The Hippo Pathway Effectors YAP/TAZ Are Essential for Mineralized Tissue Homeostasis in the Alveolar Bone/Periodontal Complex
Abstract
:1. Introduction
2. Methods
2.1. Animal Model
2.2. Unopposed Molar Model
2.3. Isolation of Primary Periodontal Ligament Cells
2.4. Radiographic Imaging
2.5. Histology
2.6. Application of Mechanical Stress on Cells
2.7. siRNA Mediated Knockdown of YAP
2.8. Immunofluorescence Microscopy
2.9. RNA Extraction and Real-Time Quantitative PCR
2.10. Statistical Analysis
3. Results
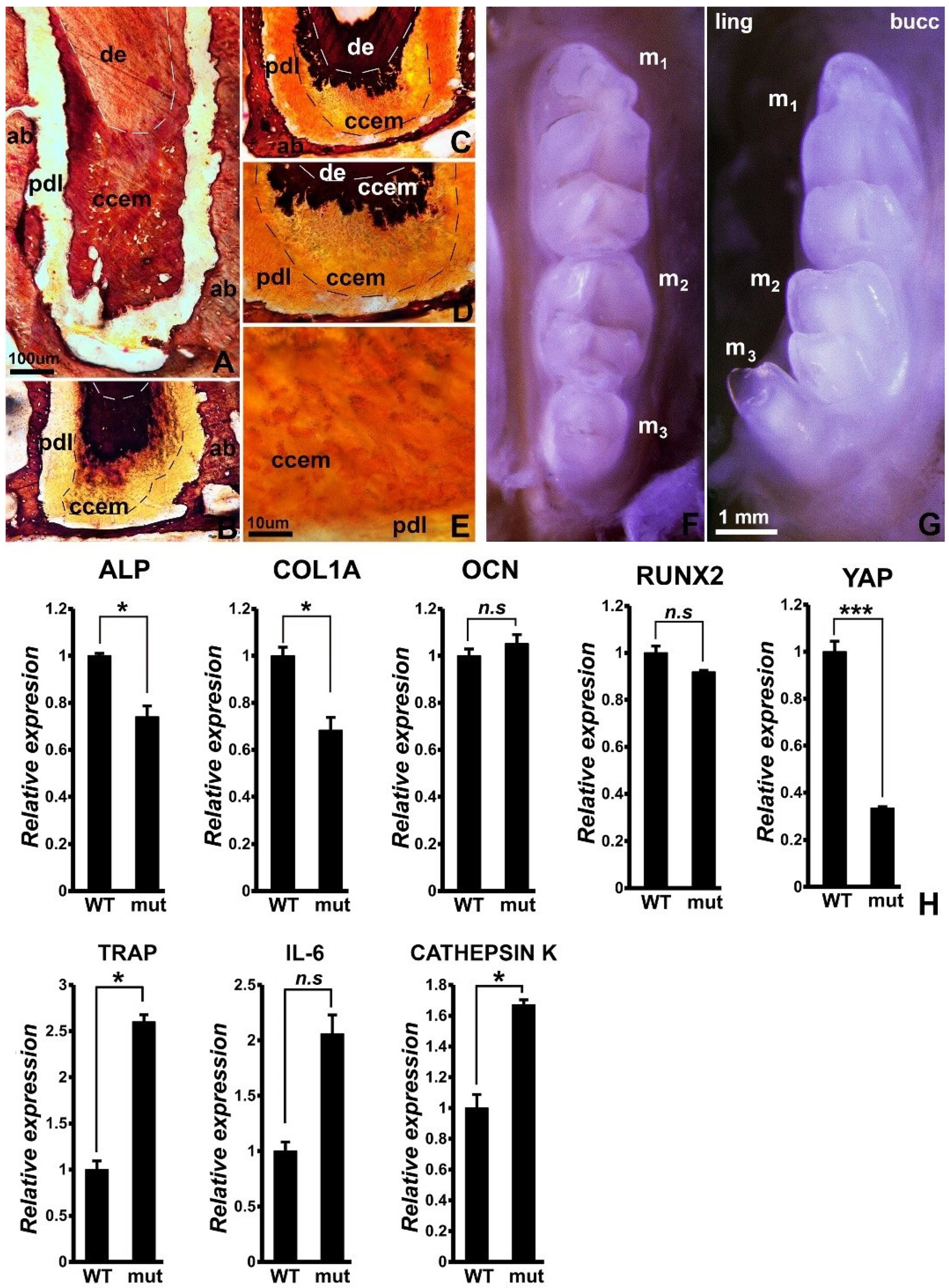
3.1. Reduced Mineralization-Related Gene Expression, Incomplete Cellular Cementum Mineralization, and 3rd Molar Tooth Row Misalignment in Wnt1cre/YAP Flox/TAZ Flox Mice
3.2. Cyclic Strain Increased Nuclear YAP, and the Proteoglycan Agrin Partially Rescued the Effect of YAP Knockdown on Nuclear YAP Expression
3.3. Significant Reduction in Mineralization-Related Gene Expression following YAP1 Knockdown, and Partial Rescue with the Agrin Proteoglycan
3.4. Reduced Mineralization-Related Gene Expression and Loss of Tooth/PDL/Alveolar Bone Tissue Contours in the Wnt1Cre/YAP/TAZ Mutant Mice Dentoalveolar Complex following Unloading
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luan, X.; Diekwisch, T.G.H. Vienna–Chicago: The Cultural Transformation of the Model System of the Un-Opposed Molar. Bioessays 2007, 29, 819–830. [Google Scholar] [CrossRef] [Green Version]
- Walker, C.G.; Dangaria, S.; Ito, Y.; Luan, X.; Diekwisch, T.G.H. Osteopontin Is Required for Unloading-Induced Osteoclast Recruitment and Modulation of RANKL Expression during Tooth Drift-Associated Bone Remodeling, but Not for Super-Eruption. Bone 2010, 47, 1020–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holliday, S.; Schneider, B.; Galang, M.T.; Fukui, T.; Yamane, A.; Luan, X.; Diekwisch, T.G.H. Bones, Teeth, and Genes: A Genomic Homage to Harry Sicher’s “Axial Movement of Teeth”. World J. Orthod. 2005, 6, 61–70. [Google Scholar] [PubMed]
- Luan, X.; Ito, Y.; Holliday, S.; Walker, C.; Daniel, J.; Galang, T.M.; Fukui, T.; Yamane, A.; Begole, E.; Evans, C.; et al. Extracellular Matrix-Mediated Tissue Remodeling Following Axial Movement of Teeth. J. Histochem. Cytochem. 2007, 55, 127–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diekwisch, T.G.H. Our Periodontal Tissue: A Masterpiece of Evolution. J. Clin. Periodontol. 2016, 43, 320–322. [Google Scholar] [CrossRef]
- Burridge, K. Focal Adhesions: A Personal Perspective on a Half Century of Progress. FEBS J. 2017, 284, 3355–3361. [Google Scholar] [CrossRef] [Green Version]
- Martinac, B. The Ion Channels to Cytoskeleton Connection as Potential Mechanism of Mechanosensitivity. Biochim. Biophys. Acta—Biomembr. 2014, 1838, 682–691. [Google Scholar] [CrossRef] [Green Version]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Fukata, Y.; Kaibuchi, K.; Amano, M.; Kaibuchi, K. Rho–Rho-Kinase Pathway in Smooth Muscle Contraction and Cytoskeletal Reorganization of Non-Muscle Cells. Trends Pharmacol. Sci. 2001, 22, 32–39. [Google Scholar] [CrossRef]
- Dong, J.-M.; Lau, L.-S.; Ng, Y.-W.; Lim, L.; Manser, E. Paxillin Nuclear-Cytoplasmic Localization Is Regulated by Phosphorylation of the LD4 Motif: Evidence That Nuclear Paxillin Promotes Cell Proliferation. Biochem. J. 2009, 418, 173–184. [Google Scholar] [CrossRef]
- Valbuena, A.; Vera, A.M.; Oroz, J.; Menéndez, M.; Carrión-Vázquez, M. Mechanical Properties of β-Catenin Revealed by Single-Molecule Experiments. Biophys. J. 2012, 103, 1744–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.E.M.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Oria, R.; Kechagia, J.Z.; Rico-Lastres, P.; Le Roux, A.-L.; et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 2017, 171, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Nardone, G.; Oliver-De La Cruz, J.; Vrbsky, J.; Martini, C.; Pribyl, J.; Skládal, P.; Pešl, M.; Caluori, G.; Pagliari, S.; Martino, F.; et al. YAP Regulates Cell Mechanics by Controlling Focal Adhesion Assembly. Nat. Commun. 2017, 8, 15321. [Google Scholar] [CrossRef]
- Morikawa, Y.; Zhang, M.; Heallen, T.; Leach, J.; Tao, G.; Xiao, Y.; Bai, Y.; Li, W.; Willerson, J.T.; Martin, J.F. Actin Cytoskeletal Remodeling with Protrusion Formation Is Essential for Heart Regeneration in Hippo-Deficient Mice. Sci. Signal. 2015, 8, ra41. [Google Scholar] [CrossRef] [Green Version]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and YAP-Dependent Matrix Remodelling Is Required for the Generation and Maintenance of Cancer-Associated Fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef]
- Chakraborty, S.; Njah, K.; Pobbati, A.V.; Lim, Y.B.; Raju, A.; Lakshmanan, M.; Tergaonkar, V.; Lim, C.T.; Hong, W. Agrin as a Mechanotransduction Signal Regulating YAP through the Hippo Pathway. Cell Rep. 2017, 18, 2464–2479. [Google Scholar] [CrossRef] [Green Version]
- Zhou, A.; Yu, H.; Liu, J.; Zheng, J.; Jia, Y.; Wu, B.; Xiang, L. Role of Hippo-YAP Signaling in Osseointegration by Regulating Osteogenesis, Angiogenesis, and Osteoimmunology. Front. Cell Dev. Biol. 2020, 8, 780. [Google Scholar] [CrossRef]
- Pan, J.-X.; Xiong, L.; Zhao, K.; Zeng, P.; Wang, B.; Tang, F.-L.; Sun, D.; Guo, H.; Yang, X.; Cui, S.; et al. YAP Promotes Osteogenesis and Suppresses Adipogenic Differentiation by Regulating β-Catenin Signaling. Bone Res. 2018, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Sun, H.; Song, F.; Wu, Y.; Wang, J. Yes-Associated Protein 1 Promotes the Differentiation and Mineralization of Cementoblast. J. Cell. Physiol. 2018, 233, 2213–2224. [Google Scholar] [CrossRef]
- Reginensi, A.; Scott, R.P.; Gregorieff, A.; Bagherie-Lachidan, M.; Chung, C.; Lim, D.-S.; Pawson, T.; Wrana, J.; McNeill, H. Yap- and Cdc42-Dependent Nephrogenesis and Morphogenesis during Mouse Kidney Development. PLoS Genet. 2013, 9, e1003380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dangaria, S.J.; Ito, Y.; Luan, X.; Diekwisch, T.G.H. Differentiation of Neural-Crest-Derived Intermediate Pluripotent Progenitors into Committed Periodontal Populations Involves Unique Molecular Signature Changes, Cohort Shifts, and Epigenetic Modifications. Stem Cells Dev. 2011, 20, 39–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diekwisch, T.G.H.; Berman, B.J.; Gentner, S.; Slavkin, H.C. Initial Enamel Crystals Are Not Spatially Associated with Mineralized Dentine. Cell Tissue Res. 1995, 279, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Prophet, E.B. (Ed.) Laboratory Methods in Histotechnology; Armed Forces Institute of Pathology: Washington, DC, USA, 1992; p. 131. ISBN 9781881041009. [Google Scholar]
- Chen, Y.; Mohammed, A.; Oubaidin, M.; Evans, C.A.; Zhou, X.; Luan, X.; Diekwisch, T.G.H.; Atsawasuwan, P. Cyclic Stretch and Compression Forces Alter MicroRNA-29 Expression of Human Periodontal Ligament Cells. Gene 2015, 566, 13–17. [Google Scholar] [CrossRef]
- Lerner, U.H. Inflammation-Induced Bone Remodeling in Periodontal Disease and the Influence of Post-Menopausal Osteoporosis. J. Dent. Res. 2006, 85, 596–607. [Google Scholar] [CrossRef]
- Graves, D.T.; Liu, R.; Alikhani, M.; Al-Mashat, H.; Trackman, P.C. Diabetes-Enhanced Inflammation and Apoptosis—Impact on Periodontal Pathology. J. Dent. Res. 2006, 85, 15–21. [Google Scholar] [CrossRef]
- Graves, D. Cytokines That Promote Periodontal Tissue Destruction. J. Periodontol. 2008, 79, 1585–1591. [Google Scholar] [CrossRef] [Green Version]
- Luan, X.; Zhou, X.; Trombetta-eSilva, J.; Francis, M.; Gaharwar, A.K.; Atsawasuwan, P.; Diekwisch, T.G.H. MicroRNAs and Periodontal Homeostasis. J. Dent. Res. 2017, 96, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Diekwisch, T.G.H. Periodontal Homeostasis: From Vienna to Texas—A Century of Periodontal Research in the Spirit of Bernhard Gottlieb. Stem Cells Dev. 2019, 28, 961–962. [Google Scholar] [CrossRef]
- Tang, Y.; Weiss, S.J. Snail/Slug-YAP/TAZ Complexes Cooperatively Regulate Mesenchymal Stem Cell Function and Bone Formation. Cell Cycle 2017, 16, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Guo, Y.; Chen, H.; Jiang, Y.; Tang, H.; Gong, P.; Xiang, L. The Influence of Receptor Activity–Modifying Protein-1 Overexpression on Angiogenesis in Mouse Brain Capillary Endothelial Cells. J. Cell Biochem. 2019, 120, 10087–10096. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.L.; Nagatomo, K.J.; Bamashmous, S.O.; Tompkins, K.A.; Fong, H.; Dunn, D.; Chu, E.Y.; Guenther, C.; Kingsley, D.M.; Rutherford, R.B.; et al. The Progressive Ankylosis Protein Regulates Cementum Apposition and Extracellular Matrix Composition. Cells Tissues Organs 2011, 194, 382–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, B.L.; Nagatomo, K.J.; Nociti, F.H.; Fong, H.; Dunn, D.; Tran, A.B.; Wang, W.; Narisawa, S.; Millán, J.L.; Somerman, M.J. Central Role of Pyrophosphate in Acellular Cementum Formation. PLoS ONE 2012, 7, e38393. [Google Scholar] [CrossRef] [Green Version]
- Lim, W.H.; Liu, B.; Cheng, D.; Williams, B.O.; Mah, S.J.; Helms, J.A. Wnt Signaling Regulates Homeostasis of the Periodontal Ligament. J. Periodont. Res. 2014, 49, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, G.; Foyle, D.; Luan, X.; Diekwisch, T.G.H. The Wnt Antagonist SFRP1: A Key Regulator of Periodontal Mineral Homeostasis. Stem Cells Dev. 2019, 28, 1004–1014. [Google Scholar] [CrossRef]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The Biology of YAP/TAZ: Hippo Signaling and Beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Kim, N.-G.; Gumbiner, B.M. Regulation of Hippo Pathway by Mitogenic Growth Factors via Phosphoinositide 3-Kinase and Phosphoinositide-Dependent Kinase-1. Proc. Natl. Acad. Sci. USA 2013, 110, 2569–2574. [Google Scholar] [CrossRef] [Green Version]
- Elbediwy, A.; Vanyai, H.; Diaz-de-la-Loza, M.-C.; Frith, D.; Snijders, A.P.; Thompson, B.J. Enigma Proteins Regulate YAP Mechanotransduction. J. Cell Sci. 2018, 131, jcs.221788. [Google Scholar] [CrossRef] [Green Version]
- Ege, N.; Dowbaj, A.M.; Jiang, M.; Howell, M.; Hooper, S.; Foster, C.; Jenkins, R.P.; Sahai, E. Quantitative Analysis Reveals That Actin and Src-Family Kinases Regulate Nuclear YAP1 and Its Export. Cell Syst. 2018, 6, 692–708.e13. [Google Scholar] [CrossRef] [Green Version]
- Cobbaut, M.; Karagil, S.; Bruno, L.; Diaz de la Loza, M.D.C.; Mackenzie, F.E.; Stolinski, M.; Elbediwy, A. Dysfunctional Mechanotransduction through the YAP/TAZ/Hippo Pathway as a Feature of Chronic Disease. Cells 2020, 9, 151. [Google Scholar] [CrossRef] [Green Version]
- Tan, F.; Huang, Y.; Pei, Q.; Liu, H.; Pei, H.; Zhu, H. Matrix Stiffness Mediates Stemness Characteristics via Activating the Yes-associated Protein in Colorectal Cancer Cells. J. Cell Biochem. 2019, 120, 2213–2225. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.G.; Ito, Y.; Dangaria, S.; Luan, X.; Diekwisch, T.G.H. RANKL, Osteopontin, and Osteoclast Homeostasis in a Hyperocclusion Mouse Model. Eur. J. Oral Sci. 2008, 116, 312–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandya, M.; Gopinathan, G.; Tillberg, C.; Wang, J.; Luan, X.; Diekwisch, T.G.H. The Hippo Pathway Effectors YAP/TAZ Are Essential for Mineralized Tissue Homeostasis in the Alveolar Bone/Periodontal Complex. J. Dev. Biol. 2022, 10, 14. https://doi.org/10.3390/jdb10010014
Pandya M, Gopinathan G, Tillberg C, Wang J, Luan X, Diekwisch TGH. The Hippo Pathway Effectors YAP/TAZ Are Essential for Mineralized Tissue Homeostasis in the Alveolar Bone/Periodontal Complex. Journal of Developmental Biology. 2022; 10(1):14. https://doi.org/10.3390/jdb10010014
Chicago/Turabian StylePandya, Mirali, Gokul Gopinathan, Connie Tillberg, Jun Wang, Xianghong Luan, and Thomas G. H. Diekwisch. 2022. "The Hippo Pathway Effectors YAP/TAZ Are Essential for Mineralized Tissue Homeostasis in the Alveolar Bone/Periodontal Complex" Journal of Developmental Biology 10, no. 1: 14. https://doi.org/10.3390/jdb10010014
APA StylePandya, M., Gopinathan, G., Tillberg, C., Wang, J., Luan, X., & Diekwisch, T. G. H. (2022). The Hippo Pathway Effectors YAP/TAZ Are Essential for Mineralized Tissue Homeostasis in the Alveolar Bone/Periodontal Complex. Journal of Developmental Biology, 10(1), 14. https://doi.org/10.3390/jdb10010014








