Lowered GnT-I Activity Decreases Complex-Type N-Glycan Amounts and Results in an Aberrant Primary Motor Neuron Structure in the Spinal Cord
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish
2.2. Dissections of Tissue
2.3. Total Membrane Preparations and Homogenate
2.4. Coomassie Blue-Stained Gels and Lectin Blots
2.5. N-Glycan Profiling
2.6. Spinner Task Assay
2.7. Zebrafish Hatching and Swim Bladder Developmental Evaluations
2.8. Motor Skill Measurements via Mean Square Difference Analysis
2.9. Microscopy of Primary Motor Neurons in Live Embryos
2.10. Statistical Analysis
3. Results
3.1. N-Glycan Profiles of Spinal Cords from Wt AB Zebrafish Lines
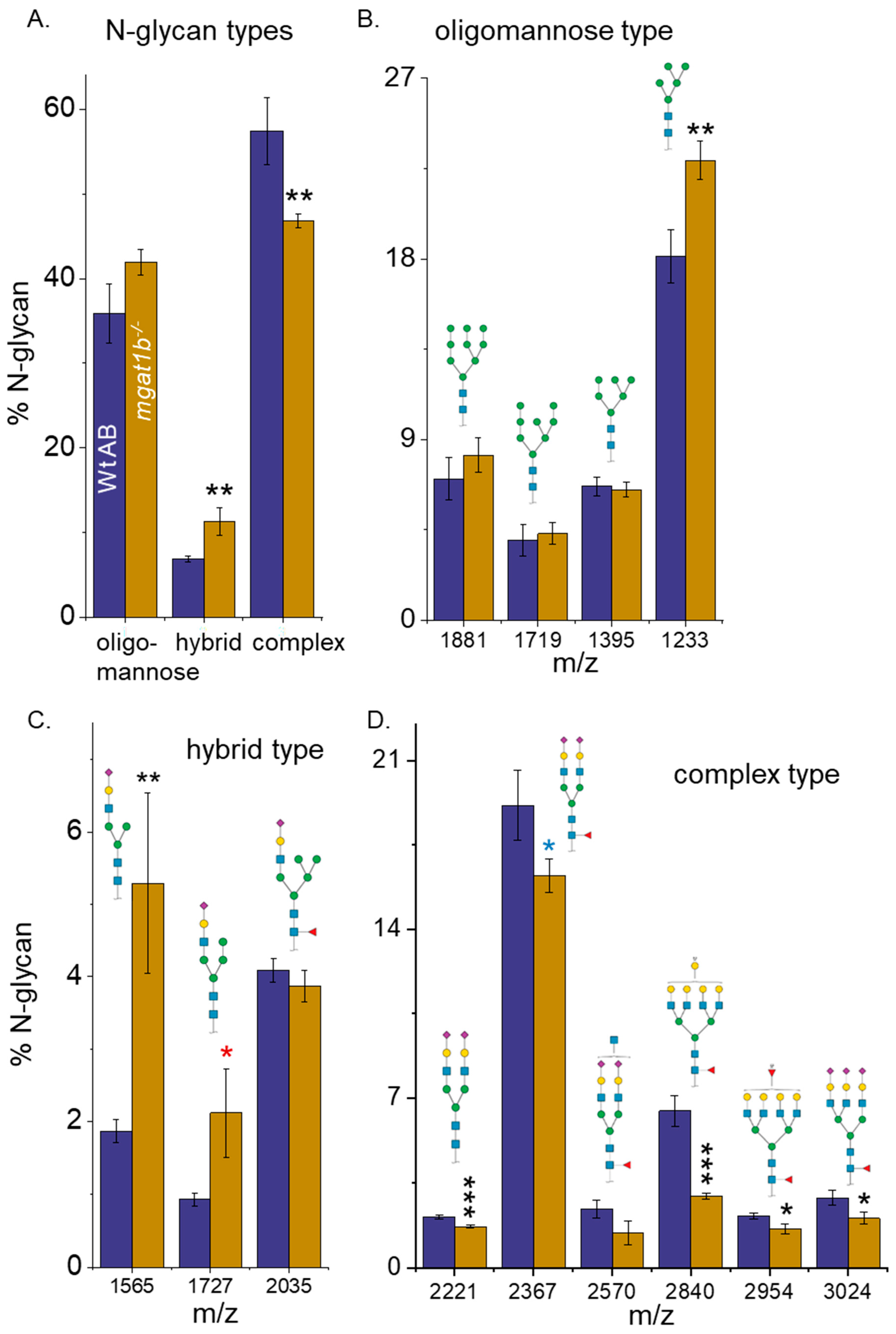
3.2. Differences in the N-Glycan Populations in Spinal Cords from Adult Wt AB and Mgat1b−/− Zebrafish
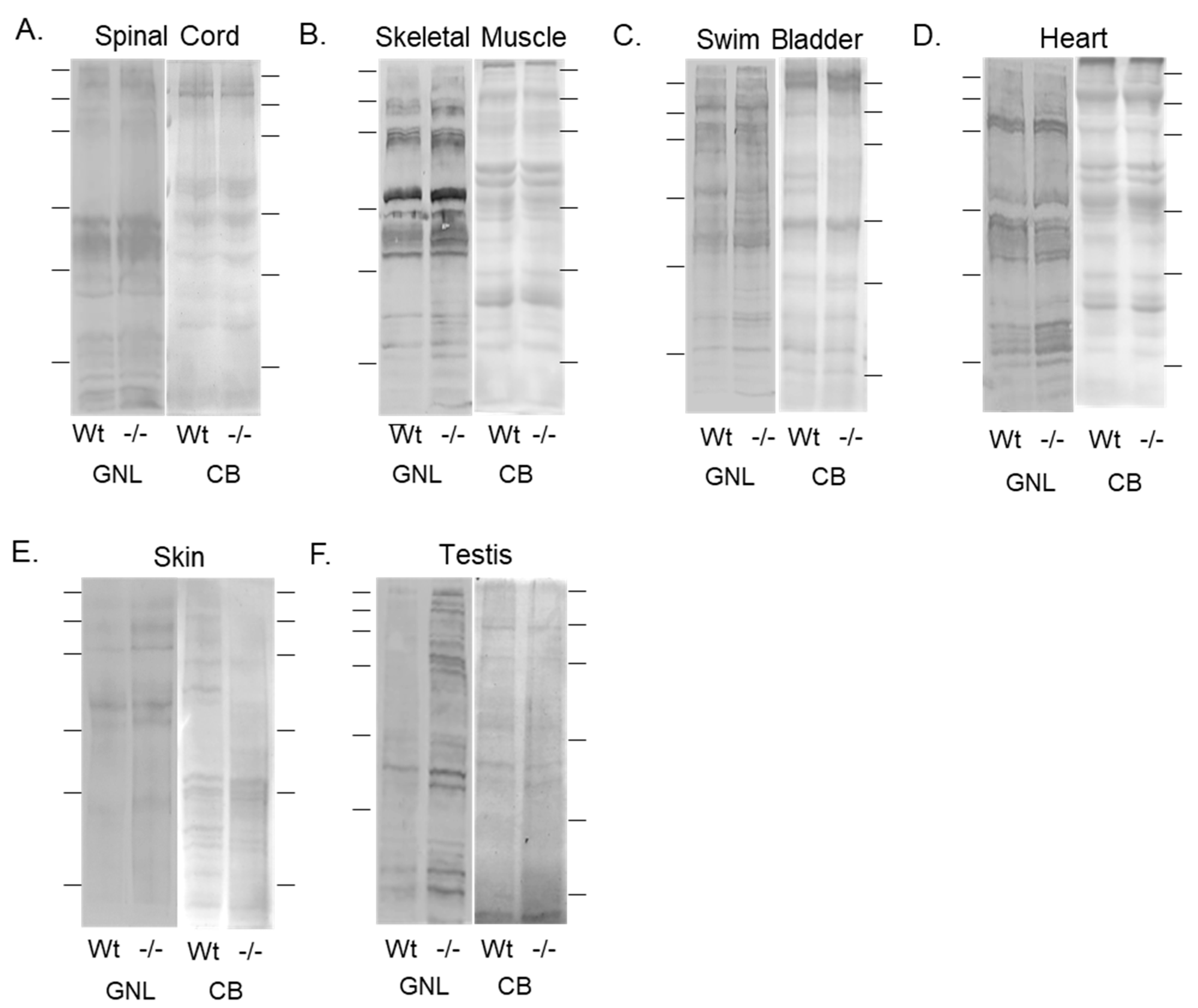
3.3. Oligomannose-Type N-Glycans Were Increased in Various Tissues of the Mgat1b Mutant Relative to the Wt AB Zebrafish
3.4. Primary Motor Neurons in the Spinal Cord of Mgat1b Mutant Fish Are Maldeveloped
3.5. Hatching and Swim Bladder Development Is Delayed
3.6. Motor Coordination and Resistance Are Impaired in the Mgat1b Mutant Fish
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varki, A. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology 1993, 3, 97–130. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.G.; Veillon, L.; Mechref, Y. N-Glycan Profile of Cerebrospinal Fluids from Alzheimer’s Disease Patients Using Liquid Chromatography with Mass Spectrometry. J. Proteome Res. 2019, 18, 3770–3779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conroy, L.R.; Hawkinson, T.R.; Young, L.E.A.; Gentry, M.S.; Sun, R.C. Emerging roles of N-linked glycosylation in brain physiology and disorders. Trends Endocrinol Metab. 2021, 32, 980–993. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cvetko, A.; Kifer, D.; Gornik, O.; Klarić, L.; Visser, E.; Lauc, G.; Wilson, J.F.; Štambuk, T. Glycosylation Alterations in Multiple Sclerosis Show Increased Proinflammatory Potential. Biomedicines 2020, 8, 410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fajersztajn, L.; Cordeiro, R.C.; Andreoni, S.; Garcia, J.T. Effects of functional physical activity on the maintenance of motor function in Alzheimer’s disease. Dement. Neuropsychol. 2008, 2, 233–240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kizuka, Y.; Kitazume, S.; Taniguchi, N. N-glycan and Alzheimer’s disease. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Grigorian, A.; Pawling, J.; Chen, I.J.; Gao, G.; Mozaffar, T.; McKerlie, C.; Demetriou, M. N-glycan processing deficiency promotes spontaneous inflammatory demyelination and neurodegeneration. J. Biol. Chem. 2007, 282, 33725–33734. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.A.; Chakravarthy, S.; Phillips, J.R.; Gupta, A.; Keri, S.; Polner, B.; Frank, M.J.; Jahanshahi, M. Motor symptoms in Parkinson’s disease: A unified framework. Neurosci. Biobehav. Rev. 2016, 68, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, P.; Kang, H.; Lee, B. Glycosylation and behavioral symptoms in neurological disorders. Transl. Psychiatry 2023, 13, 154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schedin-Weiss, S.; Winblad, B.; Tjernberg, L.O. The role of protein glycosylation in Alzheimer disease. Febs. J. 2014, 281, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Videira, P.A.Q.; Castro-Caldas, M. Linking Glycation and Glycosylation With Inflammation and Mitochondrial Dysfunction in Parkinson’s Disease. Front. Neurosci. 2018, 12, 381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wajda, D.A.; Sosnoff, J.J. Cognitive-motor interference in multiple sclerosis: A systematic review of evidence, correlates, and consequences. Biomed Res. Int. 2015, 2015, 720856. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, M.; Jin, H.; Wu, Z.; Han, Y.; Chen, J.; Mao, C.; Hao, P.; Zhang, X.; Liu, C.F.; Yang, S. Mass Spectrometry-Based Analysis of Serum N-Glycosylation Changes in Patients with Parkinson’s Disease. ACS Chem. Neurosci. 2022, 13, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Sturiale, L.; Fiumara, A.; Palmigiano, A.; Bua, R.O.; Rizzo, R.; Zappia, M.; Garozzo, D. CSF N-glycan profile reveals sialylation deficiency in a patient with GM2 gangliosidosis presenting as childhood disintegrative disorder. Autism. Res. 2016, 9, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Cast, T.P.; Boesch, D.J.; Smyth, K.; Shaw, A.E.; Ghebrial, M.; Chanda, S. An Autism-Associated Mutation Impairs Neuroligin-4 Glycosylation and Enhances Excitatory Synaptic Transmission in Human Neurons. J. Neurosci. 2021, 41, 392–407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dwyer, C.A.; Esko, J.D. Glycan susceptibility factors in autism spectrum disorders. Mol. Aspects Med. 2016, 51, 104–114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pivac, N.; Knezević, A.; Gornik, O.; Pucić, M.; Igl, W.; Peeters, H.; Crepel, A.; Steyaert, J.; Novokmet, M.; Redzić, I.; et al. Human plasma glycome in attention-deficit hyperactivity disorder and autism spectrum disorders. Mol. Cell Proteomics 2011, 10, M110.004200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, I.J.; He, M.; Lam, C.T. Congenital disorders of glycosylation. Ann. Transl. Med. 2018, 6, 477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paprocka, J.; Jezela-Stanek, A.; Tylki-Szymańska, A.; Grunewald, S. Congenital Disorders of Glycosylation from a Neurological Perspective. Brain Sci. 2021, 11, 88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verheijen, J.; Tahata, S.; Kozicz, T.; Witters, P.; Morava, E. Therapeutic approaches in Congenital Disorders of Glycosylation (CDG) involving N-linked glycosylation: An update. Genet. Med. 2020, 22, 268–279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stanley, P.; Moremen, K.W.; Lewis, N.E.; Taniguchi, N.; Aebi, M. N-Glycans. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022. [Google Scholar]
- Sur, A.; Wang, Y.; Capar, P.; Margolin, G.; Prochaska, M.K.; Farrell, J.A. Single-cell analysis of shared signatures and transcriptional diversity during zebrafish development. Dev Cell 2023, 58, 3028–3047.e12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ioffe, E.; Stanley, P. Mice lacking N-acetylglucosaminyltransferase I activity die at mid-gestation, revealing an essential role for complex or hybrid N-linked carbohydrates. Proc. Natl. Acad. Sci. USA 1994, 91, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Schachter, H.; Marth, J.D. Mice with a homozygous deletion of the Mgat2 gene encoding UDP-N-acetylglucosamine:alpha-6-D-mannoside beta1,2-N-acetylglucosaminyltransferase II: A model for congenital disorder of glycosylation type IIa. Biochim. Biophys. Acta. 2002, 1573, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tan, J.; Sutton-Smith, M.; Ditto, D.; Panico, M.; Campbell, R.M.; Varki, N.M.; Long, J.M.; Jaeken, J.; Levinson, S.R.; et al. Modeling human congenital disorder of glycosylation type IIa in the mouse: Conservation of asparagine-linked glycan-dependent functions in mammalian physiology and insights into disease pathogenesis. Glycobiology 2001, 11, 1051–1070. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Marth, J.D. N-glycan branching requirement in neuronal and postnatal viability. Glycobiology 2004, 14, 547–558. [Google Scholar] [CrossRef]
- Tan, J.; Dunn, J.; Jaeken, J.; Schachter, H. Mutations in the MGAT2 gene controlling complex N-glycan synthesis cause carbohydrate-deficient glycoprotein syndrome type II, an autosomal recessive disease with defective brain development. Am. J. Hum. Genet. 1996, 59, 810–817. [Google Scholar] [PubMed] [PubMed Central]
- Hanzawa, K.; Suzuki, N.; Natsuka, S. Structures and developmental alterations of N-glycans of zebrafish embryos. Glycobiology 2017, 27, 228–245. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, N.; Vanbeselaere, J.; Chang, L.Y.; Yu, S.Y.; Ducrocq, L.; Harduin-Lepers, A.; Kurata, J.; Aoki-Kinoshita, K.F.; Sato, C.; Khoo, K.H.; et al. Systems glycomics of adult zebrafish identifies organ-specific sialylation and glycosylation patterns. Nat. Commun. 2018, 9, 4647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hall, M.K.; Hatchett, C.J.; Shalygin, S.; Azadi, P.; Schwalbe, R.A. Reduction in N-Acetylglucosaminyltransferase-I Activity Decreases Survivability and Delays Development of Zebrafish. Curr. Issues Mol. Biol. 2023, 45, 9165–9180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, K.T.; Amin, M.R.; Shah, P.; Ali, D.W. Motor neuron development in zebrafish is altered by brief (5-hr) exposures to THC (∆(9)-tetrahydrocannabinol) or CBD (cannabidiol) during gastrulation. Sci. Rep. 2018, 8, 10518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.L.; Ribera, A.B. Zebrafish motor neuron subtypes differ electrically prior to axonal outgrowth. J. Neurophysiol. 2009, 102, 2477–2484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wen, H.; Brehm, P. Paired motor neuron-muscle recordings in zebrafish test the receptor blockade model for shaping synaptic current. J. Neurosci. 2005, 25, 8104–8111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lewis, K.E.; Eisen, J.S. From cells to circuits: Development of the zebrafish spinal cord. Prog. Neurobiol. 2003, 69, 419–449. [Google Scholar] [CrossRef] [PubMed]
- Myers, P.Z.; Eisen, J.S.; Westerfield, M. Development and axonal outgrowth of identified motoneurons in the zebrafish. J. Neurosci. 1986, 6, 2278–2289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sainath, R.; Granato, M. Plexin A3 and turnout regulate motor axonal branch morphogenesis in zebrafish. PLoS ONE 2013, 8, e54071. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Westerfield, M.; Liu, D.W.; Kimmel, C.B.; Walker, C. Pathfinding and synapse formation in a zebrafish mutant lacking functional acetylcholine receptors. Neuron 1990, 4, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.K.; Weidner, D.A.; Chen, J.; Bernetski, C.J.; Schwalbe, R.A. Glycan structures contain information for the spatial arrangement of glycoproteins in the plasma membrane. PLoS ONE 2013, 8, e75013. [Google Scholar] [CrossRef] [PubMed]
- Blazina, A.R.; Vianna, M.R.; Lara, D.R. The spinning task: A new protocol to easily assess motor coordination and resistance in zebrafish. Zebrafish 2013, 10, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.K.; Stanley, P. Lectin-resistant CHO glycosylation mutants. Methods Enzymol 2006, 416, 159–182. [Google Scholar] [PubMed]
- Rudy, B.; McBain, C.J. Kv3 channels: Voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001, 24, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Issa, F.A.; Hall, M.K.; Hatchett, C.J.; Weidner, D.A.; Fiorenza, A.C.; Schwalbe, R.A. Compromised N-Glycosylation Processing of Kv3.1b Correlates with Perturbed Motor Neuron Structure and Locomotor Activity. Biology 2021, 10, 486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ronan, R.; Kshirsagar, A.; Rebelo, A.L.; Sunny, A.; Kilcoyne, M.; Flaherty, R.O.; Rudd, P.M.; Schlosser, G.; Saldova, R.; Pandit, A.; et al. Distinct Glycosylation Responses to Spinal Cord Injury in Regenerative and Nonregenerative Models. J. Proteome Res. 2022, 21, 1449–1466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.J.; Wing, D.R.; Guile, G.R.; Dwek, R.A.; Harvey, D.J.; Zamze, S. Neutral N-glycans in adult rat brain tissue--complete characterisation reveals fucosylated hybrid and complex structures. Eur. J. Biochem. 1998, 251, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.K.; Weidner, D.A.; Dayal, S.; Pak, E.; Murashov, A.K.; Schwalbe, R.A. Membrane Distribution and Activity of a Neuronal Voltage-Gated K+ Channel is Modified by Replacement of Complex Type N-Glycans with Hybrid Type. J. Glycobiol. 2017, 6, 128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eisen, J.S.; Myers, P.Z.; Westerfield, M. Pathway selection by growth cones of identified motoneurones in live zebra fish embryos. Nature 1986, 320, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.W.; Westerfield, M. Function of identified motoneurones and co-ordination of primary and secondary motor systems during zebra fish swimming. J. Physiol. 1988, 403, 73–89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fetcho, J.R.; Higashijima, S.; McLean, D.L. Zebrafish and motor control over the last decade. Brain Res. Rev. 2008, 57, 86–93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Issa, F.A.; Mazzochi, C.; Mock, A.F.; Papazian, D.M. Spinocerebellar ataxia type 13 mutant potassium channel alters neuronal excitability and causes locomotor deficits in zebrafish. J. Neurosci. 2011, 31, 6831–6841. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
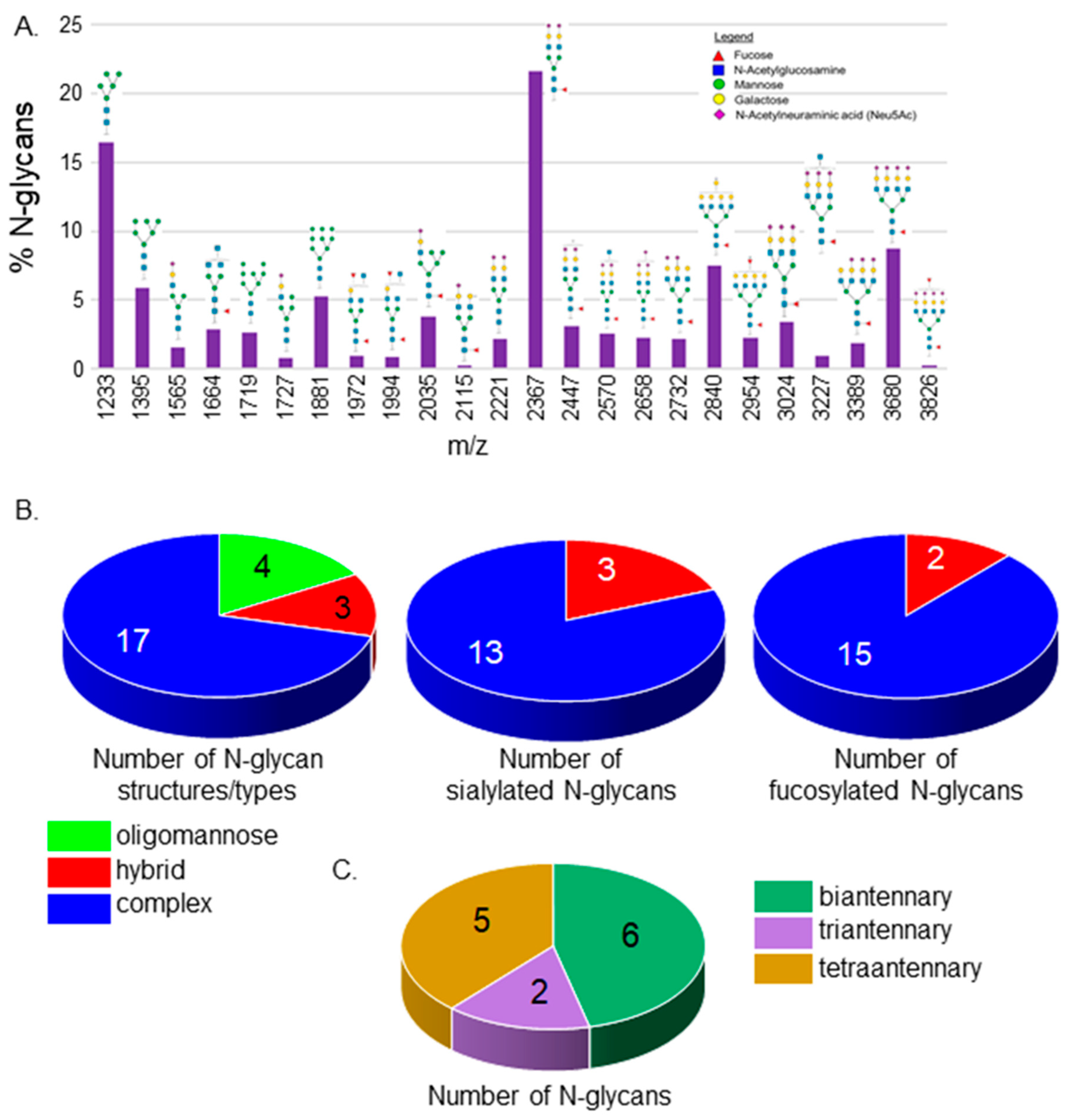


| N-Glycan | Wt AB | mgat1b−/− |
|---|---|---|
| Sialylated | 49.0 ± 4.8 | 45.6 ± 2.7 |
| Fucosylated | 59.4 ± 3.8 | 49.0 ± 0.7 ** |
| Bi-antennary | 27.6 ± 1.6 | 25.3 ± 1.1 |
| Tri-antennary | 4.8 ± 0.5 | 3.9 ± 0.4 |
| Tetra-antennary | 15.3 ± 4.0 | 7.8 ± 0.9 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatchett, C.J.; Hall, M.K.; Messer, A.R.; Schwalbe, R.A. Lowered GnT-I Activity Decreases Complex-Type N-Glycan Amounts and Results in an Aberrant Primary Motor Neuron Structure in the Spinal Cord. J. Dev. Biol. 2024, 12, 21. https://doi.org/10.3390/jdb12030021
Hatchett CJ, Hall MK, Messer AR, Schwalbe RA. Lowered GnT-I Activity Decreases Complex-Type N-Glycan Amounts and Results in an Aberrant Primary Motor Neuron Structure in the Spinal Cord. Journal of Developmental Biology. 2024; 12(3):21. https://doi.org/10.3390/jdb12030021
Chicago/Turabian StyleHatchett, Cody J., M. Kristen Hall, Abel R. Messer, and Ruth A. Schwalbe. 2024. "Lowered GnT-I Activity Decreases Complex-Type N-Glycan Amounts and Results in an Aberrant Primary Motor Neuron Structure in the Spinal Cord" Journal of Developmental Biology 12, no. 3: 21. https://doi.org/10.3390/jdb12030021
APA StyleHatchett, C. J., Hall, M. K., Messer, A. R., & Schwalbe, R. A. (2024). Lowered GnT-I Activity Decreases Complex-Type N-Glycan Amounts and Results in an Aberrant Primary Motor Neuron Structure in the Spinal Cord. Journal of Developmental Biology, 12(3), 21. https://doi.org/10.3390/jdb12030021







