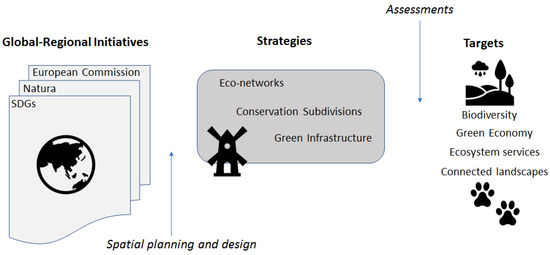
Figure 3.
Conceptual relationship between broad scale international initiatives to achieve goals in human–environment targets (e.g. connected landscapes) through various landscape-based strategies. Spatial planning and design produce plans for these strategies, including green infrastructure, while various assessments, including connectivity models, inform and evaluate the strategies in meeting targets. SDGs = global sustainable development goals.
This paper highlights that connectivity assessments are valuable contributions for GI as they aim at understanding species movement in a spatially explicit context, and the data-driven approach allows users to verify hypotheses of connectivity across landscapes. Yet, to promote their use for robust reliability of GI applications, connectivity models could profit from addressing the following challenges: we suggest reflecting on the drivers of the modeled connectivity pathways with respect to movement types and geometries used to identify movement and endogenous properties of the organisms under study. Studies that focus primarily on landscape/environmental structure as the primary driver of movement could benefit from existing movement frameworks that consider the relative importance of exogenous versus endogenous drivers [42]. Also, we encourage transparency and interpretation of the modeled connectivity pathways with respect to use and limitations of the considered movement data. A promising approach to explore use, limitation, and synergies between different data types and connectivity assessments ideally relies on a combination of complementary data sources [71]. As stressed by other authors [65,85,93,94], assessments on connectivity model robustness and sensitivity towards model parameterization require further in-depth exploration. Utilizing recommended statistical techniques that most fully tease apart alternative mechanisms can also help to improve connectivity approaches e.g., [128].
We also see several ways in which connectivity could advance with the use of additional data and tools:
- (1)
- Connectivity assessments would profit from continuous 3D landscape data. The past decade has seen remote sensing advance, and three-dimensional (3D) landscape structure is now available [129,130], UAV (unmanned aerial vehicles) and active remote sensors e.g., LiDAR, a laser detection system [131,132]. Compelling empirical evidence suggests that connectivity assessments could be supplemented with continuous 3D vegetation structure to provide functionally relevant landscape features in connectivity assessments.
- (2)
- Connectivity assessments could account for dynamic environments as static approaches are only of limited use for decision-makers, given expected significant environmental change in the future [110,133,134,135]. Accounting for dynamic processes such as urbanization and providing projections of future connectivity assessments in a changing environment may be of great value for future GI planning.
- (3)
- Individual-based models may supplement least-cost and cost-distance analyses. Individual-based approaches may help to tackle the complexity of animal movement whose behavioral and navigational ability are strong drivers of the realized movement pathways [136,137].
- (4)
- Multi-species connectivity could be important for conservation management. While most connectivity analyses focus on a single species, conservation planners with limited resources could benefit greatly from models that predict the movement patterns of multiple species [59] as they are more efficient for conservation and restoration [138].
- (5)
- Finally, we see that spatial planning and design for GI is still challenged by how to integrate multiple demands and reconcile the trade-offs between functions that GI serves in natural and human systems. Planners and designers could do well to converge on a more unified perspective and approach to integrate multifunctional goals to the extent possible, and to use tools to measure the performance and trade-offs of different spatial plans in meeting those goals. In fact, geodesign processes are rapidly advancing to meet these needs [139]. Municipalities and regional governing boards could then use these decision-support tools to move toward GI implementation (as in Stessens et al. [31].
6. Conclusions
In recent decades several planning concepts centered on maintaining connected green spaces and corridors have emerged, including ecological networks, conservation subdivisions, and green infrastructure, among others [117] (Figure 3). The focus of most attention recently, green infrastructure comprises the natural assets (parks, streams, lakes, forests, and rivers) that form a system of interconnected environmental features to sustain ecological processes and provide ecosystem services [113]. Yet how will planners and designers know which spatial plans best capture the ecological processes and ecosystem services of interest?
Connectivity assessments are among the most popular scientific tools for optimizing corridor design. Overall, connectivity assessments are valuable contributions to understand species movement in a spatially explicit context as the data-driven approach allows verification of hypotheses of organismic movement across landscapes. However, conceptual (e.g., movement ecology aspects) and methodological shortcomings (e.g., evaluation of resistance values and model outputs) currently limit ecological inference and interpretation of the method’s outputs. We argue that connectivity studies need to explicitly situate themselves in (1) a framework to identify what movement is referred to with respect to the applied movement data and geometry, and where possible, include endogenous information to supplement simple environmentally driven connectivity assessments; (2) the space and time scale that can be considered with the applied movement data to effectively delineate connectivity relevant for conservation applications; (3) connectivity studies, that require empirically-informed resistance surfaces, including: (4) sensitivity analyses and/or evaluation measures to test the validity of the method’s results. Given the rapid developments in remote sensing, applicability of the approach can be broadened with continuous 3D landscape data, behavioral aspects and multi-species assessments that will improve the functionality of connectivity assessments for conservation application.
The green infrastructure approach envisions scientists, decision makers and the public collaborating to preserve and link open spaces, watersheds, wildlife, habitats, parks, and other natural areas that enrich and sustain a community’s quality of life, economy, and sense of place [113]. Yet, while green infrastructure has attracted great interest among scholars, practitioners and decision-makers, the scope of approaches has varied, and implementation has been limited. With improved and consistent methodology, connectivity assessments could help move GI decisions to the fore.
Author Contributions
Janine Bolliger and Janet Silbernagel contributed equally to the manuscript conceptualization, visualization, writing—original draft preparation, and visualization writing—review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors’ collaboration on this paper was supported by a research fellowship from the Swiss Federal Institute for Forest, Snow, and Landscape Research (WSL) to J. Silbernagel, hosted by F. Kienast at WSL. Four reviewers provided important suggestions on an earlier version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Otto, S.P. Adaptation, speciation and extinction in the Anthropocene. Proc. R. Soc. B Biol. Sci. 2018, 285, 20182047. [Google Scholar] [CrossRef] [PubMed]
- Steffen, W.; Broadgate, W.; Deutsch, L.; Gaffney, O.; Ludwig, C. The trajectory of the Anthropocene: The Great Acceleration. Anthr. Rev. 2015, 2, 81–98. [Google Scholar] [CrossRef]
- Irwin, A. The dark side of light. Nature 2018, 553, 268. [Google Scholar] [CrossRef] [PubMed]
- Krauss, J.; Bommarco, R.; Guardiola, M.; Heikkinen, R.K.; Helm, A.; Kuussaari, M.; Lindborg, R.; Öckinger, E.; Pärtel, M.; Pino, J.; et al. Habitat fragmentation causes immediate and time-delayed biodiversity loss at different trophic levels. Ecol. Lett. 2010, 12, 597–605. [Google Scholar]
- Farneda, F.Z.; Grelle, C.E.V.; Rocha, R.; Ferreira, D.F.; Lopez-Baucells, A.; Meyer, C.F.J. Predicting biodiversity loss in island and countryside ecosystems through the lens of taxonomic and functional biogeography. Ecography 2019. [Google Scholar] [CrossRef]
- Fletcher, R.; Didham, R.; Banks-Leite, C.; Barlow, J.; Ewers, R.M.; Rosindell, J.; Holt, R.D.; Gonzalez, A.; Pardini, R.; Damschen, E.I.; et al. Is habitat fragmentation good for biodiversity? Biol. Conserv. 2018, 226, 9–15. [Google Scholar] [CrossRef]
- Verburg, R.W.; Osseweijer, F. A framework to estimate biodiversity loss and associated costs due to nitrogen emissions from single power plants. J. Clean. Prod. 2019, 239. [Google Scholar] [CrossRef]
- Sauter, I.; Kienast, F.; Bolliger, J.; Winter, B.; Pazur, R. Changes in demand and supply of ecosystem services under scenarios of future land use in Vorarlberg, Austria. J. Mt. Sci. 2019, in press. [Google Scholar] [CrossRef]
- Fahrig, L. Ecological Responses to Habitat Fragmentation Per Se. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Fahrig, L.; Arroyo-Rodríguez, V.; Bennett, J.; Boucher-Lalonde, V.; Cazetta, E.; Currie, D.J.; Eigenbrod, F.; Ford, A.T.; Harrison, S.P.; Jaeger, J.A.G.; et al. Is habitat fragmentation bad for biodiversity? Biol. Conserv. 2019, 230, 179–186. [Google Scholar] [CrossRef]
- Burkart, S.; Gugerli, F.; Senn, J.; Kuehn, R.; Bolliger, J. Evaluating the functionality of expert-assessed wildlife corridors with genetic data: Setting priorities for management measures in roe deer (Capreolus capreolus). Basic Appl. Ecol. 2016, 17, 52–60. [Google Scholar] [CrossRef]
- Luqman, H.; Muller, R.; Vaupel, A.; Brodbeck, S.; Bolliger, J.; Gugerli, F. No distinct barrier effect of highways and wide river on genetic structure of the Alpine newt (Ichthyosaura alpestris) in densely settled landscapes. Conserv. Genet. 2018, 19, 673–685. [Google Scholar] [CrossRef]
- Bolliger, J.; Edwards, T.C.; Eggenberg, S.; Ismail, S.; Seidl, I.; Kienast, F. Balancing forest-regeneration probabilities and maintenance costs in dry grassland meadows of high conservation priority. Conserv. Biol. 2011, 25, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.B.; Cabeza, M.; Thuiller, W.; Hannah, L.; Williams, P.H. Would climate change drive species out of reserves? An assessment of existing reserve-selection methods. Glob. Chang. Biol. 2004, 10, 1618–1626. [Google Scholar] [CrossRef]
- Wang, J.X.; Banzhaf, E. Towards a better understanding of Green Infrastructure: A critical review. Ecol. Indic. 2018, 85, 758–772. [Google Scholar] [CrossRef]
- Snäll, T.; Lehtomäki, J.; Arponen, A.; Elith, J.; Moilanen, A. Green infrastructure design based on spatial conservation prioritization and modeling of biodiversity features and ecosystem services. Environ. Manag. 2016, 57, 251–256. [Google Scholar]
- European Commission. Green Infrastructure (GI)—Enhancing Europe’s Natural Capital; EEA: Brussels, Belgium, 2013; p. 149. [Google Scholar]
- EEA. What Is Green Infrastructure? Available online: https://www.eea.europa.eu/themes/sustainability-transitions/urban-environment/urban-green-infrastructure/what-is-green-infrastructure (accessed on 27 March 2020).
- Privitera, R.; La Rosa, D. Reducing Seismic Vulnerability and Energy Demand of Cities through Green Infrastructure. Sustainability 2018, 10. [Google Scholar] [CrossRef]
- Lanzas, M.; Hermoso, V.; de-Miguel, S.; Bota, G.; Brotons, L. Designing a network of green infrastructure to enhance the conservation value of protected areas and maintain ecosystem services. Sci. Total Environ. 2019, 651, 541–550. [Google Scholar] [CrossRef]
- Brink, E.; Aalders, T.; Adam, D.; Feller, R.; Henselek, Y.; Hoffmann, A.; Ibe, K.; Matthey-Doret, A.; Meyer, M.; Negrut, N.L.; et al. Cascades of green: A review of ecosystem-based adaptation in urban areas. Glob. Environ. Chang. Hum. Policy Dimens. 2016, 36, 111–123. [Google Scholar] [CrossRef]
- Derkzen, M.L.; van Teeffelen, A.J.A.; Verburg, P.H. Green infrastructure for urban climate adaptation: How do residents’ views on climate impacts and green infrastructure shape adaptation preferences? Landsc. Urban Plan. 2017, 157, 106–130. [Google Scholar] [CrossRef]
- Demuzere, M.; Orru, K.; Heidrich, O.; Olazabal, E.; Geneletti, D.; Orru, H.; Bhave, A.G.; Mittal, N.; Feliu, E.; Faehnle, M. Mitigating and adapting to climate change: Multi-functional and multi-scale assessment of green urban infrastructure. J. Environ. Manag. 2014, 146, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Carlier, J.; Moran, J.; Aughney, T.; Roche, N. Effects of greenway development on functional connectivity for bats. Glob. Ecol. Conserv. 2019, 18, e00613. [Google Scholar] [CrossRef]
- Bartesaghi-Koc, C.; Osmond, P.; Peters, A. Spatio-temporal patterns in green infrastructure as driver of land surface temperature variability: The case of Sydney. Int. J. Appl. Earth Obs. Geoinf. 2019, 83. [Google Scholar] [CrossRef]
- Liu, X.L.; Wang, Y.; Li, Y.; Wu, J.S. Quantifying the Spatio-Temporal Process of Township Urbanization: A Large-Scale Data-Driven Approach. Isprs Int. J. Geo-Inf. 2019, 8. [Google Scholar] [CrossRef]
- Tischendorf, L.; Fahrig, L. On the usage and measurement of landscape connectivity. Oikos 2001, 90, 7–19. [Google Scholar] [CrossRef]
- Tischendorf, L. Can landscape indices predict ecological processes consistently? Landsc. Ecol. 2001, 16, 235–254. [Google Scholar] [CrossRef]
- Hrdalo, I.; Tomić, D.; Pereković, P. Implementation of Green Infrastructure principles in Dubrovnik, Croatia to minimize cimate change problems. Urbani Izziv 2015, 26, S38–S49. [Google Scholar] [CrossRef]
- Manna, P.; Bonfante, A.; Colandrea, M.; Di Vaio, C.; Langella, G.; Marotta, L.; Mileti, F.A.; Minieri, L.; Terribile, F.; Vingiani, S.; et al. A geospatial decision support system to assist olive growing at the landscape scale. Comput. Electron. Agric. 2020, 168. [Google Scholar] [CrossRef]
- Stessens, P.; Khan, A.Z.; Huysmans, M.; Canters, F. Analysing urban green space accessibility and quality: A GIS-based model as spatial decision support for urban ecosystem services in Brussels. Ecosyst. Serv. 2017, 28, 328–340. [Google Scholar] [CrossRef]
- Williams, A.E.; Worsley-Tonks, K.E.L.; Ezenwa, V.O. Drivers and consequences of variation in individual social connectivity. Anim. Behav. 2017, 133, 1–9. [Google Scholar] [CrossRef]
- Holyoak, M.; Casagrandi, R.; Nathan, R.; Revilla, E.; Spiegel, O. Trends and missing parts in the study of movement ecology. Proc. Natl. Acad. Sci. USA 2008, 105, 19060–19065. [Google Scholar] [CrossRef] [PubMed]
- LaPoint, S.; Gallery, P.; Wikelski, M.; Kays, R. Animal behavior, cost-based corridor models, and real corridors. Landsc. Ecol. 2013, 28, 1615–1630. [Google Scholar] [CrossRef]
- McClure, M.L.; Hansen, A.J.; Inman, R.M. Connecting models to movements: Testing connectivity model predictions against empirical migration and dispersal data. Landsc. Ecol. 2016, 31, 1419–1432. [Google Scholar] [CrossRef]
- Jaquiéry, J.; Broquet, T.; Hirzel, A.; Yearsley, J.; Perrin, N. Inferring landscape effects on dispersal from genetic distances: How far can we go? Mol. Ecol. 2011, 29, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Lee-Yaw, J.A.; Davidson, A.; McRae, B.H.; Green, D.M. Do landscape processes predict phylogeographic patterns in the wood frog? Mol. Ecol. 2009, 18, 1863–1874. [Google Scholar] [CrossRef]
- Dickson, B.G.; Albano, C.M.; Anantharaman, R.; Beier, P.; Fargione, J.; Graves, T.A.; Gray, M.E.; Hall, K.R.; Lawler, J.J.; Leonard, P.B.; et al. Circuit-theory applications to connectivity science and conservation. Conserv. Biol. 2019, 33, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.W.; Brown, W.S.; Stechert, R.; Zamudio, K.R. Integrating individual behaviour and landscape genetics: The population structure of timber rattlesnake hibernacula. Mol. Ecol. 2008, 17, 719–730. [Google Scholar] [CrossRef]
- Andreasen, A.M.; Stewart, K.M.; Longland, W.S.; Beckmann, J.P.; Forister, M.L. Identification of source-sink dynamics in mountain lions of the Great Basin. Mol. Ecol. 2012, 21, 5689–5701. [Google Scholar] [CrossRef]
- Reding, D.M.; Cushman, S.A.; Gosselink, T.E.; Clark, W.R. Linking movement behavior and fine-scale genetic structure to model landscape connectivity for bobcats (Lynx rufus). Landsc. Ecol. 2013, 28, 471–486. [Google Scholar] [CrossRef]
- Nathan, R.; Getz, W.M.; Revilla, E.; Holyoak, M.; Kadmon, R.; Saltz, D.; Smouse, P.E. A movement ecology paradigm for unifying organismal movement research. Proc. Natl. Acad. Sci. USA 2008, 105, 19052–19059. [Google Scholar] [CrossRef]
- Cushman, S.A.; Lewis, J.S. Movement behavior explains genetic differentiation in American black bears. Landsc. Ecol. 2010, 25, 1613–1625. [Google Scholar] [CrossRef]
- Fletcher, R.; Young, J.; Hutto, R.; Noson, A.; Rota, C. Insights from ecological theory on temporal dynamics and species distribution modeling. In Predictive Species and Habitat Modeling in Landscape Ecology: Concepts and Applications; Drew, A., Wiersma, Y., Huettmann, F., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Fattebert, J.; Robinson, H.S.; Balme, G.; Slotow, R.; Hunter, L. Structural habitat predicts functional dispersal habitat of a large carnivore: How leopards change spots. Ecol. Appl. 2015, 25, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Baguette, M.; Van Dyck, H. Landscape connectivity and animal behavior: Functional grain as a key determinant for dispersal. Landsc. Ecol. 2007, 22, 1117–1129. [Google Scholar] [CrossRef]
- Nixon, K.; Silbernagel, J.; Price, J.; Miller, N.; Swaty, R. Habitat availability for multiple avian species under modeled alternative conservation scenarios in the Two Hearted River watershed in Michigan, USA. J. Nat. Conserv. 2014, 22, 302–317. [Google Scholar] [CrossRef]
- Peterman, W.E.; Connette, G.M.; Semlitsch, R.D.; Eggert, L.S. Ecological resistance surfaces predict fine-scale genetic differentiation in a terrestrial woodland salamander. Mol. Ecol. 2014, 23, 2402–2413. [Google Scholar] [CrossRef]
- Jackson, C.R.; Marnewick, K.; Lindsey, P.A.; Roskaft, E.; Robertson, M.P. Evaluating habitat connectivity methodologies: A case study with endangered African wild dogs in South Africa. Landsc. Ecol. 2016, 31, 1433–1447. [Google Scholar] [CrossRef]
- Brodie, J.F.; Giordano, A.J.; Dickson, B.G.; Hebblewhite, M.; Bernard, H.; Mohd-Azlan, J.; Anderson, J.; Ambu, L. Evaluating multispecies landscape connectivity in a threatened tropical mammal community. Conserv. Biol. 2015, 29, 122–132. [Google Scholar] [CrossRef]
- Bond, M.L.; Bradley, C.M.; Kiffner, C.; Morrison, T.A.; Lee, D.E. A multi-method approach to delineate and validate migratory corridors. Landsc. Ecol. 2017, 32, 1705–1721. [Google Scholar] [CrossRef]
- Abrahms, B.; DiPietro, D.; Graffis, A.; Hollander, A. Managing biodiversity under climate change: Challenges, frameworks, and tools for adaptation. Biodivers. Conserv. 2017, 26, 2277–2293. [Google Scholar] [CrossRef]
- Lechner, A.M.; Doerr, V.; Harris, R.M.B.; Doerr, E.; Lefroy, E.C. A framework for incorporating fine-scale dispersal behaviour into biodiversity conservation planning. Landsc. Urban Plan. 2015, 141, 11–23. [Google Scholar] [CrossRef]
- Reed, G.C.; Litvaitis, J.A.; Callahan, C.; Carroll, R.P.; Litvaitis, M.K.; Broman, D.J.A. Modeling landscape connectivity for bobcats using expert-opinion and empirically derived models: How well do they work? Anim. Conserv. 2017, 20, 308–320. [Google Scholar] [CrossRef]
- Charney, N.D. Evaluating expert opinion and spatial scale in an amphibian model. Ecol. Model. 2012, 242, 37–45. [Google Scholar] [CrossRef]
- Milanesi, P.; Holderegger, R.; Caniglia, R.; Fabbri, E.; Galaverni, M.; Randi, E. Expert-based versus habitat-suitability models to develop resistance surfaces in landscape genetics. Oecologia 2017, 183, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Koen, E.L.; Bowman, J.; Sadowski, C.; Walpole, A.A. Landscape connectivity for wildlife: Development and validation of multispecies linkage maps. Methods Ecol. Evol. 2014, 5, 626–633. [Google Scholar] [CrossRef]
- Schultz, A.; Klenke, R.; Lutze, G.; Voss, M.; Wieland, R.; Wilkening, B. Habitat models as tools for situation evaluation and planning dupport in agricultural landscapes. In Landscape Theory and Resource Management: Linking Theory with Practice; Bissonette, J., Storch, I., Eds.; Island Press: Washington, DC, USA, 2003; pp. 261–282. [Google Scholar]
- Keller, D.; Holderegger, R.; Van Strien, M.J.; Bolliger, J. How to make landscape genetics beneficial for conservation management? Conserv. Genet. 2015, 16, 503–512. [Google Scholar] [CrossRef]
- Van Strien, M.J.; Keller, D.; Holderegger, R.; Ghazoul, J.; Kienast, F.; Bolliger, J. Landscape genetics as a tool for conservation planning: Predicting the effects of landscape change on gene flow. Ecol. Appl. 2014, 24, 327–339. [Google Scholar] [CrossRef]
- Bolliger, J.; Keller, D.; Holderegger, R. When landscape variables do not explain migration rates: An example from an endangered dragonfly (Leucorrhinia caudalis). Eur. J. Entomol. 2011, 108, 327–330. [Google Scholar] [CrossRef]
- Le Lay, G.; Angelone, S.; Flory, C.; Holderegger, R.; Bolliger, J. Increasing pond density to maintain a patchy habitat network of the European tree frog (Hyla arborea). J. Herpetol. 2015, 49, 217–221. [Google Scholar] [CrossRef]
- Pfeiffer, V.; Silbernagel, J.; Guédot, C.; Zalapa, J. Woodland and floral richness boost bumble bee density in cranberry resource pulse landscapes. Landsc. Ecol. 2019, 34, 979–996. [Google Scholar] [CrossRef]
- Row, J.R.; Blouin-Demers, G.; Lougheed, S.C. Habitat distribution influences dispersal and fine-scale genetic population structure of eastern foxsnakes (Mintonius gloydi) across a fragmented landscape. Mol. Ecol. 2010, 19, 5157–5171. [Google Scholar] [CrossRef] [PubMed]
- Zeller, K.A.; McGarigal, K.; Whiteley, A.R. Estimating landscape resistance to movement: A review. Landsc. Ecol. 2012, 27, 777–797. [Google Scholar] [CrossRef]
- Yumnam, B.; Jhala, Y.V.; Qureshi, Q.; Maldonado, J.E.; Gopal, R.; Saini, S.; Srinivas, Y.; Fleischer, R.C. Prioritizing tiger conservation through landscape genetics and habitat linkages. PLoS ONE 2014, 9, e111207. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [PubMed]
- Gryseels, S.; de Bellocq, J.G.; Makundi, R.; Vanmechelen, K.; Broeckhove, J.; Mazoch, V.; Sumbera, R.; Zima, J.; Leirs, H.; Baird, S.J.E. Genetic distinction between contiguous urban and rural multimammate mice in Tanzania despite gene flow. J. Evol. Biol. 2016, 29, 1952–1967. [Google Scholar] [CrossRef]
- Harrisson, K.A.; Pavlova, A.; Amos, J.N.; Radford, J.Q.; Sunnucks, P. Does reduced mobility through fragmented landscapes explain patch extinction patterns for three honeyeaters? J. Anim. Ecol. 2014, 83, 616–627. [Google Scholar] [CrossRef]
- Harrisson, K.A.; Pavlova, A.; Amos, J.N.; Takeuchi, N.; Lill, A.; Radford, J.Q.; Sunnucks, P. Disrupted fine-scale population processes in fragmented landscapes despite large-scale genetic connectivity for a widespread and common cooperative breeder: The superb fairy-wren (Malurus cyaneus). J. Anim. Ecol. 2013, 82, 322–333. [Google Scholar] [CrossRef]
- Frei, M.; Csencsics, C.; Brodbeck, S.; Schweizer, E.; Bühler, C.; Gugerli, F.; Bolliger, J. Combining landscape genetics, radio-tracking and long-term monitoring to derive management implications for Natterjack toads (Epidalea calamita) in agricultural landscapes. J. Nat. Conserv. 2016, 32, 22–34. [Google Scholar] [CrossRef]
- Naidoo, R.; Kilian, J.W.; Du Preez, P.; Beytell, P.; Aschenborn, O.; Taylor, R.D.; Stuart-Hill, G. Evaluating the effectiveness of local- and regional-scale wildlife corridors using quantitative metrics of functional connectivity. Biol. Conserv. 2018, 217, 96–103. [Google Scholar] [CrossRef]
- Wasserman, T.N.; Cushman, S.A.; Schwartz, M.K.; Wallin, D.O. Spatial scaling and multi-model inference in landscape genetics: Martes americana in northern Idaho. Landsc. Ecol. 2010, 25, 1601–1612. [Google Scholar] [CrossRef]
- Squires, J.R.; DeCesare, N.J.; Olson, L.E.; Kolbe, J.A.; Hebblewhite, M.; Parks, S.A. Combining resource selection and movement behavior to predict corridors for Canada lynx at their southern range periphery. Biol. Conserv. 2013, 157, 187–195. [Google Scholar] [CrossRef]
- Parks, L.C.; Wallin, D.O.; Cushman, S.A.; McRae, B.H. Landscape-level analysis of mountain goat population connectivity in Washington and southern British Columbia. Conserv. Genet. 2015, 16, 1195–1207. [Google Scholar] [CrossRef]
- Moran-Ordonez, A.; Pavlova, A.; Pinder, A.M.; Sim, L.; Sunnucks, P.; Thompson, R.M.; Davis, J. Aquatic communities in arid landscapes: Local conditions, dispersal traits and landscape configuration determine local biodiversity. Divers. Distrib. 2015, 21, 1230–1241. [Google Scholar] [CrossRef]
- Bleyhl, B.; Baumann, M.; Griffiths, P.; Heidelberg, A.; Manvelyan, K.; Radeloff, V.C.; Zazanashvili, N.; Kuemmerle, T. Assessing landscape connectivity for large mammals in the Caucasus using Landsat 8 seasonal image composites. Remote Sens. Environ. 2017, 193, 193–203. [Google Scholar] [CrossRef]
- Xiu, N.; Ignatieva, M.; van den Bosch, C.K.; Chai, Y.Y.; Wang, F.; Cui, T.F.; Yang, F.P. A socio-ecological perspective of urban green networks: The Stockholm case. Urban Ecosyst. 2017, 20, 729–742. [Google Scholar] [CrossRef]
- Adriaensen, F.; Chardon, J.P.; De Blust, G.; Swinnen, E.; Villalba, S.; Gulnick, H.; Matthysen, E. The application of “least cost” modelling as functional landscape models. Landsc. Urban Plan. 2003, 64, 233–247. [Google Scholar] [CrossRef]
- McRae, B.H.; Dickson, B.G.; Keitt, T.H.; Shah, V.B. Using circuit theory to model connectivity in ecology and conservation. Ecology 2008, 10, 2712–2724. [Google Scholar] [CrossRef]
- Moilanen, A.; Wilson, K.A.; Possingham, H. Spatial Conservation Prioritization: Quantitative Methods and Computational Tools; University Press: Oxford, UK, 2009. [Google Scholar]
- Pinto, N.; Keitt, T.H. Beyond the least-cost path: Evaluating corridor redundancy using a graph-theoretic approach. Landsc. Ecol. 2009, 24, 253–266. [Google Scholar] [CrossRef]
- Fattebert, J.; Baubet, E.; Slotow, R.; Fischer, C. Landscape effects on wild boar home range size under contrasting harvest regimes in a human-dominated agro-ecosystem. Eur. J. Wildl. Res. 2017, 63. [Google Scholar] [CrossRef]
- Bolliger, J.; Lander, T.; Balkenhol, N. Landscape genetics since 2003: Status, challenges and future directions. Landsc. Ecol. 2014, 29, 361–366. [Google Scholar] [CrossRef]
- Rayfield, B.; Fortin, M.J.; Fall, A. The sensitivity of least-cost habitat graphs to relative cost surface values. Landsc. Ecol. 2010, 25, 519–532. [Google Scholar] [CrossRef]
- Panzacchi, M.; Van Moorter, B.; Strand, O.; Saerens, M.; Ki, I.K.; St Clair, C.C.; Herfindal, I.; Boitani, L. Predicting the continuum between corridors and barriers to animal movements using Step Selection Functions and Randomized Shortest Paths. J. Anim. Ecol. 2016, 85, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Grafius, D.R.; Corstanje, R.; Siriwardena, G.M.; Plummer, K.E.; Harris, J.A. A bird’s eye view: Using circuit theory to study urban landscape connectivity for birds. Landsc. Ecol. 2017, 32, 1771–1787. [Google Scholar] [CrossRef] [PubMed]
- Lechner, A.M.; Sprod, D.; Carter, O.; Lefroy, E.C. Characterising landscape connectivity for conservation planning using a dispersal guild approach. Landsc. Ecol. 2017, 32, 99–113. [Google Scholar] [CrossRef]
- Bani, L.; Pisa, G.; Luppi, M.; Spilotros, G.; Fabbri, E.; Randi, E.; Orioli, V. Ecological connectivity assessment in a strongly structured fire salamander (Salamandra salamandra) population. Ecol. Evol. 2015, 5, 3472–3485. [Google Scholar] [CrossRef] [PubMed]
- Braaker, S.; Moretti, M.; Boesch, R.; Ghazoul, J.; Obrist, M.K.; Bontadina, F. Assessing habitat connectivity for ground-dwelling animals in an urban environment. Ecol. Appl. 2014, 24, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.J.; Veiman-Echeverria, M.; Kurz, D.J.; Donnelly, M.A. Evaluating connectivity for tropical amphibians using empirically derived resistance surfaces. Ecol. Appl. 2015, 25, 928–942. [Google Scholar] [CrossRef]
- Zeller, K.A.; Jennings, M.K.; Vickers, T.W.; Ernest, H.B.; Cushman, S.A.; Boyce, W.M. Are all data types and connectivity models created equal? Validating common connectivity approaches with dispersal data. Divers. Distrib. 2018, 24, 868–879. [Google Scholar] [CrossRef]
- Graves, T.; Chandler, R.B.; Royle, J.A.; Beier, P.; Kendall, K.C. Estimating landscape resistance to dispersal. Landsc. Ecol. 2014, 29, 1201–1211. [Google Scholar] [CrossRef]
- Koen, E.L.; Bowman, J.; Walpole, A.A. The effect of cost surface parameterization on landscape resistance estimates. Mol. Ecol. Resour. 2012, 12, 686–696. [Google Scholar] [CrossRef]
- Dilts, T.E.; Weisberg, P.J.; Leitner, P.; Matocq, M.D.; Inman, R.D.; Nussear, K.E.; Esque, T.C. Multiscale connectivity and graph theory highlight critical areas for conservation under climate change. Ecol. Appl. 2016, 26, 1223–1237. [Google Scholar] [CrossRef]
- Garrido-Garduno, T.; Tellez-Valdes, O.; Manel, S.; Vazquez-Dominguez, E. Role of habitat heterogeneity and landscape connectivity in shaping gene flow and spatial population structure of a dominant rodent species in a tropical dry forest. J. Zool. 2016, 298, 293–302. [Google Scholar] [CrossRef]
- Churko, G.; Kienast, F.; Bolliger, J. A multispecies assessment to identify functional connectivity in a human-dominated landscape. Int. J. Geogr. Inf. Syst. 2020, in press. [Google Scholar]
- Cushman, S.A.; McKelvey, K.S.; Hayden, J.; Schwartz, M.K. Gene flow in complex landscapes: Testing multiple hypotheses with causal modeling. Am. Nat. 2006, 168, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.A.; Cushman, S.A.; Srivastava, A.; Sarkar, M.S.; Shivaji, S. Tiger abundance and gene flow in Central India are driven by disparate combinations of topography and land cover. Divers. Distrib. 2017, 23, 863–874. [Google Scholar] [CrossRef]
- Vergara, M.; Cushman, S.A.; Ruiz-Gonzalez, A. Ecological differences and limiting factors in different regional contexts: Landscape genetics of the stone marten in the Iberian Peninsula. Landsc. Ecol. 2017, 32, 1269–1283. [Google Scholar] [CrossRef]
- Landguth, E.L.; Hand, B.K.; Glassy, J.; Cushman, S.A.; Sawaya, M.A. UNICOR: A species connectivity and corridor network simulator. Ecography 2012, 35, 9–14. [Google Scholar] [CrossRef]
- Koen, E.L.; Ellington, E.H.; Bowman, J. Mapping landscape connectivity for large spatial extents. Landsc. Ecol. 2019, 34, 2421–2433. [Google Scholar] [CrossRef]
- Leibovici, D.G.; Claramunt, C. On Integrating Size and Shape Distributions into a Spatio-Temporal Information Entropy Framework. Entropy 2019, 21. [Google Scholar] [CrossRef]
- Perkl, R.; Norman, L.M.; Mitchell, D.; Feller, M.; Smith, G.; Wilson, N.R. Urban growth and landscape connectivity threats assessment at Saguaro National Park, Arizona, USA. J. Land Use Sci. 2018, 13, 102–117. [Google Scholar] [CrossRef]
- Krosby, M.; Breckheimer, I.; Pierce, D.J.; Singleton, P.H.; Hall, S.A.; Halupka, K.C.; Gaines, W.L.; Long, R.A.; McRae, B.H.; Cosentino, B.L.; et al. Focal species and landscape “naturalness” corridor models offer complementary approaches for connectivity conservation planning. Landsc. Ecol. 2015, 30, 2121–2132. [Google Scholar] [CrossRef]
- Freeman, C.F.; Bell, K.P. Conservation versus cluster subdivisions and implications for habitat connectivity. Landsc. Urban Plan. 2011, 10, 30–42. [Google Scholar] [CrossRef]
- Sawyer, S.C.; Epps, C.W.; Brashares, J.S. Placing linkages among fragmented habitats: Do least-cost models reflect how animals use landscapes? J. Appl. Ecol. 2011, 48, 668–678. [Google Scholar] [CrossRef]
- Marrotte, R.R.; Bowman, J.; Brown, M.G.C.; Cordes, C.; Morris, K.Y.; Prentice, M.B.; Wilson, P.J. Multi-species genetic connectivity in a terrestrial habitat network. Mov. Ecol. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Vukomanovic, J.; Skrip, M.; Meentenmeyer, R. Making It Spatial Makes It Personal: Engaging Stakeholders with Geospatial Participatory Modeling. Land 2019, 8, 38. [Google Scholar] [CrossRef]
- Lechner, A.M.; Harris, R.M.S.; Doerr, V.; Doerr, E.; Drielsma, M.; Lefroy, E.C. From static connectivity modelling to scenario-based planning at local and regional scales. J. Nat. Conserv. 2015, 28, 78–88. [Google Scholar] [CrossRef]
- Zhang, Z.; Meerow, S.; Newell, J.; Lindquist, M. Enhancing landscape connectivity through multifunctional green infrastructure corridor modeling and design. Urban For. Urban Green. 2019, 38, 305–317. [Google Scholar] [CrossRef]
- Perkl, R.M. Geodesigning landscape linkages: Coupling GIS with wildlife corridor design in conservation planning. Landsc. Urban Plan. 2016, 156, 44–58. [Google Scholar] [CrossRef]
- Firehock, K.E.; Walker, R.A. Green Infrastructure: Map and Plan the Natural World with GIS; Esri Press: Redlands, CA, USA, 2019. [Google Scholar]
- Wisconsin, G. Tackling Barriers to Green Infrastructure: An Audit of Local Codes and Ordinances; WI, USA, 2017; Available online: https://www.seagrant.wisc.edu/our-work/focus-areas/coastal-communities/green-infrastructure/ (accessed on 1 January 2020).
- Benedict, M.; McMahon, E.; Bergen, L. Green Infrastructure: Linking Landscapes and Communities; Island Press: Washington, DC, USA, 2006. [Google Scholar]
- Lynch, A. Is it good to be green? Assessing the ecological results of county green infrastructure planning. J. Plan. Educ. Res. 2016, 36, 90–104. [Google Scholar] [CrossRef]
- Szulczewska, B.; Giedych, R.; Maksymiuk, G. Can we face the challenge: How to implement a theoretical concept of green infrastructure into planning practice? Warsaw case study. Landsc. Res. 2017, 42, 76–194. [Google Scholar] [CrossRef]
- Meerow, S.; Newell, J. Spatial planning for multifunctional green infrastructure: Growing resilience in Detroit. Landsc. Urban Plan. 2017, 159, 62–75. [Google Scholar] [CrossRef]
- Lai, S.; Leone, F. Bridging Biodiversity Conservation Objectives with Landscape Planning Through Green Infrastructures: A Case Study from Sardinia, Italy. In Lecture Notes in Computer Science, Proceedings of theComputational Science and Its Applications, Trieste, Italy, 3–6 July 2017; Springer: Cham, Switzerland, 2017; Volume 10409, pp. 10456–10472. [Google Scholar]
- Lafortezza, R.; Davies, C.; Sanesi, G.; Konijnendijk, C. Green Infrastructure as a tool to support spatial planning in European urban regions. iForest Biogeosci. For. 2013, 6, 102–108. [Google Scholar] [CrossRef]
- Reimer, M.; Rusche, K. Green infrastructure under pressure. A global narrative between regional vision and local implementation. Eur. Plan. Stud. 2019, 27, 1542–1563. [Google Scholar] [CrossRef]
- Liquete, C.; Kleeschulte, S.; Dige, G.; Maes, J.; Grizzetti, B.; Olah, B.; Zulian, G. Mapping green infrastructure based on ecosystem services and ecological networks: A Pan-European case study. Environ. Sci. Policy 2015, 54, 268–280. [Google Scholar] [CrossRef]
- Vasiljevic, N.; Radic, B.; Gavrilovic, S.; Sljukic, B.; Medarevic, M.; Ristic, R. The concept of green infrastructure and urban landscape planning: A challenge for urban forestry planning in Belgrade, Serbia. iForest Biogeosci. For. 2018, 11, 491–498. [Google Scholar] [CrossRef]
- Liu, S.; Yin, Y.; Li, J.; Cheng, F.; Dong, S.; Zhang, Y. Using cross-scale landscape connectivity indices to identify key habitat resource patches for Asian elephants in Xishuangbanna, China. Landsc. Urban Plan. 2018, 171, 80–87. [Google Scholar] [CrossRef]
- United Nations. About the Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 15 May 2009).
- Bai, X.M.; van der Leeuw, S.; O’Brien, K.; Berkhout, F.; Biermann, F.; Brondizio, E.S.; Cudennec, C.; Dearing, J.; Duraiappah, A.; Glaser, M.; et al. Plausible and desirable futures in the Anthropocene: A new research agenda. Glob. Environ. Chang. Hum. Policy Dimens. 2016, 39, 351–362. [Google Scholar] [CrossRef]
- Future Earth. Research Agenda. 2014. Available online: http://www.futureearth.org/sites/default/files/strategic_research_agenda_2014.pdf (accessed on 27 March 2020).
- Van Strien, M.J.; Keller, D.; Holderegger, R. A new analytical approach to landscape genetic modelling: Least-cost transect analysis and linear mixed models. Mol. Ecol. 2012, 21, 4010–4023. [Google Scholar] [CrossRef]
- Ginzler, C.; Hobi, M.L. Countrywide stereo-image matching for updating digital surface models in the framework of the Swiss National Forest Inventory. Remote Sens. 2015, 7, 4343–4370. [Google Scholar] [CrossRef]
- Randin, C.F.; Ashcroft, M.; Bolliger, J.; Cavender-Bares, J.; Coops, N.; Dullinger, S.; Dirnböck, T.; Eckert, S.; Ellis, E.; Giuliani, G.; et al. Towards improved monitoring of terrestrial ecosystems in the Anthropocene through the closer integration of remotely sensed data into species distribution models. Remote Sens. Environ. 2020, in press. [Google Scholar]
- Bergen, K.M.; Goetz, S.J.; Dubayah, R.O.; Henebry, G.M.; Hunsaker, C.T.; Imhoff, M.L.; Nelson, R.F.; Parker, G.G.; Radeloff, V.C. Remote sensing of vegetation 3-D structure for biodiversity and habitat: Review and implications for lidar and radar spaceborne missions. J. Geophys. Res. 2009, 114, G00E06. [Google Scholar] [CrossRef]
- Merrick, M.J.; Koprowski, J.L. Circuit theory to estimate natal dispersal routes and functional landscape connectivity for an endangered small mammal. Landsc. Ecol. 2017, 32, 1163–1179. [Google Scholar] [CrossRef]
- Maggini, R.; Lehmann, A.; Zbinden, N.; Zimmermann, N.E.; Bolliger, J.; Schroder, B.; Foppen, R.; Schmid, H.; Beniston, M.; Jenni, L. Assessing species vulnerability to climate and land use change: The case of the Swiss breeding birds. Divers. Distrib. 2014, 20, 708–719. [Google Scholar] [CrossRef]
- Leonard, P.; Sutherland, R.; Baldwin, R.; Fedak, D.; Carnes, R.; Montgomery, A. Landscape connectivity losses due to sea levelrise and land use change. Anim. Conserv. 2017, 20, 80–90. [Google Scholar] [CrossRef]
- Nor, A.N.M.; Corstanje, R.; Harris, J.A.; Grafius, D.R.; Siriwardena, G.M. Ecological connectivity networks in rapidly expanding cities. Heliyon 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Kanagaraj, R.; Wiegand, T.; Kramer-Schadt, S.; Goyal, S.P. Using individual-based movement models to assess inter-patch connectivity for large carnivores in fragmented landscapes. Biol. Conserv. 2013, 167, 298–309. [Google Scholar] [CrossRef]
- Polansky, L.; Kilian, W.; Wittemyer, G. Elucidating the significance of spatial memory on movement decisions by African savannah elephants using state-space models. Proc. R. Soc. B Biol. Sci. 2015, 282, 20143042. [Google Scholar] [CrossRef]
- Fleishman, E.; Anderson, J.; Dickson, B.G. Single-species and multiple-species connectivity models for large mammals on the Navajo nation. West. N. Am. Nat. 2017, 77, 237–251. [Google Scholar] [CrossRef]
- Rodriguez-Espinos, V.M.; Aguilera-Benavente, F.; Gimez-Delgado, M. Green infrastructure design using GIS and spatial analysis: A proposal for the Henares Corridor (Madrid-Guadalajara, Spain). Landsc. Res. 2020, 45, 26–43. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).