Getting to the Core: Exploring the Embryonic Development from Notochord to Nucleus Pulposus
Abstract
1. Introduction
2. Embryonic Development of the NP
3. Transcription Factors in NP Development
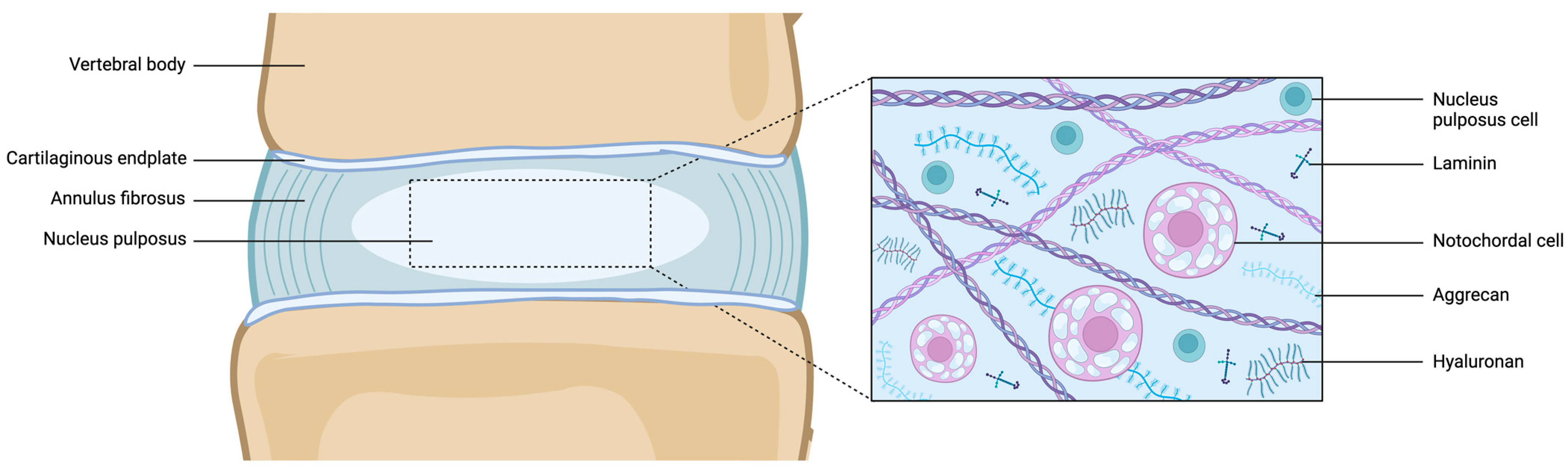
4. Cellular Composition of Notochord to NP
5. The Impact of Aging and Degeneration on the NP
6. The Pathogenesis of Chordoma
7. Potential Therapeutic Strategies: Taking Inspiration from NP Development
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vo, N.V.; Hartman, R.A.; Patil, P.R.; Risbud, M.V.; Kletsas, D.; Iatridis, J.C.; Hoyland, J.A.; Le Maitre, C.L.; Sowa, G.A.; Kang, J.D. Molecular mechanisms of biological aging in intervertebral discs. J. Orthop. Res. 2016, 34, 1289–1306. [Google Scholar] [CrossRef]
- Nerurkar, N.L.; Elliott, D.M.; Mauck, R.L. Mechanical design criteria for intervertebral disc tissue engineering. J. Biomech. 2010, 43, 1017–1030. [Google Scholar] [CrossRef]
- Tilotta, V.; Vadalà, G.; Ambrosio, L.; Cicione, C.; Di Giacomo, G.; Russo, F.; Papalia, R.; Denaro, V. Mesenchymal stem cell-derived secretome enhances nucleus pulposus cell metabolism and modulates extracellular matrix gene expression in vitro. Front. Bioeng. Biotechnol. 2023, 11, 1152207. [Google Scholar] [CrossRef]
- Risbud, M.V.; Schoepflin, Z.R.; Mwale, F.; Kandel, R.A.; Grad, S.; Iatridis, J.C.; Sakai, D.; Hoyland, J.A. Defining the phenotype of young healthy nucleus pulposus cells: Recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. J. Orthop. Res. 2015, 33, 283–293. [Google Scholar] [CrossRef]
- Soma, H.; Sakai, D.; Nakamura, Y.; Tamagawa, S.; Warita, T.; Schol, J.; Matsushita, E.; Naiki, M.; Sato, M.; Watanabe, M. Recombinant Laminin-511 Fragment (iMatrix-511) Coating Supports Maintenance of Human Nucleus Pulposus Progenitor Cells In Vitro. Int. J. Mol. Sci. 2023, 24, 16713. [Google Scholar] [CrossRef]
- Trout, J.J.; Buckwalter, J.A.; Moore, K.C.; Landas, S.K. Ultrastructureofthe human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell 1982, 14, 359–369. [Google Scholar] [CrossRef]
- Matta, A.; Karim, M.Z.; Isenman, D.E.; Erwin, W.M. Molecular Therapy for Degenerative Disc Disease: Clues from Secretome Analysis of the Notochordal Cell-Rich Nucleus Pulposus. Sci. Rep. 2017, 7, srep45623. [Google Scholar] [CrossRef]
- Ambrosio, L.; Mazzuca, G.; Maguolo, A.; Russo, F.; Cannata, F.; Vadalà, G.; Maffeis, C.; Papalia, R.; Denaro, V. The burden of low back pain in children and adolescents with overweight and obesity: From pathophysiology to prevention and treatment strategies. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720X231188831. [Google Scholar] [CrossRef]
- Fournier, D.E.; Kiser, P.K.; Shoemaker, J.K.; Battié, M.C.; Séguin, C.A. Vascularization of the human intervertebral disc: A scoping review. Jor Spine 2020, 3, e1123. [Google Scholar] [CrossRef]
- Cornejo, M.C.; Cho, S.K.; Giannarelli, C.; Iatridis, J.C.; Purmessur, D. Soluble factors from the notochordal-rich intervertebral disc inhibit endothelial cell invasion and vessel formation in the presence and absence of pro-inflammatory cytokines. Osteoarthr. Cartil. 2015, 23, 487–496. [Google Scholar] [CrossRef]
- de Vries, S.; Doeselaar, M.V.; Meij, B.; Tryfonidou, M.; Ito, K. Notochordal Cell Matrix As a Therapeutic Agent for Intervertebral Disc Regeneration. Tissue Eng. Part A 2019, 25, 830–841. [Google Scholar] [CrossRef]
- Reese, D.E.; Hall, C.E.; Mikawa, T. Negative Regulation of Midline Vascular Development by the Notochord. Dev. Cell 2004, 6, 699–708. [Google Scholar] [CrossRef]
- Chen, S.; Fu, P.; Wu, H.; Pei, M. Meniscus, articular cartilage and nucleus pulposus: A comparative review of cartilage-like tissues in anatomy, development and function. Cell Tissue Res. 2017, 370, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Lyu, F.J.; Wang, H.; Zheng, Z. The involvement of immune system in intervertebral disc herniation and degeneration. JOR Spine 2022, 5, e1196. [Google Scholar] [CrossRef]
- Williams, R.J.; Laagland, L.T.; Bach, F.C.; Ward, L.; Chan, W.; Tam, V.; Medzikovic, A.; Basatvat, S.; Paillat, L.; Vedrenne, N.; et al. Recommendations for intervertebral disc notochordal cell investigation: From isolation to characterization. JOR Spine 2023, 6, e1272. [Google Scholar] [CrossRef]
- Schol, J.; Sakai, D. Comprehensive narrative review on the analysis of outcomes from cell transplantation clinical trials for discogenic low back pain. N. Am. Spine Soc. J. 2023, 13, 100195. [Google Scholar] [CrossRef]
- Alkhatib, B.; Ban, G.I.; Williams, S.; Serra, R. IVD Development: Nucleus Pulposus Development and Sclerotome Specification. Curr. Mol. Biol. Rep. 2018, 4, 132–141. [Google Scholar] [CrossRef]
- Stemple, D.L. Structure and function of the notochord: An essential organ for chordate development. Development 2005, 132, 2503–2512. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Tamplin, O.J.; Beckers, A.; Gossler, A.; Rossant, J. Live Imaging and Genetic Analysis of Mouse Notochord Formation Reveals Regional Morphogenetic Mechanisms. Dev. Cell 2007, 13, 884–896. [Google Scholar] [CrossRef]
- Imuta, Y.; Koyama, H.; Shi, D.; Eiraku, M.; Fujimori, T.; Sasaki, H. Mechanical control of notochord morphogenesis by extra-embryonic tissues in mouse embryos. Mech. Dev. 2014, 132, 44–58. [Google Scholar] [CrossRef]
- Smits, P.; Li, P.; Mandel, J.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B.; Lefebvre, V. The Transcription Factors L-Sox5 and Sox6 Are Essential for Cartilage Formation. Dev. Cell 2001, 1, 277–290. [Google Scholar] [CrossRef]
- Choi, K.-S.; Harfe, B.D. Hedgehog signaling is required for formation of the notochord sheath and patterning of nuclei pulposi within the intervertebral discs. Proc. Natl. Acad. Sci. USA 2011, 108, 9484–9489. [Google Scholar] [CrossRef]
- Williams, S.; Alkhatib, B.; Serra, R. Development of the axial skeleton and intervertebral disc. In Vertebrate Skeletal Development; Elsevier: Amsterdam, The Netherlands, 2019; pp. 49–90. [Google Scholar] [CrossRef]
- Kalcheim, C.; Ben-Yair, R. Cell rearrangements during development of the somite and its derivatives. Curr. Opin. Genet. Dev. 2005, 15, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; O’Rahilly, R. The Primitive Streak, the Caudal Eminence and Related Structures in Staged Human Embryos. Cells Tissues Organs 2004, 177, 2–20. [Google Scholar] [CrossRef]
- Lawson, L.; Harfe, B.D. Notochord to Nucleus Pulposus Transition. Curr. Osteoporos. Rep. 2015, 13, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.; Pang, A.S.W.; Evans, S.E.; Stern, C.D. The role of the notochord in amniote vertebral column segmentation. Dev. Biol. 2018, 439, 3–18. [Google Scholar] [CrossRef]
- Sivakamasundari, V.; Lufkin, T. Bridging the Gap: Understanding Embryonic Intervertebral Disc Development. Cell Dev. Biol. 2012, 1, 103. [Google Scholar] [CrossRef]
- Aszódi, A.; Chan, D.; Hunziker, E.; Bateman, J.F.; Fässler, R. Collagen II Is Essential for the Removal of the Notochord and the Formation of Intervertebral Discs. J. Cell Biol. 1998, 143, 1399–1412. [Google Scholar] [CrossRef]
- Ghazanfari, S.; Werner, A.; Ghazanfari, S.; Weaver, J.C.; Smit, T.H. Morphogenesis of aligned collagen fibers in the annulus fibrosus: Mammals versus avians. Biochem. Biophys. Res. Commun. 2018, 503, 1168–1173. [Google Scholar] [CrossRef]
- Spitz, F.; Furlong, E.E. Transcription factors: From enhancer binding to developmental control. Nat. Rev. Genet. 2012, 13, 613–626. [Google Scholar] [CrossRef]
- Nakamichi, R.; Asahara, H. The transcription factors regulating intervertebral disc development. JOR Spine 2020, 3, e1081. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Lo, Y.; Harfe, B.D. Foxa1 and Foxa2 are required for formation of the intervertebral discs. PLoS ONE 2013, 8, e55528. [Google Scholar] [CrossRef]
- Pennimpede, T.; Proske, J.; Konig, A.; Vidigal, J.A.; Morkel, M.; Bramsen, J.B.; Herrmann, B.G.; Wittler, L. In vivo knockdown of Brachyury results in skeletal defects and urorectal malformations resembling caudal regression syndrome. Dev. Biol. 2012, 372, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Kwan, K.M.; Mackem, S. Putative oncogene Brachyury (T) is essential to specify cell fate but dispensable for notochord progenitor proliferation and EMT. Proc. Natl. Acad. Sci. USA 2016, 113, 3820–3825. [Google Scholar] [CrossRef] [PubMed]
- Vujovic, S.; Henderson, S.; Presneau, N.; Odell, E.; Jacques, T.S.; Tirabosco, R.; Boshoff, C.; Flanagan, A.M. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J. Pathol. 2006, 209, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xia, Y.; Yue, C.; Xin, T.; Wang, Q.; Zhang, H.; Shen, C.; Shen, M.; Gu, Y.; Shen, J. Brachyury positively regulates extracellular matrix synthesis via directly promoting aggrecan transcription in nucleus pulposus. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2023, 37, e22976. [Google Scholar] [CrossRef]
- Tang, S.; Richards, J.; Khan, S.; Hoyland, J.; Gallego-Perez, D.; Higuita-Castro, N.; Walter, B.; Purmessur, D. Nonviral Transfection with Brachyury Reprograms Human Intervertebral Disc Cells to a Pro-Anabolic Anti-Catabolic/Inflammatory Phenotype: A Proof of Concept Study. J. Orthop. Res. 2019, 37, 2389–2400. [Google Scholar] [CrossRef]
- Colombier, P.; Halgand, B.; Chedeville, C.; Chariau, C.; Francois-Campion, V.; Kilens, S.; Vedrenne, N.; Clouet, J.; David, L.; Guicheux, J.; et al. NOTO Transcription Factor Directs Human Induced Pluripotent Stem Cell-Derived Mesendoderm Progenitors to a Notochordal Fate. Cells 2020, 9, 509. [Google Scholar] [CrossRef]
- Barrionuevo, F.; Taketo, M.M.; Scherer, G.; Kispert, A. Sox9 is required for notochord maintenance in mice. Dev. Biol. 2006, 295, 128–140. [Google Scholar] [CrossRef]
- Sivakamasundari, V.; Kraus, P.; Sun, W.; Hu, X.; Lim, S.L.; Prabhakar, S.; Lufkin, T. A developmental transcriptomic analysis of Pax1 and Pax9 in embryonic intervertebral disc development. Biol. Open 2017, 6, 187–199. [Google Scholar] [CrossRef]
- Takimoto, A.; Kokubu, C.; Watanabe, H.; Sakuma, T.; Yamamoto, T.; Kondoh, G.; Hiraki, Y.; Shukunami, C. Differential transactivation of the upstream aggrecan enhancer regulated by PAX1/9 depends on SOX9-driven transactivation. Sci. Rep. 2019, 9, 4605. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, I.; Hill, R.E.; Balling, R.; Munsterberg, A.; Imai, K. Pax1 and Pax9 activate Bapx1 to induce chondrogenic differentiation in the sclerotome. Development 2003, 130, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Peters, H.; Wilm, B.; Sakai, N.; Imai, K.; Maas, R.; Balling, R. Pax1 and Pax9 synergistically regulate vertebral column development. Development 1999, 126, 5399–5408. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, E.E.; Wilson, N.; Barcellona, M.N.; Ni Neill, T.; Bagnall, J.; Brama, P.A.J.; Cunniffe, G.M.; Darwish, S.L.; Butler, J.S.; Buckley, C.T. Preclinical to clinical translation for intervertebral disc repair: Effects of species-specific scale, metabolism, and matrix synthesis rates on cell-based regeneration. JOR Spine 2023, 6, e1279. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, E.E.; Buckley, C.T. Consolidating and re-evaluating the human disc nutrient microenvironment. JOR Spine 2022, 5, e1192. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Schipani, E.; Shapiro, I.M. Hypoxic regulation of nucleus pulposus cell survival: From niche to notch. Am. J. Pathol. 2010, 176, 1577–1583. [Google Scholar] [CrossRef]
- Merceron, C.; Mangiavini, L.; Robling, A.; Wilson, T.L.; Giaccia, A.J.; Shapiro, I.M.; Schipani, E.; Risbud, M.V. Loss of HIF-1alpha in the notochord results in cell death and complete disappearance of the nucleus pulposus. PLoS ONE 2014, 9, e110768. [Google Scholar] [CrossRef]
- Thorpe, A.A.; Binch, A.L.; Creemers, L.B.; Sammon, C.; Le Maitre, C.L. Nucleus pulposus phenotypic markers to determine stem cell differentiation: Fact or fiction? Oncotarget 2016, 7, 2189–2200. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Chen, P.B.; Xu, Q.Y.; Li, B.; Jiang, S.D.; Jiang, L.S.; Zheng, X.F. Constitutive and conditional gene knockout mice for the study of intervertebral disc degeneration: Current status, decision considerations, and future possibilities. JOR Spine 2023, 6, e1242. [Google Scholar] [CrossRef]
- Ionescu, A.; Kozhemyakina, E.; Nicolae, C.; Kaestner, K.H.; Olsen, B.R.; Lassar, A.B. FoxA family members are crucial regulators of the hypertrophic chondrocyte differentiation program. Dev. Cell 2012, 22, 927–939. [Google Scholar] [CrossRef]
- Weinstein, D.C.; Ruiz i Altaba, A.; Chen, W.S.; Hoodless, P.; Prezioso, V.R.; Jessell, T.M.; Darnell, J.E., Jr. The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell 1994, 78, 575–588. [Google Scholar] [CrossRef]
- Beckers, A.; Alten, L.; Viebahn, C.; Andre, P.; Gossler, A. The mouse homeobox gene Noto regulates node morphogenesis, notochordal ciliogenesis, and left right patterning. Proc. Natl. Acad. Sci. USA 2007, 104, 15765–15770. [Google Scholar] [CrossRef]
- Abdelkhalek, H.B.; Beckers, A.; Schuster-Gossler, K.; Pavlova, M.N.; Burkhardt, H.; Lickert, H.; Rossant, J.; Reinhardt, R.; Schalkwyk, L.C.; Muller, I.; et al. The mouse homeobox gene Not is required for caudal notochord development and affected by the truncate mutation. Genes Dev. 2004, 18, 1725–1736. [Google Scholar] [CrossRef]
- McCann, M.R.; Tamplin, O.J.; Rossant, J.; Seguin, C.A. Tracing notochord-derived cells using a Noto-cre mouse: Implications for intervertebral disc development. Dis. Models Mech. 2012, 5, 73–82. [Google Scholar] [CrossRef]
- Tsingas, M.; Ottone, O.K.; Haseeb, A.; Barve, R.A.; Shapiro, I.M.; Lefebvre, V.; Risbud, M.V. Sox9 deletion causes severe intervertebral disc degeneration characterized by apoptosis, matrix remodeling, and compartment-specific transcriptomic changes. Matrix Biol. J. Int. Soc. Matrix Biol. 2020, 94, 110–133. [Google Scholar] [CrossRef]
- Smits, P.; Lefebvre, V. Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus of intervertebral discs. Development 2003, 130, 1135–1148. [Google Scholar] [CrossRef]
- Adham, I.M.; Gille, M.; Gamel, A.J.; Reis, A.; Dressel, R.; Steding, G.; Brand-Saberi, B.; Engel, W. The scoliosis (sco) mouse: A new allele of Pax1. Cytogenet. Genome Res. 2005, 111, 16–26. [Google Scholar] [CrossRef]
- Wu, W.J.; Zhang, X.K.; Zheng, X.F.; Yang, Y.H.; Jiang, S.D.; Jiang, L.S. SHH-dependent knockout of HIF-1 alpha accelerates the degenerative process in mouse intervertebral disc. Int. J. Immunopathol. Pharmacol. 2013, 26, 601–609. [Google Scholar] [CrossRef]
- Bach, F.C.; Poramba-Liyanage, D.W.; Riemers, F.M.; Guicheux, J.; Camus, A.; Iatridis, J.C.; Chan, D.; Ito, K.; Le Maitre, C.L.; Tryfonidou, M.A. Notochordal Cell-Based Treatment Strategies and Their Potential in Intervertebral Disc Regeneration. Front. Cell Dev. Biol. 2021, 9, 780749. [Google Scholar] [CrossRef]
- Basatvat, S.; Bach, F.C.; Barcellona, M.N.; Binch, A.L.; Buckley, C.T.; Bueno, B.; Chahine, N.O.; Chee, A.; Creemers, L.B.; Dudli, S.; et al. Harmonization and standardization of nucleus pulposus cell extraction and culture methods. JOR Spine 2023, 6, e1238. [Google Scholar] [CrossRef]
- Mwale, F.; Roughley, P.; Antoniou, J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: A requisite for tissue engineering of intervertebral disc. Eur. Cells Mater. 2004, 8, 58–63, discussion 63–54. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Ludwinski, F.E.; Gnanalingham, K.K.; Atkinson, R.A.; Freemont, A.J.; Hoyland, J.A. Notochordal and nucleus pulposus marker expression is maintained by sub-populations of adult human nucleus pulposus cells through aging and degeneration. Sci. Rep. 2017, 7, 1501. [Google Scholar] [CrossRef] [PubMed]
- Volleman, T.N.E.; Schol, J.; Morita, K.; Sakai, D.; Watanabe, M. Wnt3a and wnt5a as Potential Chondrogenic Stimulators for Nucleus Pulposus Cell Induction: A Comprehensive Review. Neurospine 2020, 17, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; Cohn, M.J.; Harfe, B.D. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: Implications for disk degeneration and chordoma formation. Dev. Dyn. 2008, 237, 3953–3958. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Lim, T.H.; Kim, J.G.; Jeong, S.T.; Masuda, K.; An, H.S. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine (Phila Pa 1976) 2003, 28, 982–990. [Google Scholar] [CrossRef]
- Rodrigues-Pinto, R.; Berry, A.; Piper-Hanley, K.; Hanley, N.; Richardson, S.M.; Hoyland, J.A. Spatiotemporal analysis of putative notochordal cell markers reveals CD24 and keratins 8, 18, and 19 as notochord-specific markers during early human intervertebral disc development. J. Orthop. Res. 2016, 34, 1327–1340. [Google Scholar] [CrossRef]
- Bagwell, J.; Norman, J.; Ellis, K.; Peskin, B.; Hwang, J.; Ge, X.; Nguyen, S.V.; McMenamin, S.K.; Stainier, D.Y.; Bagnat, M. Notochord vacuoles absorb compressive bone growth during zebrafish spine formation. Elife 2020, 9, e51221. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.C.; de Vries, S.A.; Krouwels, A.; Creemers, L.B.; Ito, K.; Meij, B.P.; Tryfonidou, M.A. The species-specific regenerative effects of notochordal cell-conditioned medium on chondrocyte-like cells derived from degenerated human intervertebral discs. Eur. Cells Mater. 2015, 30, 132–146, discussion 146-137. [Google Scholar] [CrossRef] [PubMed]
- Alini, M.; Diwan, A.D.; Erwin, W.M.; Little, C.B.; Melrose, J. An update on animal models of intervertebral disc degeneration and low back pain: Exploring the potential of artificial intelligence to improve research analysis and development of prospective therapeutics. JOR Spine 2023, 6, e1230. [Google Scholar] [CrossRef]
- Alini, M.; Eisenstein, S.M.; Ito, K.; Little, C.; Kettler, A.A.; Masuda, K.; Melrose, J.; Ralphs, J.; Stokes, I.; Wilke, H.J. Are animal models useful for studying human disc disorders/degeneration? Eur. Spine J. 2008, 17, 2–19. [Google Scholar] [CrossRef]
- Thompson, K.; Moore, S.; Tang, S.; Wiet, M.; Purmessur, D. The chondrodystrophic dog: A clinically relevant intermediate-sized animal model for the study of intervertebral disc-associated spinal pain. JOR Spine 2018, 1, e1011. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Schol, J.; Bach, F.C.; Tekari, A.; Sagawa, N.; Nakamura, Y.; Chan, S.C.W.; Nakai, T.; Creemers, L.B.; Frauchiger, D.A.; et al. Successful fishing for nucleus pulposus progenitor cells of the intervertebral disc across species. JOR Spine 2018, 1, e1018. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Chen, P.; Ma, C.Y.; Li, C.; Au, T.Y.K.; Tam, V.; Peng, Y.; Wu, R.; Cheung, K.M.C.; et al. Directed Differentiation of Notochord-like and Nucleus Pulposus-like Cells Using Human Pluripotent Stem Cells. Cell Rep. 2020, 30, 2791–2806.e5. [Google Scholar] [CrossRef]
- Tamagawa, S.; Sakai, D.; Nojiri, H.; Nakamura, Y.; Warita, T.; Matsushita, E.; Schol, J.; Soma, H.; Ogasawara, S.; Munesada, D.; et al. SOD2 orchestrates redox homeostasis in intervertebral discs: A novel insight into oxidative stress-mediated degeneration and therapeutic potential. Redox Biol. 2024, 71, 103091. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.S.; Li, D.D.; Wang, C.G.; Ying, L.W.; Wang, J.K.; Yang, B.; Shu, J.W.; Huang, X.P.; Zhang, Y.A.; Yu, C.; et al. An esterase-responsive ibuprofen nano-micelle pre-modified embryo derived nucleus pulposus progenitor cells promote the regeneration of intervertebral disc degeneration. Bioact. Mater. 2023, 21, 69–85. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Shi, X.; Han, J.; Chen, J.; Zhang, X.; Xie, D.; Li, Z.; Niu, X.; Chen, L.; et al. Characterization of the Nucleus Pulposus Progenitor Cells via Spatial Transcriptomics. Adv. Sci. 2024, 11, e2303752. [Google Scholar] [CrossRef] [PubMed]
- Gogate, S.S.; Fujita, N.; Skubutyte, R.; Shapiro, I.M.; Risbud, M.V. Tonicity enhancer binding protein (TonEBP) and hypoxia-inducible factor (HIF) coordinate heat shock protein 70 (Hsp70) expression in hypoxic nucleus pulposus cells: Role of Hsp70 in HIF-1alpha degradation. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2012, 27, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Johnson, Z.I.; Risbud, M.V. Understanding nucleus pulposus cell phenotype: A prerequisite for stem cell based therapies to treat intervertebral disc degeneration. Curr. Stem Cell Res. Ther. 2015, 10, 307–316. [Google Scholar] [CrossRef]
- Kelsey, R. Targeting NP cell senescence in IVDD. Nat. Rev. Rheumatol. 2024, 20, 197. [Google Scholar] [CrossRef]
- Diwan, A.D.; Melrose, J. Intervertebral disc degeneration and how it leads to low back pain. JOR Spine 2023, 6, e1231. [Google Scholar] [CrossRef]
- Oichi, T.; Taniguchi, Y.; Oshima, Y.; Tanaka, S.; Saito, T. Pathomechanism of intervertebral disc degeneration. JOR Spine 2020, 3, e1076. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Cai, W.; Liu, F.; Cheng, K.; Guo, D.; Liu, Z. An in-depth analysis of the immunomodulatory mechanisms of intervertebral disc degeneration. JOR Spine 2022, 5, e1233. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Schol, J.; Sakai, D.; Warita, T.; Nukaga, T.; Sako, K.; Wangler, S.; Tamagawa, S.; Zeiter, S.; Alini, M.; Grad, S. Homing of vertebral-delivered mesenchymal stromal cells for degenerative intervertebral discs repair—An in vivo proof-of-concept study. JOR Spine 2023, 6, e1228. [Google Scholar] [CrossRef]
- Croft, A.S.; Illien-Junger, S.; Grad, S.; Guerrero, J.; Wangler, S.; Gantenbein, B. The Application of Mesenchymal Stromal Cells and Their Homing Capabilities to Regenerate the Intervertebral Disc. Int. J. Mol. Sci. 2021, 22, 3519. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, M.; Shi, D.; Chen, K.; Zhao, J.; He, S.; Bai, Y.; Shen, P.; Ni, H. Single-cell RNA Seq reveals cellular landscape-specific characteristics and potential etiologies for adolescent idiopathic scoliosis. JOR Spine 2021, 4, e1184. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Glaeser, J.D.; Salehi, K.; Kaneda, G.; Mathkar, P.; Wagner, A.; Ho, R.; Sheyn, D. Single-cell atlas unveils cellular heterogeneity and novel markers in human neonatal and adult intervertebral discs. iScience 2022, 25, 104504. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; He, J.; Zhu, J.; Xu, Z.; Wang, Z.; Yan, J.; Hu, O.; Bai, Z.; Chen, L.; Xie, Y.; et al. Spatially defined single-cell transcriptional profiling characterizes diverse chondrocyte subtypes and nucleus pulposus progenitors in human intervertebral discs. Bone Res. 2021, 9, 37. [Google Scholar] [CrossRef]
- Jiang, W.; Glaeser, J.D.; Kaneda, G.; Sheyn, J.; Wechsler, J.T.; Stephan, S.; Salehi, K.; Chan, J.L.; Tawackoli, W.; Avalos, P.; et al. Intervertebral disc human nucleus pulposus cells associated with back pain trigger neurite outgrowth in vitro and pain behaviors in rats. Sci. Transl. Med. 2023, 15, eadg7020. [Google Scholar] [CrossRef]
- Han, S.; Zhang, Y.; Zhang, X.; Zhang, H.; Meng, S.; Kong, M.; Liu, X.; Ma, X. Single-Cell RNA Sequencing of the Nucleus Pulposus Reveals Chondrocyte Differentiation and Regulation in Intervertebral Disc Degeneration. Front. Cell Dev. Biol. 2022, 10, 824771. [Google Scholar] [CrossRef]
- Li, Z.; Ye, D.; Dai, L.; Xu, Y.; Wu, H.; Luo, W.; Liu, Y.; Yao, X.; Wang, P.; Miao, H.; et al. Single-Cell RNA Sequencing Reveals the Difference in Human Normal and Degenerative Nucleus Pulposus Tissue Profiles and Cellular Interactions. Front. Cell Dev. Biol. 2022, 10, 910626. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Z.; Zhang, H.; Cui, P.; Li, Y.; Chen, X.; Kong, C.; Wang, W.; Lu, S. Single-cell sequencing: New insights for intervertebral disc degeneration. Biomed. Pharmacother. Biomed. Pharmacother. 2023, 165, 115224. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.L.; de Luca, K.; Haile, L.M.; Steinmetz, J.D.; Culbreth, G.T.; Cross, M.; Kopec, J.A.; Ferreira, P.H.; Blyth, F.M.; Buchbinder, R.; et al. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e316–e329. [Google Scholar] [CrossRef] [PubMed]
- Tessier, S.; Risbud, M.V. Understanding embryonic development for cell-based therapies of intervertebral disc degeneration: Toward an effort to treat disc degeneration subphenotypes. Dev. Dyn. 2020, 250, 302–317. [Google Scholar] [CrossRef]
- Walsh, A.J.L.; Lotz, J.C. Biological response of the intervertebral disc to dynamic loading. J. Biomech. 2004, 37, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, L.; Yang, M.; Li, B.; Yi, J.; Ai, X.; Zhang, Y.; Huang, B.; Li, C.; Feng, C.; et al. A positive feedback loop between EZH2 and NOX4 regulates nucleus pulposus cell senescence in age-related intervertebral disc degeneration. Cell Div. 2020, 15, s13008–s13020. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fernández, C.; Francisco, V.; Pino, J.; Mera, A.; González-Gay, M.A.; Gómez, R.; Lago, F.; Gualillo, O. Molecular Relationships among Obesity, Inflammation and Intervertebral Disc Degeneration: Are Adipokines the Common Link? Int. J. Mol. Sci. 2019, 20, 2030. [Google Scholar] [CrossRef] [PubMed]
- Schumann, B.; Bolm-Audorff, U.; Bergmann, A.; Ellegast, R.; Elsner, G.; Grifka, J.; Haerting, J.; Jäger, M.; Michaelis, M.; Seidler, A. Lifestyle factors and lumbar disc disease: Results of a German multi-center case-control study (EPILIFT). Arthritis Res. Ther. 2010, 12, R193. [Google Scholar] [CrossRef]
- Chan, D.; Song, Y.; Sham, P.; Cheung, K.M.C. Genetics of disc degeneration. Eur. Spine J. 2006, 15, 317–325. [Google Scholar] [CrossRef]
- Bonnaire, F.C.; Danalache, M.; Sigwart, V.A.; Breuer, W.; Rolauffs, B.; Hofmann, U.K. The intervertebral disc from embryonic development to disc degeneration: Insights into spatial cellular organization. Spine J. 2021, 21, 1387–1398. [Google Scholar] [CrossRef]
- Peck, S.H.; McKee, K.K.; Tobias, J.W.; Malhotra, N.R.; Harfe, B.D.; Smith, L.J. Whole Transcriptome Analysis of Notochord-Derived Cells during Embryonic Formation of the Nucleus Pulposus. Sci. Rep. 2017, 7, 10504. [Google Scholar] [CrossRef] [PubMed]
- Vergroesen, P.P.; Kingma, I.; Emanuel, K.S.; Hoogendoorn, R.J.; Welting, T.J.; van Royen, B.J.; van Dieen, J.H.; Smit, T.H. Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarthr. Cartil. 2015, 23, 1057–1070. [Google Scholar] [CrossRef]
- Sampara, P.; Banala, R.R.; Vemuri, S.K.; Av, G.R.; Gpv, S. Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: A review. Gene Ther. 2018, 25, 67–82. [Google Scholar] [CrossRef]
- Bachmeier, B.E.; Nerlich, A.; Mittermaier, N.; Weiler, C.; Lumenta, C.; Wuertz, K.; Boos, N. Matrix metalloproteinase expression levels suggest distinct enzyme roles during lumbar disc herniation and degeneration. Eur. Spine J. 2009, 18, 1573–1586. [Google Scholar] [CrossRef]
- Pockert, A.J.; Richardson, S.M.; Le Maitre, C.L.; Lyon, M.; Deakin, J.A.; Buttle, D.J.; Freemont, A.J.; Hoyland, J.A. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 2009, 60, 482–491. [Google Scholar] [CrossRef]
- Francisco, V.; Pino, J.; González-Gay, M.Á.; Lago, F.; Karppinen, J.; Tervonen, O.; Mobasheri, A.; Gualillo, O. A new immunometabolic perspective of intervertebral disc degeneration. Nat. Rev. Rheumatol. 2021, 18, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Mohd Isa, I.L.; Teoh, S.L.; Mohd Nor, N.H.; Mokhtar, S.A. Discogenic Low Back Pain: Anatomy, Pathophysiology and Treatments of Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2022, 24, 208. [Google Scholar] [CrossRef] [PubMed]
- Walcott, B.P.; Nahed, B.V.; Mohyeldin, A.; Coumans, J.V.; Kahle, K.T.; Ferreira, M.J. Chordoma: Current concepts, management, and future directions. Lancet Oncol. 2012, 13, e69–e76. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Sommer, J. Building a global consensus approach to chordoma: A position paper from the medical and patient community. Lancet Oncol. 2015, 16, e71–e83. [Google Scholar] [CrossRef]
- Ulici, V.; Hart, J. Chordoma. Arch. Pathol. Lab. Med. 2022, 146, 386–395. [Google Scholar] [CrossRef]
- Tarpey, P.S.; Behjati, S.; Young, M.D.; Martincorena, I.; Alexandrov, L.B.; Farndon, S.J.; Guzzo, C.; Hardy, C.; Latimer, C.; Butler, A.P.; et al. The driver landscape of sporadic chordoma. Nat. Commun. 2017, 8, 890. [Google Scholar] [CrossRef] [PubMed]
- Mammar, H.; Polivka, M.; Belkacemi, Y.; Lot, G.; Froelich, S.; Carpentier, A.; Clemenceau, S.; Gaillard, S.; Paquis, P.; Birtwisle-Peyrottes, I.; et al. Hypoxia and Metabolism Regulation in Chordomas: Correlation Between Biology and Clinical Features for Potential Targeted Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, E89–E90. [Google Scholar] [CrossRef]
- He, G.; Liu, X. Hypoxia-Inducible Factor-1α (HIF-1α) as a Factor to Predict the Prognosis of Spinal Chordoma. Spine (Phila Pa 1976) 2024, 49, 661–669. [Google Scholar] [CrossRef]
- Kabolizadeh, P.; Chen, Y.-L.; Liebsch, N.; Hornicek, F.J.; Schwab, J.H.; Choy, E.; Rosenthal, D.I.; Niemierko, A.; DeLaney, T.F. Updated Outcome and Analysis of Tumor Response in Mobile Spine and Sacral Chordoma Treated With Definitive High-Dose Photon/Proton Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 254–262. [Google Scholar] [CrossRef]
- Williams, D.; Ford, C. Carbon Ion Beam Therapy for Chordoma: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines; CADTH: Ottawa, ON, Canada, 2018. [Google Scholar]
- Barber, S.M.; Sadrameli, S.S.; Lee, J.J.; Fridley, J.S.; Teh, B.S.; Oyelese, A.A.; Telfeian, A.E.; Gokaslan, Z.L. Chordoma—Current Understanding and Modern Treatment Paradigms. J. Clin. Med. 2021, 10, 1054. [Google Scholar] [CrossRef]
- Dong, M.; Liu, R.; Zhang, Q.; Wang, D.; Luo, H.; Wang, Y.; Chen, J.; Ou, Y.; Wang, X. Efficacy and safety of carbon ion radiotherapy for chordomas: A systematic review and meta-analysis. Radiat. Oncol. 2023, 18, s13014–s13023. [Google Scholar] [CrossRef] [PubMed]
- Härtl, R.; Bonassar, L.; Bonassar, L.J. Biological Approaches to Spinal Disc Repair and Regeneration for Clinicians; Thieme Medical Publishers, Incorporated: Leipzig, Germany, 2017. [Google Scholar]
- Williams, R.J.; Tryfonidou, M.A.; Snuggs, J.W.; Le Maitre, C.L. Cell sources proposed for nucleus pulposus regeneration. JOR Spine 2021, 4, e1175. [Google Scholar] [CrossRef]
- Purmessur, D.; Cornejo, M.C.; Cho, S.K.; Hecht, A.C.; Iatridis, J.C. Notochordal cell-derived therapeutic strategies for discogenic back pain. Glob. Spine J. 2013, 3, 201–218. [Google Scholar] [CrossRef]
- Purmessur, D.; Schek, R.M.; Abbott, R.D.; Ballif, B.A.; Godburn, K.E.; Iatridis, J.C. Notochordal conditioned media from tissue increases proteoglycan accumulation and promotes a healthy nucleus pulposus phenotype in human mesenchymal stem cells. Arthritis Res. Ther. 2011, 13, R81. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Acevedo, L.; Wang, X.; Karim, M.Z.; Matta, A.; Mehrkens, A.; Schaeren, S.; Feliciano, S.; Jakob, M.; Martin, I.; et al. Notochordal cell conditioned medium (NCCM) regenerates end-stage human osteoarthritic articular chondrocytes and promotes a healthy phenotype. Arthritis Res. Ther. 2016, 18, 125. [Google Scholar] [CrossRef]
- Sheyn, D.; Ben-David, S.; Tawackoli, W.; Zhou, Z.; Salehi, K.; Bez, M.; De Mel, S.; Chan, V.; Roth, J.; Avalos, P.; et al. Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model. Theranostics 2019, 9, 7506–7524. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Jing, L.; Willard, V.P.; Wu, C.L.; Guilak, F.; Chen, J.; Setton, L.A. Differentiation of human induced pluripotent stem cells into nucleus pulposus-like cells. Stem Cell Res. Ther. 2018, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lee, E.J.; Jing, L.; Christoforou, N.; Leong, K.W.; Setton, L.A. Differentiation of mouse induced pluripotent stem cells (iPSCs) into nucleus pulposus-like cells in vitro. PLoS ONE 2013, 8, e75548. [Google Scholar] [CrossRef] [PubMed]
- Sako, K.; Sakai, D.; Nakamura, Y.; Schol, J.; Matsushita, E.; Warita, T.; Horikita, N.; Sato, M.; Watanabe, M. Effect of Whole Tissue Culture and Basic Fibroblast Growth Factor on Maintenance of Tie2 Molecule Expression in Human Nucleus Pulposus Cells. Int. J. Mol. Sci. 2021, 22, 4723. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Sakai, D.; Schol, J.; Nakai, T.; Suyama, K.; Watanabe, M. Sciatic nerve regeneration by transplantation of in vitro differentiated nucleus pulposus progenitor cells. Regen Med. 2017, 12, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.; Hackel, S.; Croft, A.S.; Albers, C.E.; Gantenbein, B. The effects of 3D culture on the expansion and maintenance of nucleus pulposus progenitor cell multipotency. JOR Spine 2021, 4, e1131. [Google Scholar] [CrossRef] [PubMed]
- Sako, K.; Sakai, D.; Nakamura, Y.; Matsushita, E.; Schol, J.; Warita, T.; Horikita, N.; Sato, M.; Watanabe, M. Optimization of Spheroid Colony Culture and Cryopreservation of Nucleus Pulposus Cells for the Development of Intervertebral Disc Regenerative Therapeutics. Appl. Sci. 2021, 11, 3309. [Google Scholar] [CrossRef]
- Zhang, X.; Guerrero, J.; Croft, A.S.; Albers, C.E.; Hackel, S.; Gantenbein, B. Spheroid-Like Cultures for Expanding Angiopoietin Receptor-1 (aka. Tie2) Positive Cells from the Human Intervertebral Disc. Int. J. Mol. Sci. 2020, 21, 9423. [Google Scholar] [CrossRef]
- Mehrkens, A.; Matta, A.; Karim, M.Z.; Kim, S.; Fehlings, M.G.; Schaeren, S.; Mark Erwin, W. Notochordal cell-derived conditioned medium protects human nucleus pulposus cells from stress-induced apoptosis. Spine J. Off. J. North Am. Spine Soc. 2017, 17, 579–588. [Google Scholar] [CrossRef]
- Arkesteijn, I.T.; Smolders, L.A.; Spillekom, S.; Riemers, F.M.; Potier, E.; Meij, B.P.; Ito, K.; Tryfonidou, M.A. Effect of coculturing canine notochordal, nucleus pulposus and mesenchymal stromal cells for intervertebral disc regeneration. Arthritis Res. Ther. 2015, 17, 60. [Google Scholar] [CrossRef]
- Erwin, W.M.; Ashman, K.; O’Donnel, P.; Inman, R.D. Nucleus pulposus notochord cells secrete connective tissue growth factor and up-regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum. 2006, 54, 3859–3867. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, T.J.; Vaso, K.; Danias, G.; Chionuma, H.N.; Weiser, J.R.; Iatridis, J.C. Extracellular Vesicles as an Emerging Treatment Option for Intervertebral Disc Degeneration: Therapeutic Potential, Translational Pathways, and Regulatory Considerations. Adv. Healthc. Mater. 2022, 11, e2100596. [Google Scholar] [CrossRef] [PubMed]
- Krut, Z.; Pelled, G.; Gazit, D.; Gazit, Z. Stem Cells and Exosomes: New Therapies for Intervertebral Disc Degeneration. Cells 2021, 10, 2241. [Google Scholar] [CrossRef]
- Tilotta, V.; Vadalà, G.; Ambrosio, L.; Di Giacomo, G.; Cicione, C.; Russo, F.; Darinskas, A.; Papalia, R.; Denaro, V. Wharton’s Jelly mesenchymal stromal cell-derived extracellular vesicles promote nucleus pulposus cell anabolism in an in vitro 3D alginate-bead culture model. JOR Spine 2024, 7, e1274. [Google Scholar] [CrossRef] [PubMed]
- Tilotta, V.; Vadalà, G.; Ambrosio, L.; Russo, F.; Cicione, C.; Di Giacomo, G.; Papalia, R.; Denaro, V. Mesenchymal Stem Cell-Derived Exosomes: The New Frontier for the Treatment of Intervertebral Disc Degeneration. Appl. Sci. 2021, 11, 11222. [Google Scholar] [CrossRef]
- Bach, F.; Libregts, S.; Creemers, L.; Meij, B.; Ito, K.; Wauben, M.; Tryfonidou, M. Notochordal-cell derived extracellular vesicles exert regenerative effects on canine and human nucleus pulposus cells. Oncotarget 2017, 8, 88845–88856. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.C.; de Vries, S.A.; Riemers, F.M.; Boere, J.; van Heel, F.W.; van Doeselaar, M.; Goerdaya, S.S.; Nikkels, P.G.; Benz, K.; Creemers, L.B.; et al. Soluble and pelletable factors in porcine, canine and human notochordal cell-conditioned medium: Implications for IVD regeneration. Eur. Cell Mater. 2016, 32, 163–180. [Google Scholar] [CrossRef]
- Ambrosio, L.; Schol, J.; Ruiz-Fernandez, C.; Tamagawa, S.; Soma, H.; Tilotta, V.; Di Giacomo, G.; Cicione, C.; Nakayama, S.; Kamiya, K.; et al. ISSLS PRIZE in Basic Science 2024: Superiority of nucleus pulposus cell-versus mesenchymal stromal cell-derived extracellular vesicles in attenuating disc degeneration and alleviating pain. Eur. Spine J. 2024, 33, 1713–1727. [Google Scholar] [CrossRef]
- Hubert, M.G.; Vadala, G.; Sowa, G.; Studer, R.K.; Kang, J.D. Gene therapy for the treatment of degenerative disk disease. J. Am. Acad. Orthop. Surg. 2008, 16, 312–319. [Google Scholar] [CrossRef]
- Vadala, G.; Sowa, G.A.; Smith, L.; Hubert, M.G.; Levicoff, E.A.; Denaro, V.; Gilbertson, L.G.; Kang, J.D. Regulation of transgene expression using an inducible system for improved safety of intervertebral disc gene therapy. Spine (Phila Pa 1976) 2007, 32, 1381–1387. [Google Scholar] [CrossRef]
- Seki, S.; Iwasaki, M.; Makino, H.; Yahara, Y.; Miyazaki, Y.; Kamei, K.; Futakawa, H.; Nogami, M.; Tran Canh Tung, N.; Hirokawa, T.; et al. Direct Reprogramming and Induction of Human Dermal Fibroblasts to Differentiate into iPS-Derived Nucleus Pulposus-like Cells in 3D Culture. Int. J. Mol. Sci. 2022, 23, 4059. [Google Scholar] [CrossRef]
- Cicione, C.; Tilotta, V.; Giacomo, G.D.; Ambrosio, L.; Russo, F.; Papalia, R.; Vadalà, G.; Denaro, V. Mesendoderm Progenitor Cells Derived from Pluripotent Stem Cells for Disc Regeneration: A Preliminary Study in an Ovine Model. Orthop. Proc. 2024, 106-B, 104. [Google Scholar] [CrossRef]
- Vadala, G.; Sowa, G.A.; Kang, J.D. Gene therapy for disc degeneration. Expert Opin. Biol. Ther. 2007, 7, 185–196. [Google Scholar] [CrossRef]
- Levicoff, E.A.; Kim, J.S.; Sobajima, S.; Wallach, C.J.; Larson, J.W., 3rd; Robbins, P.D.; Xiao, X.; Juan, L.; Vadala, G.; Gilbertson, L.G.; et al. Safety assessment of intradiscal gene therapy II: Effect of dosing and vector choice. Spine (Phila Pa 1976) 2008, 33, 1509–1516, discussion 1517. [Google Scholar] [CrossRef]
- Bach, F.C.; Tellegen, A.R.; Beukers, M.; Miranda-Bedate, A.; Teunissen, M.; de Jong, W.A.M.; de Vries, S.A.H.; Creemers, L.B.; Benz, K.; Meij, B.P.; et al. Biologic canine and human intervertebral disc repair by notochordal cell-derived matrix: From bench towards bedside. Oncotarget 2018, 9, 26507–26526. [Google Scholar] [CrossRef]
- Potier, E.; de Vries, S.; van Doeselaar, M.; Ito, K. Potential application of notochordal cells for intervertebral disc regeneration: An in vitro assessment. Eur. Cells Mater. 2014, 28, 68–80, discussion 80-61. [Google Scholar] [CrossRef]
- Schmitz, T.C.; van Genabeek, B.; Pouderoijen, M.J.; Janssen, H.M.; van Doeselaar, M.; Crispim, J.F.; Tryfonidou, M.A.; Ito, K. Semi-synthetic degradable notochordal cell-derived matrix hydrogel for use in degenerated intervertebral discs: Initial in vitro characterization. J. Biomed. Mater. Res. Part A 2023, 111, 1903–1915. [Google Scholar] [CrossRef]
- Schmitz, T.C.; van Doeselaar, M.; Tryfonidou, M.A.; Ito, K. Detergent-Free Decellularization of Notochordal Cell-Derived Matrix Yields a Regenerative, Injectable, and Swellable Biomaterial. ACS Biomater. Sci. Eng. 2022, 8, 3912–3923. [Google Scholar] [CrossRef]
- Thorpe, A.A.; Bach, F.C.; Tryfonidou, M.A.; Le Maitre, C.L.; Mwale, F.; Diwan, A.D.; Ito, K. Leaping the hurdles in developing regenerative treatments for the intervertebral disc from preclinical to clinical. JOR Spine 2018, 1, e1027. [Google Scholar] [CrossRef]
- Takahashi, T.; Donahue, R.P.; Nordberg, R.C.; Hu, J.C.; Currall, S.C.; Athanasiou, K.A. Commercialization of regenerative-medicine therapies. Nat. Rev. Bioeng. 2023, 1, 906–929. [Google Scholar] [CrossRef]
- Silverman, L.I.; Flanagan, F.; Rodriguez-Granrose, D.; Simpson, K.; Saxon, L.H.; Foley, K.T. Identifying and Managing Sources of Variability in Cell Therapy Manufacturing and Clinical Trials. Regen. Eng. Transl. Med. 2019, 5, 354–361. [Google Scholar] [CrossRef]



| Factors | Role in Embryogenesis | KO Mice [50] | Regulates | References |
|---|---|---|---|---|
| FOXA1/FOXA2 | Spatiotemporal regulation NP development Activator of NC phenotype Regulates transition from NCs to NPCs | Single KO: no change Double KO: NP deformation/notochord fails to form Deformity most evident posteriorly High cell death in posterior somites Shorter tails | NOTO TBXT | [33,51,52] |
| TBXT | Regulates mesodermal precursor differentiation into NCs Supports maintenance of the notochord Regulates transition from NCs to NPCs Promotes aggrecan expression | Loss of somite formation | NOTO | [34,35,36] |
| NOTO | Regulates specification and formation of the notochord Left/right patterning | Moderate notochord malformations | [53,54,55] | |
| SOX5/SOX6/SOX9 | Regulates notochordal sheath formation Promotes transition from NCs to NPCs Defines the inner and outer AF region Promotes aggrecan and type II collagen expression | Severe chondrodysplasia Prevents proper segmentation Prevents NP formation Postnatal KO induces significant IDD | PAX1/9 | [21,56,57] |
| PAX1/PAX9 | Somite patterning Interferes with Sox5/6/9-induced aggrecan expression Defines the inner and outer AF region | No separation between vertebrae and discs Prevents sclerotome chondrogenesis Scoliotic-like deformity | SOX5/6 | [41,42,44,58] |
| HIF-1α | Regulates the adaptation to hypoxia | Normal notochord development Reduced NP size (at E15.5) Fibrocartilaginous NP (at 1M) NPCs lacking vacuoles High cell death in NP | [48,59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosio, L.; Schol, J.; Ruiz-Fernández, C.; Tamagawa, S.; Joyce, K.; Nomura, A.; de Rinaldis, E.; Sakai, D.; Papalia, R.; Vadalà, G.; et al. Getting to the Core: Exploring the Embryonic Development from Notochord to Nucleus Pulposus. J. Dev. Biol. 2024, 12, 18. https://doi.org/10.3390/jdb12030018
Ambrosio L, Schol J, Ruiz-Fernández C, Tamagawa S, Joyce K, Nomura A, de Rinaldis E, Sakai D, Papalia R, Vadalà G, et al. Getting to the Core: Exploring the Embryonic Development from Notochord to Nucleus Pulposus. Journal of Developmental Biology. 2024; 12(3):18. https://doi.org/10.3390/jdb12030018
Chicago/Turabian StyleAmbrosio, Luca, Jordy Schol, Clara Ruiz-Fernández, Shota Tamagawa, Kieran Joyce, Akira Nomura, Elisabetta de Rinaldis, Daisuke Sakai, Rocco Papalia, Gianluca Vadalà, and et al. 2024. "Getting to the Core: Exploring the Embryonic Development from Notochord to Nucleus Pulposus" Journal of Developmental Biology 12, no. 3: 18. https://doi.org/10.3390/jdb12030018
APA StyleAmbrosio, L., Schol, J., Ruiz-Fernández, C., Tamagawa, S., Joyce, K., Nomura, A., de Rinaldis, E., Sakai, D., Papalia, R., Vadalà, G., & Denaro, V. (2024). Getting to the Core: Exploring the Embryonic Development from Notochord to Nucleus Pulposus. Journal of Developmental Biology, 12(3), 18. https://doi.org/10.3390/jdb12030018










