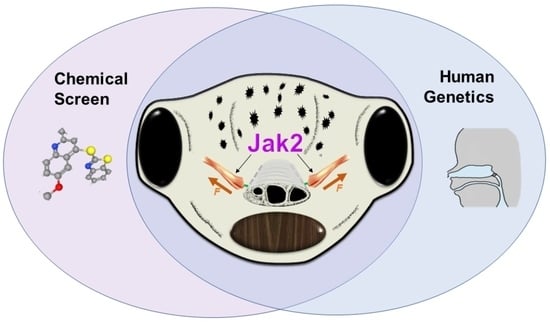
Jak2 and Jaw Muscles Are Required for Buccopharyngeal Membrane Perforation during Mouth Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Gathering Embryos and Staging
2.2. Chemical Screen
2.3. Copy Number Variant Screen in Patients with Choanal Atresia
2.4. Imaging the Facial Features
2.5. Morpholino
2.6. Phalloidin Labeling, Immunofluorescence Auto Fluorescent Rendering
2.7. Tissue Sectioning
2.8. Micro Incisions in the Face
3. Results
3.1. Buccopharyngeal Membrane Perforation in Xenopus Laevis
3.2. Chemical Screen Identified Known and New Candidate Regulators of Buccopharyngeal Membrane Perforation
3.3. Human Genetic Data from Patients with Choanal Atresia Identified Potential Regulators of Buccopharyngeal Membrane Perforation
3.4. Overlap in Chemical and Genetic Screens Identifies JAK2 as a Candidate Regulator of Buccopharyngeal Membrane Perforation
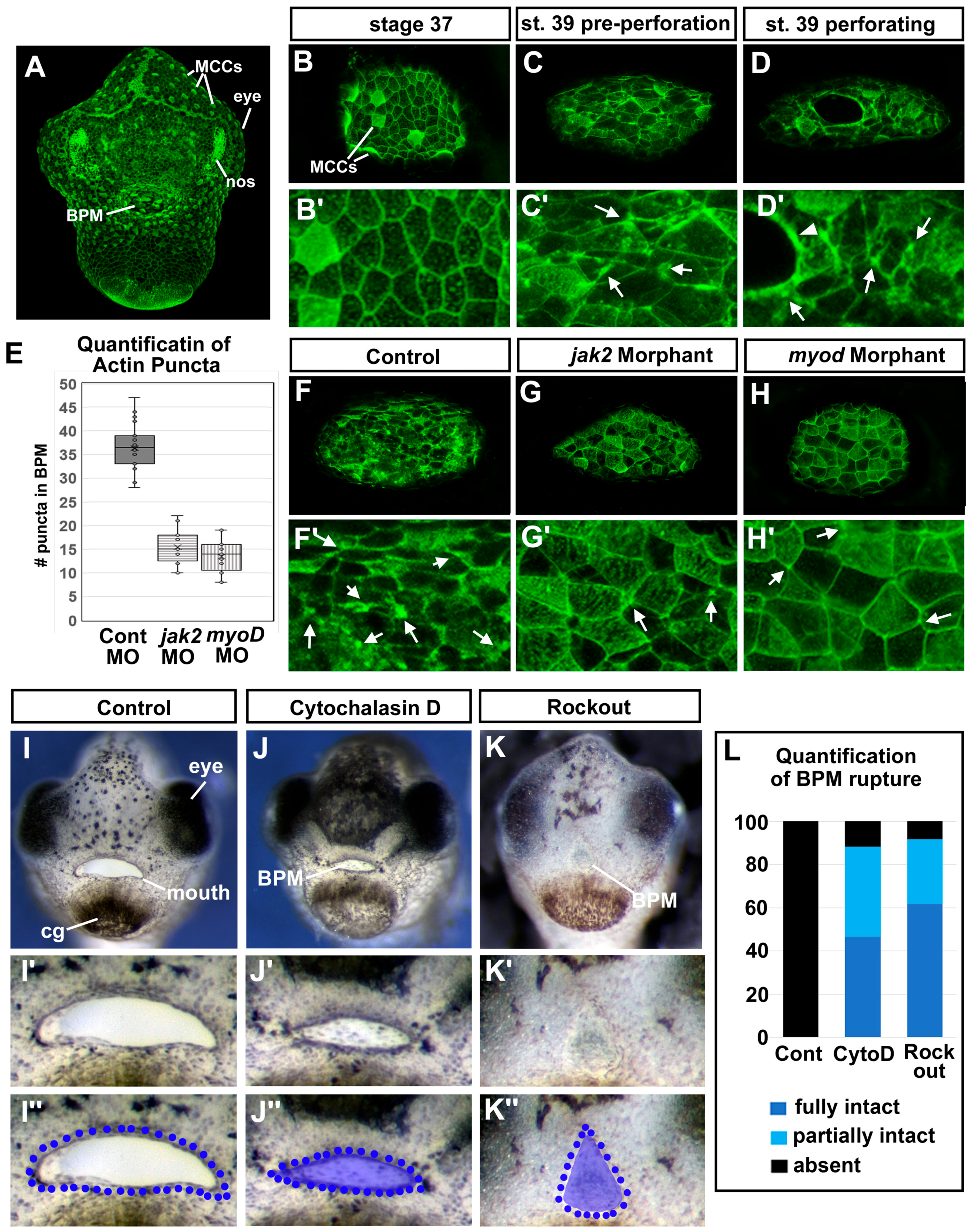
3.5. Deficient Jak2 Causes a Persistent Buccopharyngeal Membrane
3.6. Jak2 Is Specifically Required for Buccopharyngeal Membrane Rupture Rather Than Earlier Steps in Embryonic Mouth Development
3.7. Jak2 Is Required for Cranial Muscle Development
3.8. The Cranial Muscles Are Required for Buccopharyngeal Membrane Rupture
3.9. Jaw Muscles Are Connected to the Oral Epithelium by Laminin
3.10. Incisions around the Oral Cavity Result in a Persistent Buccopharyngeal Membrane
3.11. F-actin Puncta Were Observed in the Cells of the Buccopharyngeal Membrane Prior to Rupture
3.12. Perturbing Actin Dynamics Results in a Persistent Buccopharyngeal Membrane
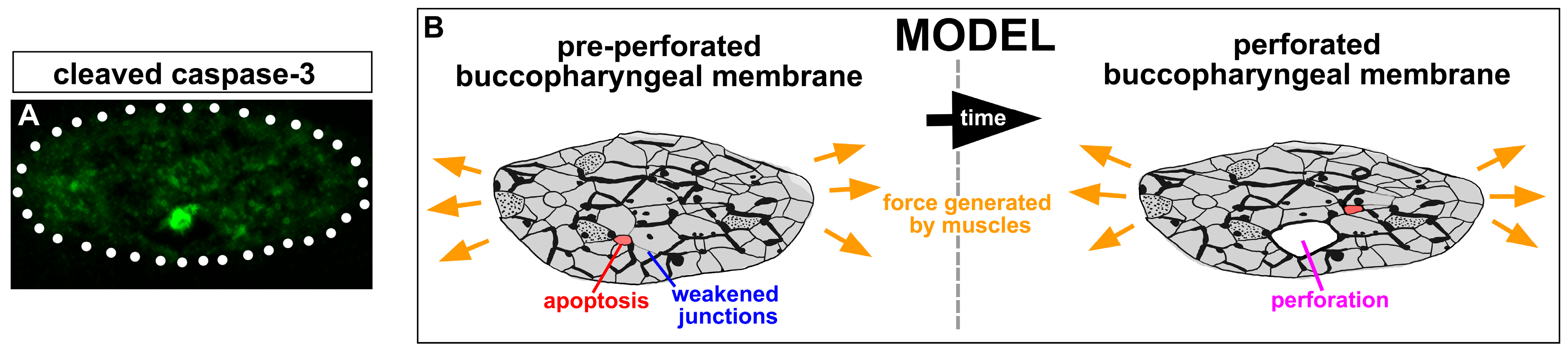
3.13. Apoptotic Cells Are Present in the Buccopharyngeal Membrane just Prior to Perforation
4. Discussion
5. Limitations of the Study
6. Conclusions
7. Patents
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dickinson, A.J. Using frogs faces to dissect the mechanisms underlying human orofacial defects. Semin. Cell Dev. Biol. 2016, 51, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, A.J.; Sive, H. Development of the primary mouth in Xenopus laevis. Dev. Biol. 2006, 295, 700–713. [Google Scholar] [CrossRef]
- Soukup, V.; Horácek, I.; Cerny, R. Development and evolution of the vertebrate primary mouth. J. Anat. 2013, 222, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.M.; Lee, S.H. About face: Signals and genes controlling jaw patterning and identity in vertebrates. BioEssays News Rev. Mol. Cell. Dev. Biol. 2003, 25, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Maxson, R.E., Jr. Recent advances in craniofacial morphogenesis. Dev. Dyn. 2006, 235, 2353–2375. [Google Scholar] [CrossRef] [PubMed]
- Francis-West, P.H.; Robson, L.; Evans, D.J.R. Craniofacial Development: The Tissue and Molecular Interactions That Control Development of the Head; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2003. [Google Scholar]
- Gitton, Y.; Heude, E.; Vieux-Rochas, M.; Benouaiche, L.; Fontaine, A.; Sato, T.; Kurihara, Y.; Kurihara, H.; Couly, G.; Levi, G. Evolving maps in craniofacial development. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2010; Volume 21, pp. 301–308. [Google Scholar] [CrossRef]
- Helms, J.A.; Cordero, D.; Tapadia, M.D. New insights into craniofacial morphogenesis. Development 2005, 132, 851–861. [Google Scholar] [CrossRef]
- Chen, J.; Jacox, L.A.; Saldanha, F.; Sive, H. Mouth development. Wiley Interdiscip. Rev. Dev. Biol. 2017, 6, e275. [Google Scholar] [CrossRef]
- Jacox, L.; Chen, J.; Rothman, A.; Lathrop-Marshall, H.; Sive, H. Formation of a “Pre-mouth Array” from the Extreme Anterior Domain Is Directed by Neural Crest and Wnt/PCP Signaling. Cell Rep. 2016, 16, 1445–1455. [Google Scholar] [CrossRef][Green Version]
- Jacox, L.; Sindelka, R.; Chen, J.; Rothman, A.; Dickinson, A.; Sive, H. The extreme anterior domain is an essential craniofacial organizer acting through Kinin-Kallikrein signaling. Cell Rep. 2014, 8, 596–609. [Google Scholar] [CrossRef]
- Mulvihill, S.J.; Stone, M.M.; Debas, H.T.; Fonkalsrud, E.W. The role of amniotic fluid in fetal nutrition. J. Pediatr. Surg. 1985, 20, 668–672. [Google Scholar] [CrossRef]
- Dickinson, A.; Sive, H. Positioning the extreme anterior in Xenopus: Cement gland, primary mouth and anterior pituitary. Semin. Cell Dev. Biol. 2007, 18, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, A.J.; Sive, H.L. The Wnt antagonists Frzb-1 and Crescent locally regulate basement membrane dissolution in the developing primary mouth. Development 2009, 136, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Edwards, N.A.; Zorn, A.M. Modeling endoderm development and disease in Xenopus. Curr. Top. Dev. Biol. 2021, 145, 61–90. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Sasaki, F.; Takahama, H. The ultrastructure of oral (buccopharyngeal) membrane formation and rupture in the anuran embryo. Anat. Rec. 1984, 210, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Waterman, R.E. Ultrastructure of oral (buccopharyngeal) membrane formation and rupture in the hamster embryo. Dev. Biol. 1977, 58, 219–229. [Google Scholar] [CrossRef]
- Waterman, R.E. Formation and perforation of closing plates in the chick embryo. Anat. Rec. 1985, 211, 450–457. [Google Scholar] [CrossRef]
- Waterman, R.E.; Balian, G. Indirect immunofluorescent staining of fibronectin associated with the floor of the foregut during formation and rupture of the oral membrane in the chick embryo. Anat. Rec. 1980, 198, 619–635. [Google Scholar] [CrossRef]
- Waterman, R.E.; Schoenwolf, G.C. The ultrastructure of oral (buccopharyngeal) membrane formation and rupture in the chick embryo. Anat. Rec. 1980, 197, 441–470. [Google Scholar] [CrossRef]
- Agarwal, R.; Kumar, P.; Kalra, G.S.; Bhushan, S.; Chandra, R. Persistent buccopharyngeal membrane: A report of two cases. Plast. Reconstr. Surg. 1996, 98, 866–868. [Google Scholar] [CrossRef]
- Arcand, P.; Haikal, J. Persistent buccopharyngeal membrane. J. Otolaryngol. 1988, 17, 125–127. [Google Scholar]
- Chandra, R.; Yadava, V.N.; Sharma, R.N. Persistent buccopharyngeal membrane. Case report. Plast. Reconstr. Surg. 1974, 54, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Cotton, R.T. Persistent buccopharyngeal membrane. J. Otolaryngol. 1988, 17, 260. [Google Scholar] [PubMed]
- Ferril, G.R.; Barham, H.P.; Prager, J.D. Novel airway findings in a patient with 1p36 deletion syndrome. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Legius, E.; Moerman, P.; Fryns, J.P.; Vandenberghe, K.; Eggermont, E. Holzgreve-Wagner-Rehder syndrome: Potter sequence associated with persistent buccopharyngeal membrane. A second observation. Am. J. Med. Genet. 1988, 31, 269–272. [Google Scholar] [CrossRef]
- Pillai, K.G.; Kamath, V.V.; Kumar, G.S.; Nagamani, N. Persistent buccopharyngeal membrane with cleft palate. A case report. Oral Surg. Oral Med. Oral Pathol. 1990, 69, 164–166. [Google Scholar] [CrossRef]
- Gartlan, M.G.; Davies, J.; Smith, R.J. Congenital oral synechiae. Ann. Otol. Rhinol. Laryngol. 1993, 102, 186–197. [Google Scholar] [CrossRef]
- Assanasen, P.; Metheetrairut, C. Choanal atresia. J. Med. Assoc. Thai 2009, 92, 699–706. [Google Scholar]
- Chia, S.H.; Carvalho, D.S.; Jaffe, D.M.; Pransky, S.M. Unilateral choanal atresia in identical twins: Case report and literature review. Int. J. Pediatr. Otorhinolaryngol. 2002, 62, 249–252. [Google Scholar] [CrossRef]
- Kwong, K.M. Current Updates on Choanal Atresia. Front. Pediatr. 2015, 3, 52. [Google Scholar] [CrossRef]
- Katz, E.S.; Mitchell, R.B.; D’Ambrosio, C.M. Obstructive sleep apnea in infants. Am. J. Respir. Crit. Care Med. 2012, 185, 805–816. [Google Scholar] [CrossRef]
- Lesciotto, K.M.; Heuzé, Y.; Jabs, E.W.; Bernstein, J.M.; Richtsmeier, J.T. Choanal Atresia and Craniosynostosis: Development and Disease. Plast. Reconstr. Surg. 2018, 141, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Kurosaka, H.; Mushiake, J.; Saha, M.; Wu, Y.; Wang, Q.; Kikuchi, M.; Nakaya, A.; Yamamoto, S.; Inubushi, T.; Koga, S.; et al. Synergistic role of retinoic acid signaling and Gata3 during primitive choanae formation. Hum. Mol. Genet. 2021, 30, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Kurosaka, H.; Wang, Q.; Sandell, L.; Yamashiro, T.; Trainor, P.A. Rdh10 loss-of-function and perturbed retinoid signaling underlies the etiology of choanal atresia. Hum. Mol. Genet. 2017, 26, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Jacox, L.A.; Dickinson, A.J.; Sive, H. Facial transplants in Xenopus laevis embryos. J. Vis. Exp. 2014, 85, e50697. [Google Scholar] [CrossRef]
- Houssin, N.S.; Bharathan, N.K.; Turner, S.D.; Dickinson, A.J. Role of JNK during buccopharyngeal membrane perforation, the last step of embryonic mouth formation. Dev. Dyn. 2017, 246, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Tabler, J.M.; Bolger, T.G.; Wallingford, J.; Liu, K.J. Hedgehog activity controls opening of the primary mouth. Dev. Biol. 2014, 396, 1–7. [Google Scholar] [CrossRef]
- Sive, H.; Grainger, R.; Harland, R. Early Development of Xenopus laevis: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2000. [Google Scholar]
- Nieuwkoop, P.; Faber, J. Normal Table of Xenopus Laevis (Daudin): A Systematic and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis; Faber, J., Ed.; North-Holland: New York, NY, USA, 1967. [Google Scholar]
- Nieuwkoop, P.; Faber, J. Normal table of Xenopus Laevis.(1967); Daudin: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Thomas, P.D.; Ebert, D.; Muruganujan, A.; Mushayahama, T.; Albou, L.P.; Mi, H. PANTHER: Making genome-scale phylogenetics accessible to all. Protein Sci. 2022, 31, 8–22. [Google Scholar] [CrossRef]
- Firth, H.V.; Richards, S.M.; Bevan, A.P.; Clayton, S.; Corpas, M.; Rajan, D.; Van Vooren, S.; Moreau, Y.; Pettett, R.M.; Carter, N.P. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am. J. Hum. Genet. 2009, 84, 524–533. [Google Scholar] [CrossRef]
- Della Gaspera, B.; Armand, A.S.; Lecolle, S.; Charbonnier, F.; Chanoine, C. Mef2d acts upstream of muscle identity genes and couples lateral myogenesis to dermomyotome formation in Xenopus laevis. PLoS ONE 2012, 7, e52359. [Google Scholar] [CrossRef]
- Sullivan, K.G.; Levin, M. Neurotransmitter signaling pathways required for normal development in Xenopus laevis embryos: A pharmacological survey screen. J. Anat. 2016, 229, 483–502. [Google Scholar] [CrossRef]
- Adams, D.S.; Uzel, S.G.; Akagi, J.; Wlodkowic, D.; Andreeva, V.; Yelick, P.C.; Devitt-Lee, A.; Pare, J.F.; Levin, M. Bioelectric signalling via potassium channels: A mechanism for craniofacial dysmorphogenesis in KCNJ2-associated Andersen-Tawil Syndrome. J. Physiol. 2016, 594, 3245–3270. [Google Scholar] [CrossRef] [PubMed]
- Michailovici, I.; Eigler, T.; Tzahor, E. Craniofacial Muscle Development. Curr. Top. Dev. Biol. 2015, 115, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Schuff, M.; Olsson, L. A role for FoxN3 in the development of cranial cartilages and muscles in Xenopus laevis (Amphibia: Anura: Pipidae) with special emphasis on the novel rostral cartilages. J. Anat. 2011, 218, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.A.; Ostap, E.M.; Goldman, Y.E. Mechanism of inhibition of skeletal muscle actomyosin by N-benzyl-p-toluenesulfonamide. Biochemistry 2003, 42, 6128–6135. [Google Scholar] [CrossRef] [PubMed]
- Galkin, V.E.; Orlova, A.; Egelman, E.H. Actin filaments as tension sensors. Curr. Biol. 2012, 22, R96–R101. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef]
- Shwartz, Y.; Farkas, Z.; Stern, T.; Aszódi, A.; Zelzer, E. Muscle contraction controls skeletal morphogenesis through regulation of chondrocyte convergent extension. Dev. Biol. 2012, 370, 154–163. [Google Scholar] [CrossRef]
- Subramanian, A.; Kanzaki, L.F.; Galloway, J.L.; Schilling, T.F. Mechanical force regulates tendon extracellular matrix organization and tenocyte morphogenesis through TGFbeta signaling. eLife 2018, 7, e38069. [Google Scholar] [CrossRef]
- Matsuyuki, T.; Kitahara, T.; Nakashima, A. Developmental changes in craniofacial morphology in subjects with Duchenne muscular dystrophy. Eur. J. Orthod. 2006, 28, 42–50. [Google Scholar] [CrossRef][Green Version]
- Lightfoot, P.S.; German, R.Z. The effects of muscular dystrophy on craniofacial growth in mice: A study of heterochrony and ontogenetic allometry. J. Morphol. 1998, 235, 1–16. [Google Scholar] [CrossRef]
- Jones, D.C.; Zelditch, M.L.; Peake, P.L.; German, R.Z. The effects of muscular dystrophy on the craniofacial shape of Mus musculus. J. Anat. 2007, 210, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Bhojwani, A.; Hu, J.K. FACEts of mechanical regulation in the morphogenesis of craniofacial structures. Int. J. Oral Sci. 2021, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.; Parada, C.; Chai, Y. A Comprehensive Study of Soft Palate Development in Mice. PLoS ONE 2015, 10, e0145018. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.A. Embryology Buccopharyngeal Membrane. Available online: https://embryology.med.unsw.edu.au/embryology/index.php/Buccopharyngeal_membrane (accessed on 5 August 2022).
- Amin, N.M.; Womble, M.; Ledon-Rettig, C.; Hull, M.; Dickinson, A.; Nascone-Yoder, N. Budgett’s frog (Lepidobatrachus laevis): A new amphibian embryo for developmental biology. Dev. Biol. 2015, 405, 291–303. [Google Scholar] [CrossRef]
- Kindberg, A.; Hu, J.K.; Bush, J.O. Forced to communicate: Integration of mechanical and biochemical signaling in morphogenesis. Curr. Opin. Cell Biol. 2020, 66, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Duchemin, A.L.; Vignes, H.; Vermot, J. Mechanically activated piezo channels modulate outflow tract valve development through the Yap1 and Klf2-Notch signaling axis. eLife 2019, 8, e44706. [Google Scholar] [CrossRef]
- Friedland, F.; Babu, S.; Springer, R.; Konrad, J.; Herfs, Y.; Gerlach, S.; Gehlen, J.; Krause, H.J.; De Laporte, L.; Merkel, R.; et al. ECM-transmitted shear stress induces apoptotic cell extrusion in early breast gland development. Front. Cell Dev. Biol. 2022, 10, 947430. [Google Scholar] [CrossRef]
- Zeng, Y.; Du, X.; Yao, X.; Qiu, Y.; Jiang, W.; Shen, J.; Li, L.; Liu, X. Mechanism of cell death of endothelial cells regulated by mechanical forces. J. Biomech. 2022, 131, 110917. [Google Scholar] [CrossRef]
- Ambrosini, A.; Gracia, M.; Proag, A.; Rayer, M.; Monier, B.; Suzanne, M. Apoptotic forces in tissue morphogenesis. Mech. Dev. 2017, 144, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, F.; Gao, Z.; Zhang, P.; Gao, J.; Wu, X. Regulation of Hippo Signaling by Mechanical Signals and the Cytoskeleton. DNA Cell Biol. 2020, 39, 159–166. [Google Scholar] [CrossRef]
- Vorisek, C.; Weixler, V.; Dominguez, M.; Axt-Fliedner, R.; Hammer, P.E.; Lin, R.Z.; Melero-Martin, J.M.; Del Nido, P.J.; Friehs, I. Mechanical strain triggers endothelial-to-mesenchymal transition of the endocardium in the immature heart. Pediatr. Res. 2022, 92, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Compagnucci, C.; Martinus, K.; Griffin, J.; Depew, M.J. Programmed Cell Death Not as Sledgehammer but as Chisel: Apoptosis in Normal and Abnormal Craniofacial Patterning and Development. Front. Cell Dev. Biol. 2021, 9, 717404. [Google Scholar] [CrossRef] [PubMed]
- Bousoik, E.; Montazeri Aliabadi, H. “Do We Know Jack” About JAK? A Closer Look at JAK/STAT Signaling Pathway. Front. Oncol. 2018, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Park, S.O.; Wamsley, H.L.; Bae, K.; Hu, Z.; Li, X.; Choe, S.W.; Slayton, W.B.; Oh, S.P.; Wagner, K.U.; Sayeski, P.P. Conditional deletion of Jak2 reveals an essential role in hematopoiesis throughout mouse ontogeny: Implications for Jak2 inhibition in humans. PLoS ONE 2013, 8, e59675. [Google Scholar] [CrossRef]
- Jang, Y.N.; Baik, E.J. JAK-STAT pathway and myogenic differentiation. Jakstat 2013, 2, e23282. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dickinson, A.J.G. Jak2 and Jaw Muscles Are Required for Buccopharyngeal Membrane Perforation during Mouth Development. J. Dev. Biol. 2023, 11, 24. https://doi.org/10.3390/jdb11020024
Dickinson AJG. Jak2 and Jaw Muscles Are Required for Buccopharyngeal Membrane Perforation during Mouth Development. Journal of Developmental Biology. 2023; 11(2):24. https://doi.org/10.3390/jdb11020024
Chicago/Turabian StyleDickinson, Amanda J. G. 2023. "Jak2 and Jaw Muscles Are Required for Buccopharyngeal Membrane Perforation during Mouth Development" Journal of Developmental Biology 11, no. 2: 24. https://doi.org/10.3390/jdb11020024
APA StyleDickinson, A. J. G. (2023). Jak2 and Jaw Muscles Are Required for Buccopharyngeal Membrane Perforation during Mouth Development. Journal of Developmental Biology, 11(2), 24. https://doi.org/10.3390/jdb11020024