Varanid Teeth Asymmetry and Correlation to Body Size
Abstract
1. Introduction
1.1. Stress and Asymmetry
1.2. Dental Asymmetry
1.3. Body Size as a Stressor
2. Materials and Methods
2.1. Materials
2.2. Nomenclature
2.3. Photography and Data Collection
2.4. Tooth Asymmetry Calculation and Statistical Analyses
3. Results
3.1. Tooth Asymmetry Based on Position
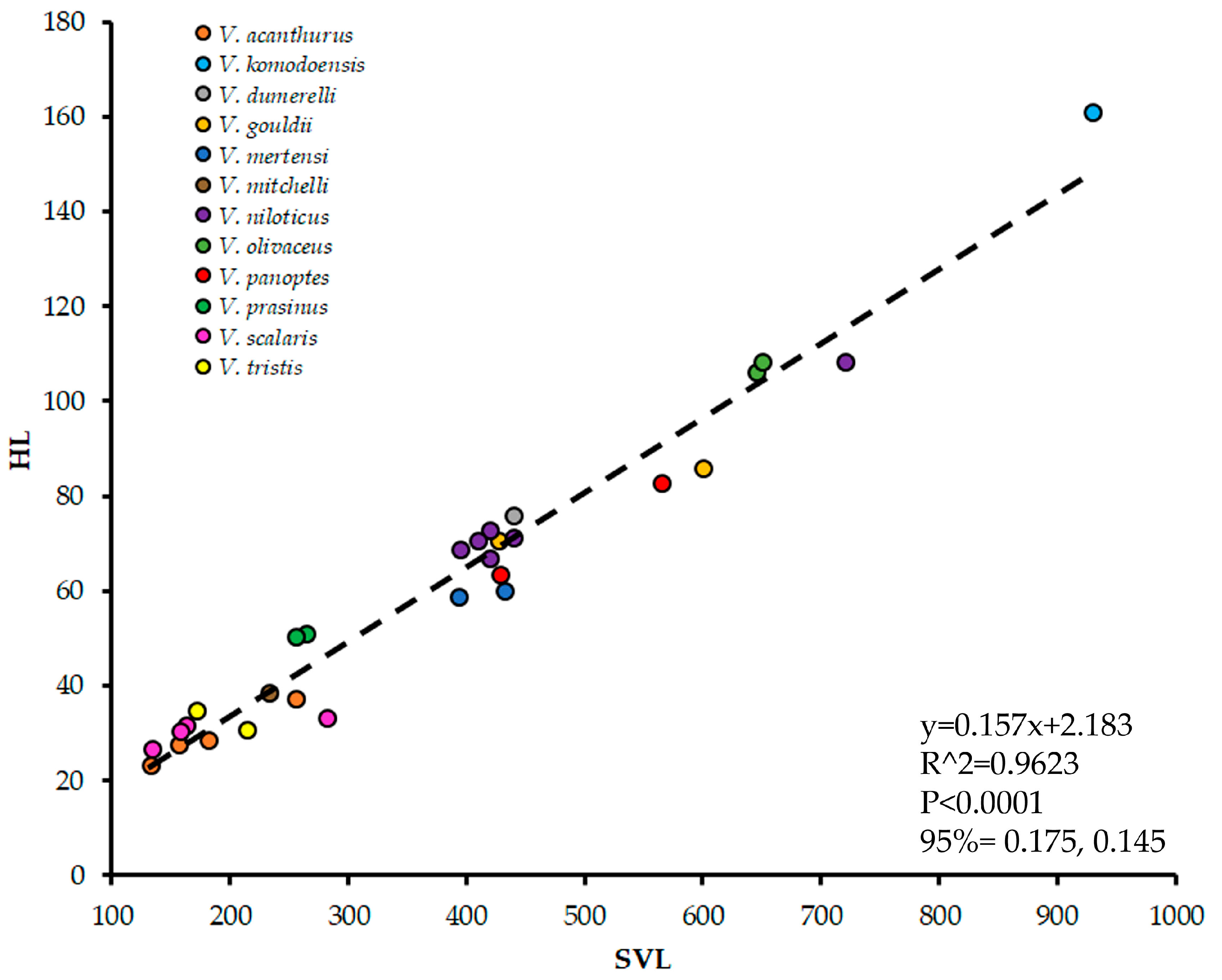
3.2. Asymmetry and Body Size
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seligmann, H. Evidence that minor directional asymmetry is functional in lizard hindlimbs. J. Zool. 1998, 245, 205–208. [Google Scholar] [CrossRef]
- Seligmann, H.; Moravec, J.; Werner, Y.L. Morphological, functional and evolutionary aspects of tail autotomy and regeneration in the ‘living fossil’ Sphenodon (Reptilia: Rhynchocephalia). Biol. J. Linn. Soc. 2008, 93, 721–743. [Google Scholar] [CrossRef]
- Sion, G.; Tal, R.; Meiri, S. Asymmetric behavior in Ptyodactylus guttatus: Can a digit ratio reflect brain laterality? Symmetry 2020, 12, 1490. [Google Scholar] [CrossRef]
- Kaczmarski, M.; Ziemblińska, K.; Tryjanowski, P. Sand lizards Lacerta agilis with higher digit ratios are more likely to autotomy. J. Anat. 2020, 237, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Razzetti, E.; Faiman, R.; Werner, Y.L. Directional asymmetry and correlation of tail injury with left-side dominance occur in Serpentes (Sauropsida). Zoomorphology 2007, 126, 31–43. [Google Scholar] [CrossRef]
- Sion, G. Directional asymmetry in lateral eye diameter and risk-taking strategy of the gecko Ptyodactylus guttatus. Isreali J. Ecol. Evol. 2011, 57, 268. [Google Scholar]
- Sion, G. Inter-Relations Among Behavior, Physiology, Morphology and Directional Asymmetry in the Gecko Ptyodactylus guttatus. Ph.D. Thesis, The Hebrew University, Jerusalem, Israel, 2015. [Google Scholar]
- Kark, S. Shifts in bilateral asymmetry within a distribution range: The case of the chukar partridge. Evolution 2001, 55, 2088–2096. [Google Scholar]
- Aakvaag, A.; Bentdal, Ø.; Quigstad, K.; Walstad, P.; Rønningen, H.; Fonnum, F. Testosterone and testosterone binding globulin (TeBG) in young men during prolonged stress. Int. J. Androl. 1978, 1, 22–31. [Google Scholar] [CrossRef]
- Moore, M.C.; Thompson, C.W.; Marler, C.A. Reciprocal changes in corti-costerone and testosterone levels following acute and chronic handling stress in the tree lizard, Urosaurus ornatus. Gen. Comp. Endocrinol. 1991, 81, 217–226. [Google Scholar] [CrossRef]
- Kieser, J.A. Fluctuating odontometric asymmetry and maternal alcohol consumption. Ann. Hum. Biol. 1992, 19, 513–520. [Google Scholar] [CrossRef]
- Barden, H.S. Dental asymmetry and mental retardation: A comparison of subjects with mental retardation resulting from prenatal or postnatal influences. J. Ment. Defic. Res. 1980, 24, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Barden, H.S. Fluctuating dental asymmetry: A measure of developmental instability in Down syndrome. Am. J. Phys. Anthropol. 1980, 52, 169. [Google Scholar] [CrossRef] [PubMed]
- Collar, D.C.; Schulte, J.A., 2nd; Losos, J.B. Evolution of extreme body size disparity in monitor lizards (Varanus). Evolution 2011, 65, 2664–2680. [Google Scholar] [CrossRef]
- Di Bennardo, R.; Bailit, H.L. Stress and dental asymmetry in a population of Japanese children. Am. J. Phys. Anthropol. 1978, 46, 89–94. [Google Scholar] [CrossRef]
- Osborne, R.H.; Horowitz, S.L.; De George, F.V. Genetic variation in tooth dimensions: A twin study of the permanent anterior teeth. Am. J. Hum. Genet. 1958, 10, 350. [Google Scholar] [PubMed]
- Perzigian, A.J. Fluctuating dental asymmetry: Variation among skeletal populations. Am. J. Phys. Anthropol. 1977, 47, 81–88. [Google Scholar] [CrossRef]
- Siegel, M.I.; Mooney, M.P. Perinatal stress and increased fluctuating asymmetry of dental calcium in laboratory rat. Am. J. Phys. Anthropol. 1987, 73, 267–270. [Google Scholar] [CrossRef]
- Sofaer, J.A.; Chung, C.S.; Niswander, J.D.; Runck, D.W. Developmental interaction, size and agenesis among permanent maxillary incisors. Hum. Biol. 1971, 43, 36–45. [Google Scholar]
- Harris, E.F.; Bodford, K. Bilateral asymmetry in the tooth relationships of orthodontic patients. Angle Orthod. 2007, 77, 779–786. [Google Scholar] [CrossRef]
- Mayhall, J.T.; Saunders, S.R. Dimensional and discrete dental trait asymmetry relationships. Am. J. Phys. Anthropol. 1986, 69, 403–411. [Google Scholar] [CrossRef]
- Nissan, J.; Gross, M.D.; Shifman, A.; Tzadok, L.; Assif, D. Chewing side preference as a type of hemispheric laterality. J. Oral Rehabil. 2004, 31, 412–416. [Google Scholar] [CrossRef]
- Reisz, R.R.; MacDougall, M.J.; LeBlanc, A.R.; Scott, D.; Nagesan, R.S. Lateralized feeding behavior in a paleozoic reptile. Curr. Biol. 2020, 30, 2374–2378. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, T.; Alvesalo, L.; Osborne, R.H.; Tienari, J. Tooth eruption symmetry in functional lateralities. Arch. Oral Biol. 2001, 46, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Townsend, G.C. Fluctuating dental asymmetry in Downs syndrome. Aust. Dent. J. 1983, 28, 39–43. [Google Scholar] [CrossRef]
- Schalk-Van der Weide, Y.; Steen, W.H.A.; Beemer, F.A.; Bosman, F. Reductions in size and left-right asymmetry of teeth in human oligodontia. Arch. Oral Biol. 1994, 39, 935–939. [Google Scholar] [CrossRef]
- Woodward, G.U.Y.; Hildrew, A.G. Body-size determinants of niche overlap and intraguild predation within a complex food web. J. Anim. Ecol. 2002, 71, 1063–1074. [Google Scholar] [CrossRef]
- Christian, K.A.; Waldschmidt, S. The relationship between lizard home range and body size: A reanalysis of the data. Herpetologica 1984, 40, 68–75. [Google Scholar]
- Guarino, F. Spatial ecology of a large carnivorous lizard, Varanus varius (Squamata: Varanidae). J. Zool. 2002, 258, 449–457. [Google Scholar] [CrossRef]
- Castilla, J.C.; Duran, L.R. Human exclusion from the rocky intertidal zone of central Chile: The effects on Concholepas concholepas (Gastropoda). Oikos 1985, 45, 391–399. [Google Scholar] [CrossRef]
- Roy, K.; Collins, A.G.; Becker, B.J.; Begovic, E.; Engle, J.M. Anthropogenic impacts and historical decline in body size of rocky intertidal gastropods in southern California. Ecol. Lett. 2003, 6, 205–211. [Google Scholar] [CrossRef]
- Blake, S.; Foster, G.N.; Eyre, M.D.; Luff, M.L. Effects of habitat type and grassland management practices on the body size distribution of carabid beetles. Pedobiologia 1994, 38, 502–512. [Google Scholar] [CrossRef]
- Bochaton, C.; Bailon, S.; Ineich, I.; Breuil, M.; Tresset, A.; Grouard, S. From a thriving past to an uncertain future: Zooarchaeological evidence of two millennia of human impact on a large emblematic lizard (Iguana delicatissima) on the Guadeloupe Islands (French West Indies). Quat. Sci. Rev. 2016, 150, 172–183. [Google Scholar] [CrossRef]
- Bochaton, C.; Bailon, S.; Herrel, A.; Grouard, S.; Ineich, I.; Tresset, A.; Cornette, R. Human impacts reduce morphological diversity in an insular species of lizard. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170921. [Google Scholar] [CrossRef]
- Putman, B.J.; Tippie, Z.A. Big city living: A global meta-analysis reveals positive impact of urbanization on body size in lizards. Front. Ecol. Evol. 2020, 8, 580745. [Google Scholar] [CrossRef]
- Doherty, T.S.; Balouch, S.; Bell, K.; Burns, T.J.; Feldman, A.; Fist, C.; Garvey, T.F.; Jessop, T.S.; Meiri, S.; Driscoll, D.A. Reptile responses to anthropogenic habitat modification: A global meta-analysis. Glob. Ecol. Biogeogr. 2020, 29, 1265–1279. [Google Scholar] [CrossRef]
- Herrel, A.; Vanhooydonck, B.; Joachim, R.; Irschick, D.J. Frugivory in polychrotid lizards: Effects of body size. Oecologia 2004, 140, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Herrel, A.; Joachim, R.; Vanhooydonck, B.; Irschick, D.J. Ecological consequences of ontogenetic changes in head shape and bite performance in the Jamaican lizard Anolis lineatopus. Biol. J. Linn. Soc. 2006, 89, 443–454. [Google Scholar] [CrossRef]
- Verwaijen, D.; Van Damme, R.; Herrel, A. Relationships between head size, bite force, prey handling efficiency and diet in two sympatric lacertid lizards. Funct. Ecol. 2002, 16, 842–850. [Google Scholar] [CrossRef]
- Metzger, K.A.; Herrel, A. Correlations between lizard cranial shape and diet: A quantitative, phylogenetically informed analysis. Biol. J. Linn. Soc. 2005, 86, 433–466. [Google Scholar] [CrossRef]
- Brennan, I.G.; Lemmon, A.R.; Lemmon, E.M.; Portik, D.M.; Weijola, V.; Welton, L.; Donnellan, S.C.; Keogh, J.S. Phylogenomics of monitor lizards and the role of competition in dictating body size disparity. Syst. Biol. 2021, 70, 120–132. [Google Scholar] [CrossRef]
- Collier, A.D.; Halkina, V.; Min, S.S.; Roberts, M.Y.; Campbell, S.D.; Camidge, K.; Leibowitz, S.F. Embryonic ethanol exposure affects the early development, migration, and location of hypocretin/orexin neurons in zebrafish. Alcohol Clin. Exp. Res. 2019, 43, 1702–1713. [Google Scholar] [CrossRef]
- Pianka, E.R. Evolution of body size: Varanid lizards as a model system. Am. Nat. 1995, 146, 398–414. [Google Scholar] [CrossRef]
- Palmer, A.R.; Strobeck, C. Fluctuating asymmetry as a measure of developmental stability: Implications of non-normal distributions and power of statistical tests. Acta Zool. Fenn. 1992, 191, 13. [Google Scholar]
- Moodie, G.E.E.; Reimchen, T.E. Phenetic variation and habitat differences in Gasterosteus populations of the Queen Charlotte Islands. Syst. Zool. 1976, 25, 49–61. [Google Scholar] [CrossRef]
- Bergstrom, C.A.; Reimchen, T.E. Functional implications of fluctuating asymmetry among endemic populations of Gasterosteus aculeatus. Behaviour 2000, 137, 1097–1112. [Google Scholar] [CrossRef]
- Van Valen, L. A study of fluctuating asymmetry. Evolution 1962, 16, 125–142. [Google Scholar] [CrossRef]
- Norberg, R.A. Occurrence and independent evolution of bilateral ear asymmetry in owls and implications on owl taxonomy. Philos. Trans. R. Soc. (B) 1977, 280, 375–408. [Google Scholar]
- Simmons, L.W.; Rhodes, G.; Peters, M.; Koehler, N. Are human preferences for facial symmetry focused on signals of developmental instability? Behav. Ecol. 2004, 15, 864–871. [Google Scholar] [CrossRef]
- Garn, S.M.; Lewis, A.B.; Kerewsky, R.S. The meaning of bilateral asymmetry in the permanent dentition. Angle Orthod. 1966, 36, 55–62. [Google Scholar]
- Pianka, E.; King, D. (Eds.) Varanoid Lizards of the World; Indiana University Press: Bloomington, IN, USA, 2004. [Google Scholar]
- Uetz, P.; Freed, P.; Aguilar, R.; Reyes, F.; Kudera, J.; Hošek, J. (Eds.) The Reptile Database. 2025. Available online: http://www.reptile-database.org (accessed on 13 February 2024).
- Smith, J.B.; Dodson, P. A proposal for a standard terminology of anatomical notation and orientation in fossil vertebrate dentitions. J. Vertebr. Paleontol. 2003, 23, 1–12. [Google Scholar] [CrossRef]
- Hendrickx, C.; Mateus, O.; Araújo, R. A proposed terminology of theropod teeth (Dinosauria, Saurischia). J. Vertebr. Paleontol. 2015, 35, e982797. [Google Scholar] [CrossRef]
- Gomez-Robles, A.; Martinon-Torres, M.; Bermudez de Castro, J.M.; Margvelashvili, A.; Bastir, M.; Arsuaga, J.L.; Pérez-Pérez, A.; Estebaranz, F.; Martínez, L.M. A geometric morphometric analysis of hominin upper first molar shape. J. Hum. Evol. 2007, 53, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Gunz, P.; Mitteroecker, P. Semilandmarks: A method for quantifying curves and surfaces. Hystrix 2013, 24, 103–109. [Google Scholar]
- McGuire, J.L. Geometric morphometrics of vole (Microtus californicus) dentition as a new paleoclimate proxy: Shape change along geographic and climatic clines. Quatern Int. 2010, 212, 198–205. [Google Scholar] [CrossRef]
- Skinner, M.M.; Gunz, P. The presence of accessory cusps in chimpanzee lower molars is consistent with a patterning cascade model of development. J. Anat. 2010, 217, 245–253. [Google Scholar] [CrossRef]
- D’Amore, D.C. Illustrating ontogenetic change in the dentition of the Nile monitor lizard, Varanus niloticus: A case study in the application of geometric morphometric methods for the quantification of shapesize heterodonty. J. Anat. 2015, 226, 403–419. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, D.C.; McHenry, C.; Doody, J.; Clulow, S.; Rhind, D. Claw morphometrics in monitor lizards: Variable substrate and habitat use correlate to shape diversity within a predator guild. Ecol. Evolution. 2018, 8, 6766–6778. [Google Scholar] [CrossRef]
- D’Amore, D.C.; Harmon, M.; Drumheller, S.K.; Testin, J.J. Quantitative heterodonty in Crocodylia: Assessing size and shape across modern and extinct taxa. PeerJ 2019, 7, e6485. [Google Scholar] [CrossRef]
- D’Amore, D.C.; Johnson-Ransom, E.; Snively, E.; Hone, D.W.E. Prey size and ecological separation in spinosaurid theropods based on heterodonty and rostrum shape. Anat. Rec. 2024, 1–18. [Google Scholar] [CrossRef]
- Becerra, M.G.; Pol, D.; Marsicano, C.A.; Rauhut, O.W. The dentition of Manidens condorensis (Ornithischia; Heterodontosauridae) from the Jurassic Cañadón Asfalto Formation of Patagonia: Morphology, heterodonty and the use of statistical methods for identifying isolated teeth. Hist. Biol. 2014, 26, 480–492. [Google Scholar] [CrossRef]
- Rohlf, F.J. TpsDig2.16. SB Morphometrics. 2017. Available online: https://sbmorphometrics.org/ (accessed on 6 January 2025).
- Tinius, A.; Russell, P.A. Points on the curve: An analysis of methods for assessing the shape of vertebrate claws. J. Morph. 2017, 278, 150–169. [Google Scholar] [CrossRef] [PubMed]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D.; Fink, W.L. Geometric Morphometrics for Biologists: A Primer; Elsevier Academic Press: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Auffenberg, W. The Behavioral Ecology of the Komodo Monitor; Indiana University Press: Bloomington, IN, USA, 1981. [Google Scholar]
- Georgalis, G.L.; Mennecart, B.; Smith, K.T. First fossil record of Varanus (Reptilia, Squamata) from Switzerland and the earliest occurrences of the genus in Europe. Swiss J. Geosci. 2023, 116, 9. [Google Scholar] [CrossRef]
- LeBlanc, A.R.; Morrell, A.P.; Sirovica, S.; Al-Jawad, M.; Labonte, D.; D’Amore, D.C.; Clemente, C.; Wang, S.; Giuliani, F.; McGilvery, C.M.; et al. Iron-coated Komodo dragon teeth and the complex dental enamel of carnivorous reptiles. Nat. Ecol. Evol. 2024, 8, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Rohlf, F.J. TpsRelw1.53. SB Morphometrics. 2017. Available online: https://sbmorphometrics.org/ (accessed on 6 January 2025).
- Werner, Y.L.; Rothenstein, D.; Sivan, N. Directional asymmetry in reptiles (Sauria: Gekkonidae: Ptyodactylus) and its possible evolutionary role, with implications for biometrical methodology. J. Zool. 1991, 225, 647–658. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A. Past: Paleontological statistics software package for educaton and data anlysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- Sion, G. Digit asymmetry and digit ratio (2:4) derived from brain laterality: The lizard Ptyodactylus guttatus as a model. In Proceedings of the 10th Symposium on the Lacertid, Tel-Aviv, Israel, 18–21 June 2018. [Google Scholar]
- Ciofi, C. Varanus komodoensis. In Varanoid Lizards of the World; Pianka, E., King, D., Eds.; Indiana University Press: Bloomington, IN, USA, 2004. [Google Scholar]
- Erickson, G.M.; Lappin, A.K.; Vliet, K.A. The ontogeny of bite-force performance in American alligator (Alligator mississippiensis). J. Zool. 2003, 260, 317–327. [Google Scholar] [CrossRef]
- Schroeder, K.; Lyons, S.K.; Smith, F.A. The influence of juvenile dinosaurs on community structure and diversity. Science 2021, 371, 941–944. [Google Scholar] [CrossRef]
- Ariefiandy, A.; Purwandana, D.; Azmi, M.; Nasu, S.A.; Mardani, J.; Ciofi, C.; Jessop, T.S. Human activities associated with reduced Komodo dragon habitat use and range loss on Flores. Biodivers. Conserv. 2021, 30, 461–479. [Google Scholar] [CrossRef]
- Ardiantiono; Jessop, T.S.; Purwandana, D.; Ciofi, C.; Jeri Imansyah, M.; Panggur, M.R.; Ariefiandy, A. Effects of human activities on Komodo dragons in Komodo National Park. Biodivers. Conserv. 2018, 27, 3329–3347. [Google Scholar] [CrossRef]
- Hall, P.M. Brachycephalic growth and dental anomalies in the New Guinea crocodile (Crocodylus novaeguineae). J. Herpetol. 1985, 19, 300–303. [Google Scholar] [CrossRef]
- Rogers, L.J.; Vallortigara, G.; Andrew, R.J. Divided Brains: The Biology and Behaviour of Brain Asymmetries; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Vallortigara, G. Comparative neuropsychology of the dual brain: A stroll through animals’ left and right perceptual worlds. Brain Lang. 2000, 73, 189–219. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.T.; Scutt, D.; Wilson, J.; Lewis-Jones, D.I. The ratio of 2nd to 4th digit length: A predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum. Reprod. (Oxf. Engl.) 1998, 13, 3000–3004. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Cantalupo, C.; Taglialatela, J. Handedness is associated with asymmetries in gyrification of the cerebral cortex of chimpanzees. Cereb. Cortex 2007, 17, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Sion, G. Lizards birds and Simpson’s paradox. In Proceedings of the Gekkota Mundi III, Chaing Mai, Thailand, 31 July–1 August 2024. [Google Scholar]
- Edmund, A.G. Sequence and rate of tooth replacement in the Crocodilia. R. Ont. Mus. Life Sci. Div. Contr. 1962, 56, 1–42. [Google Scholar]
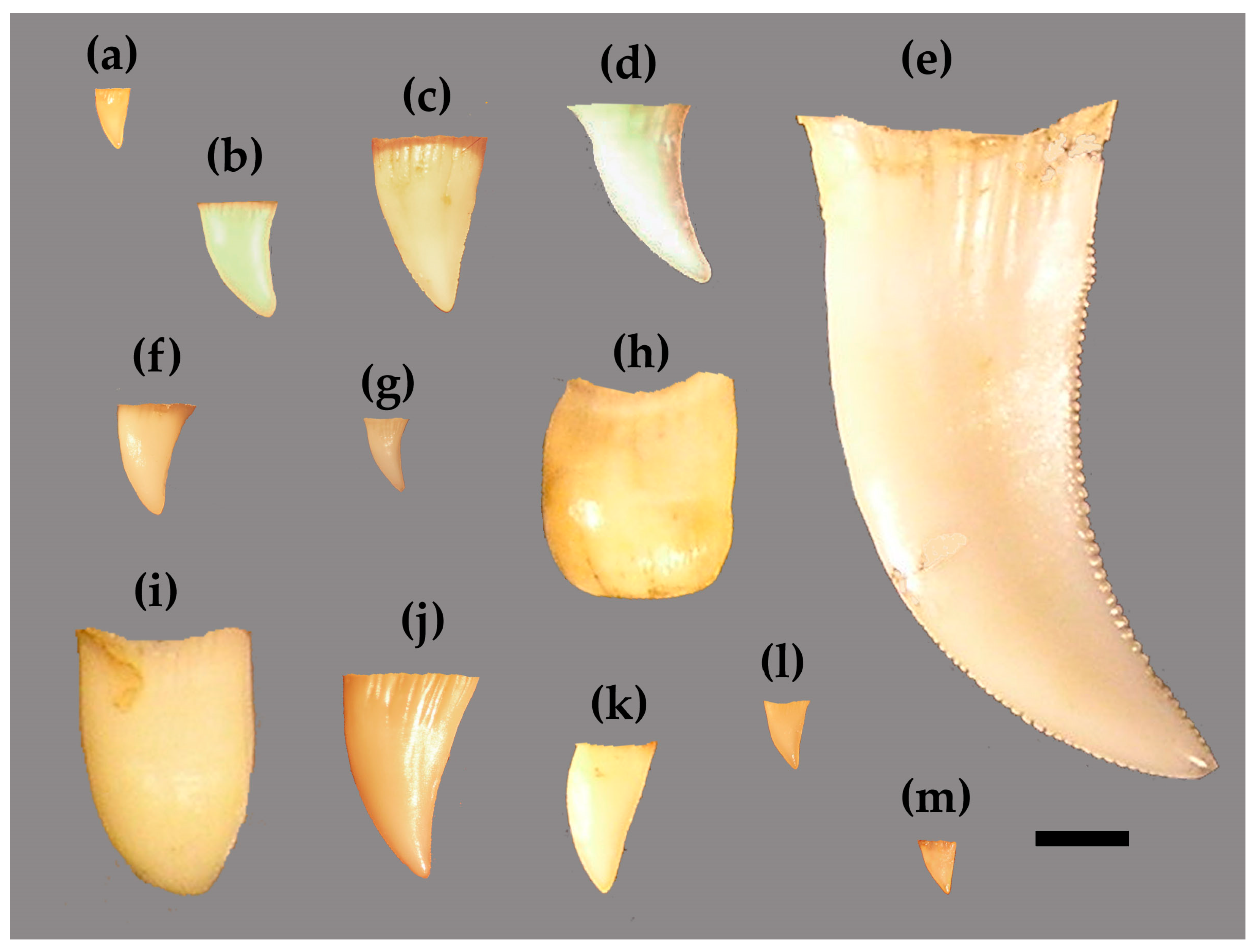



| Varanus Species | Specimens | Tooth Pairs | Tooth Positions |
|---|---|---|---|
| V. acanthurus | 6 | 60 | P = 3, M = 9, D = 10 |
| V. dumerelli | 3 | 43 | P = 3, M = 9, D = 10 |
| V. gouldii | 4 | 55 | P = 4, M = 11, D = 12 |
| V. griseus | 2 | 12 | P = 4, M = 10, D = 11 |
| V. komodoensis | 13 | 101 | P = 4, M = 13, D = 13 |
| V. mertensi | 2 | 21 | P = 4, M = 12, D = 12 |
| V. mitchelli | 1 | 10 | P = 3, M = 10, D = 11 |
| V. niloticus | 16 | 209 | P = 4, M = 11, D = 12 |
| V. olivaceus | 4 | 50 | P = 4, M = 10, D = 12 |
| V. panoptes | 2 | 16 | P = 4, M = 12, D = 12 |
| V. prasinus | 2 | 24 | P = 4, M = 10, D = 11 |
| V. scalaris | 4 | 40 | P = 4, M = 10, D = 10 |
| V. tristis | 3 | 30 | P = 4, M = 10, D = 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sion, G.; D’Amore, D.C. Varanid Teeth Asymmetry and Correlation to Body Size. J. Dev. Biol. 2025, 13, 9. https://doi.org/10.3390/jdb13010009
Sion G, D’Amore DC. Varanid Teeth Asymmetry and Correlation to Body Size. Journal of Developmental Biology. 2025; 13(1):9. https://doi.org/10.3390/jdb13010009
Chicago/Turabian StyleSion, Guy, and Domenic C. D’Amore. 2025. "Varanid Teeth Asymmetry and Correlation to Body Size" Journal of Developmental Biology 13, no. 1: 9. https://doi.org/10.3390/jdb13010009
APA StyleSion, G., & D’Amore, D. C. (2025). Varanid Teeth Asymmetry and Correlation to Body Size. Journal of Developmental Biology, 13(1), 9. https://doi.org/10.3390/jdb13010009







