Forced Expression of Foxg1 in the Cortical Hem Leads to the Transformation of Cajal-Retzius Cells into Dentate Granule Neurons
Abstract
:1. Introduction
2. Materials and Methods
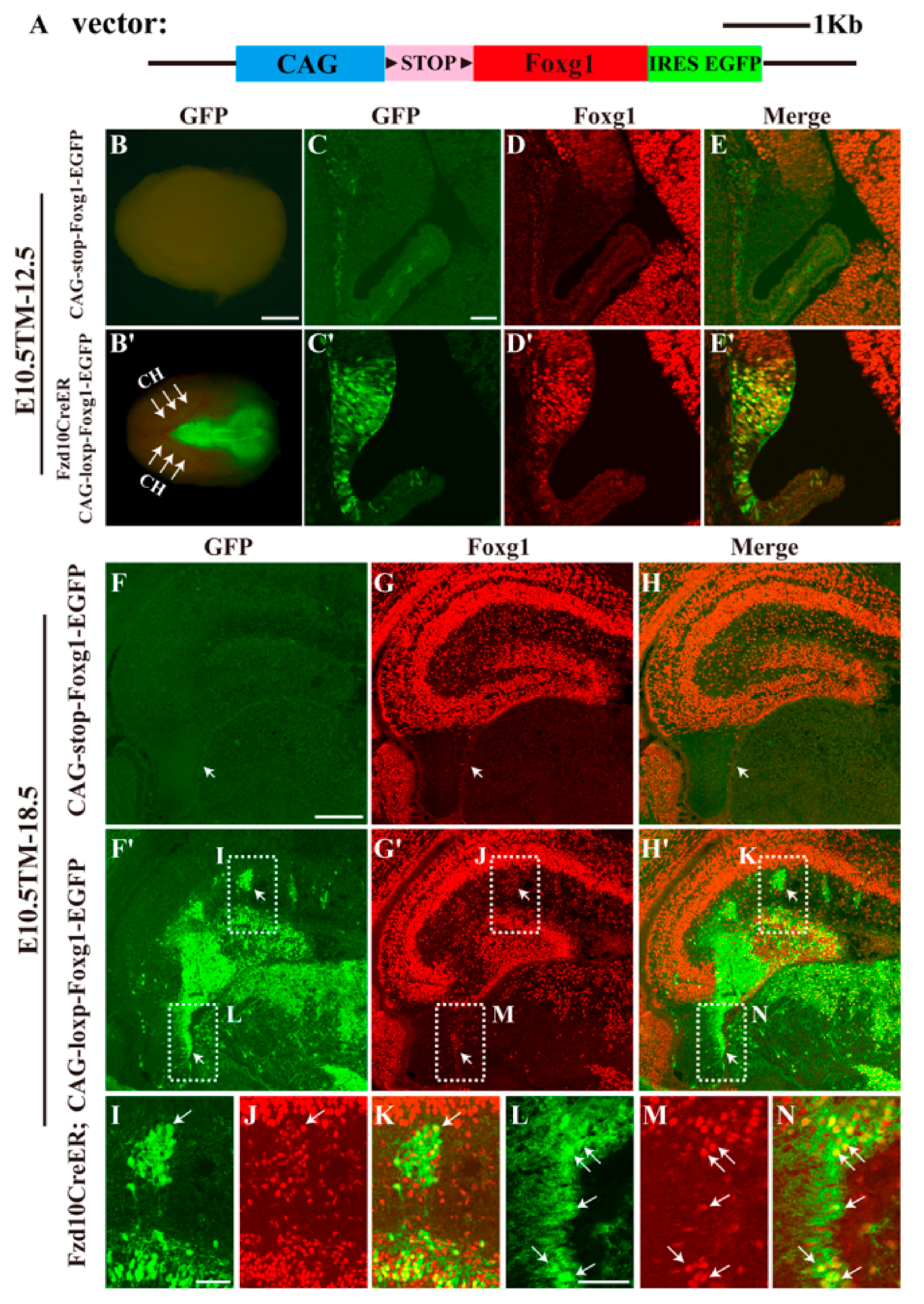
2.1. Generation of CAG-loxp-stop-loxp-Foxg1-IRES-EGFP Mouse Line
2.2. Mouse Breeding and Tamoxifen Administering
2.3. Tissue Processing
2.4. In Situ Hybridization
2.5. Immunofluorescence
3. Results
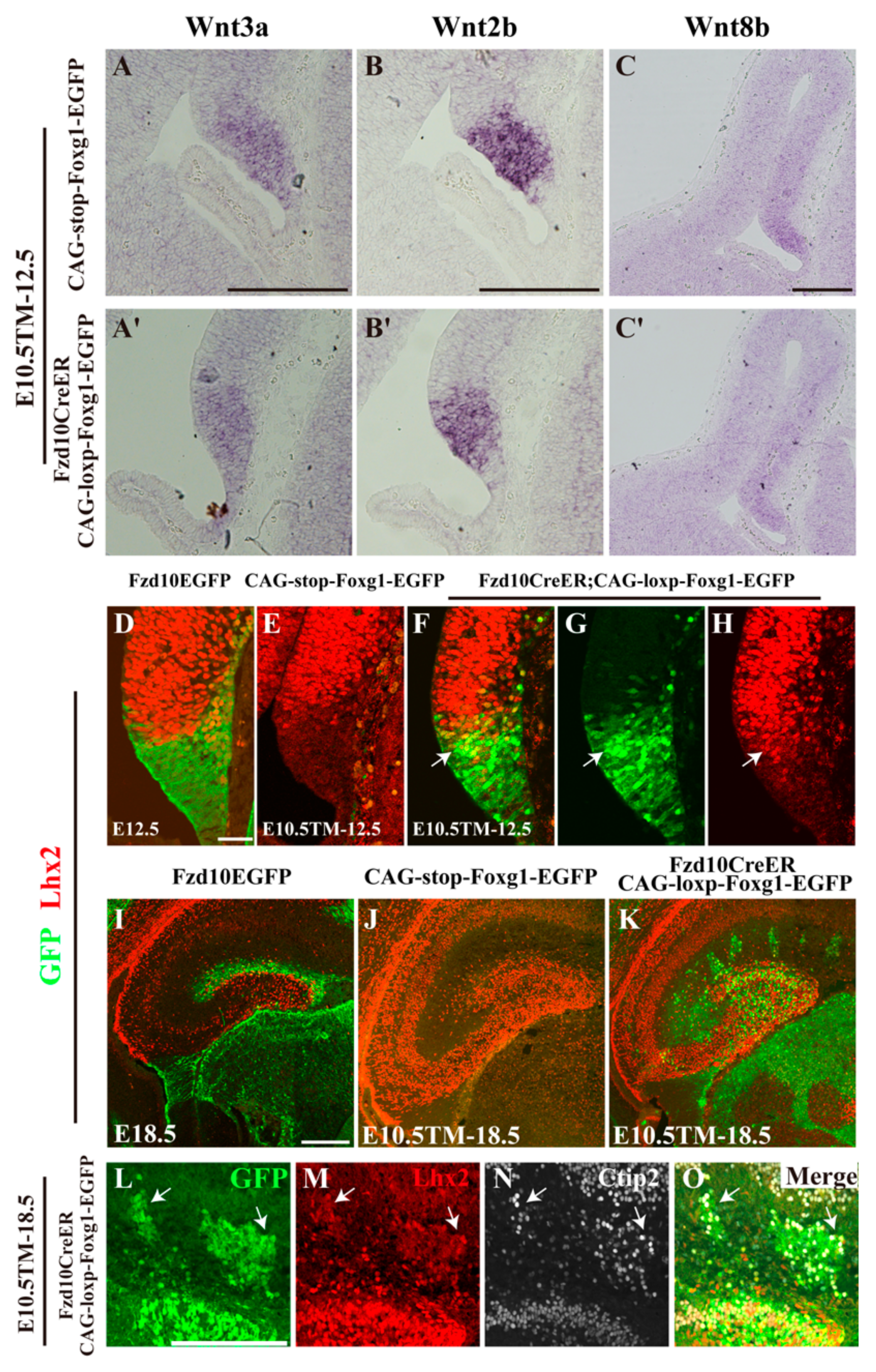
3.1. Forced Expression of Foxg1 in the Cortical Hem and Impaired Development of the DG
3.2. Most Hem-Derived Cells Lost CR Cell Fate and Mis-Distributed in the DG Area
3.3. Hem-Derived CR Cells Switched Their Fates into Dentate Granule Neurons
3.4. Normal Morphology of the Hem and Ectopic Expression of Lhx2 after Foxg1 Overexpression
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borello, U.; Pierani, A. Patterning the cerebral cortex: Traveling with morphogens. Curr. Opin. Genet. Dev. 2010, 20, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Shimogori, T.; Banuchi, V.; Ng, H.Y.; Strauss, J.B.; Grove, E.A. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development 2004, 131, 5639–5647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuta, Y.; Piston, D.W.; Hogan, B.L. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development 1997, 124, 2203–2212. [Google Scholar] [PubMed]
- Grove, E.A.; Tole, S.; Limon, J.; Yip, L.; Ragsdale, C.W. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development 1998, 125, 2315–2325. [Google Scholar] [PubMed]
- Yoshida, M.; Assimacopoulos, S.; Jones, K.R.; Grove, E.A. Massive loss of Cajal-Retzius cells does not disrupt neocortical layer order. Development 2006, 133, 537–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.M.; Tole, S.; Grove, E.; McMahon, A.P. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development 2000, 127, 457–467. [Google Scholar] [PubMed]
- Galceran, J.; Miyashita-Lin, E.M.; Devaney, E.; Rubenstein, J.L.; Grosschedl, R. Hippocampus development and generation of dentate gyrus granule cells is regulated by LEF1. Development 2000, 127, 469–482. [Google Scholar] [PubMed]
- Mangale, V.S.; Hirokawa, K.E.; Satyaki, P.R.; Gokulchandran, N.; Chikbire, S.; Subramanian, L.; Shetty, A.S.; Martynoga, B.; Paul, J.; Mai, M.V.; et al. Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science 2008, 319, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.; Perez-Garcia, C.G.; Abraham, H.; Caput, D. Expression of p73 and Reelin in the developing human cortex. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 4973–4986. [Google Scholar] [CrossRef]
- Takiguchi-Hayashi, K.; Sekiguchi, M.; Ashigaki, S.; Takamatsu, M.; Hasegawa, H.; Suzuki-Migishima, R.; Yokoyama, M.; Nakanishi, S.; Tanabe, Y. Generation of reelin-positive marginal zone cells from the caudomedial wall of telencephalic vesicles. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 2286–2295. [Google Scholar] [CrossRef] [PubMed]
- Bielas, S.; Higginbotham, H.; Koizumi, H.; Tanaka, T.; Gleeson, J.G. Cortical neuronal migration mutants suggest separate but intersecting pathways. Annu. Rev. Cell Dev. Biol. 2004, 20, 593–618. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.S.; Curran, T. Role of the reelin signaling pathway in central nervous system development. Annu. Rev. Neurosci. 2001, 24, 1005–1039. [Google Scholar] [CrossRef] [PubMed]
- Xuan, S.; Baptista, C.A.; Balas, G.; Tao, W.; Soares, V.C.; Lai, E. Winged helix transcription factor bf-1 is essential for the development of the cerebral hemispheres. Neuron 1995, 14, 1141–1152. [Google Scholar] [CrossRef]
- Bulchand, S.; Grove, E.A.; Porter, F.D.; Tole, S. Lim-homeodomain gene lhx2 regulates the formation of the cortical hem. Mech. Dev. 2001, 100, 165–175. [Google Scholar] [CrossRef]
- Muzio, L.; Mallamaci, A. Foxg1 confines Cajal-Retzius neuronogenesis and hippocampal morphogenesis to the dorsomedial pallium. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 4435–4441. [Google Scholar] [CrossRef] [PubMed]
- Godbole, G.; Shetty, A.S.; Roy, A.; D’Souza, L.; Chen, B.; Miyoshi, G.; Fishell, G.; Tole, S. Hierarchical genetic interactions between FOXG1 and LHX2 regulate the formation of the cortical hem in the developing telencephalon. Development 2018, 145. [Google Scholar] [CrossRef] [PubMed]
- Martynoga, B.; Morrison, H.; Price, D.J.; Mason, J.O. Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Dev. Biol. 2005, 283, 113–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanashima, C.; Fernandes, M.; Hebert, J.M.; Fishell, G. The role of foxg1 and dorsal midline signaling in the generation of cajal-retzius subtypes. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 11103–11111. [Google Scholar] [CrossRef] [PubMed]
- Correia, K.M.; Conlon, R.A. Whole-mount in situ hybridization to mouse embryos. Methods 2001, 23, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, K.; Wu, X.; Zhao, C. Foxg1 deletion impairs the development of the epithalamus. Mol. Brain 2018, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shen, W.; Ni, Y.; Su, Y.; Yang, Z.; Zhao, C. Impaired interneuron development after Foxg1 disruption. Cereb. Cortex 2017, 27, 793–808. [Google Scholar] [PubMed]
- Tian, C.; Gong, Y.; Yang, Y.; Shen, W.; Wang, K.; Liu, J.; Xu, B.; Zhao, J.; Zhao, C. Foxg1 has an essential role in postnatal development of the dentate gyrus. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 2931–2949. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, Y.; Hu, C.; Gu, X.; Liu, J.; Hu, Y.A.; Yang, Y.; Wei, Y.; Zhao, C. Expression of Frizzled10 in mouse central nervous system. Gene Expr. Patterns 2009, 9, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Liu, B.; Wu, X.; Yan, Y.; Zhang, Y.; Wei, Y.; Pleasure, S.J.; Zhao, C. Inducible genetic lineage tracing of cortical hem derived Cajal-Retzius cells reveals novel properties. PLoS ONE 2011, 6, e28653. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Guan, W.; Pleasure, S.J. A transgenic marker mouse line labels Cajal-Retzius cells from the cortical hem and thalamocortical axons. Brain Res. 2006, 1077, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Yan, Y.; Li, H.; He, D.; Pleasure, S.J.; Zhao, C. Characterization of the Frizzled10-CreER transgenic mouse: An inducible Cre line for the study of Cajal-Retzius cell development. Genesis 2009, 47, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Verney, C.; Derer, P. Cajal-Retzius neurons in human cerebral cortex at midgestation show immunoreactivity for neurofilament and calcium-binding proteins. J. Comp. Neurol. 1995, 359, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Galan, J.R.; Moncho-Bogani, J.; Caminos, E. Expression of calcium-binding proteins in layer 1 reelin-immunoreactive cells during rat and mouse neocortical development. J. Histochem. Cytochem. 2014, 62, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.D.; Jessberger, S.; Steiner, B.; Kronenberg, G.; Reuter, K.; Bick-Sander, A.; von der Behrens, W.; Kempermann, G. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol. Cell. Neurosci. 2003, 24, 603–613. [Google Scholar] [CrossRef]
- Lavado, A.; Lagutin, O.V.; Chow, L.M.; Baker, S.J.; Oliver, G. Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. PLoS Biol. 2010, 8, e1000460. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Brylka, H.; Schwegler, H.; Venkataramanappa, S.; Andratschke, J.; Wiegreffe, C.; Liu, P.; Fuchs, E.; Jenkins, N.A.; Copeland, N.G.; et al. A dual function of Bcl11b/Ctip2 in hippocampal neurogenesis. EMBO J. 2012, 31, 2922–2936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motro, B.; van der Kooy, D.; Rossant, J.; Reith, A.; Bernstein, A. Contiguous patterns of c-kit and steel expression: Analysis of mutations at the W and Sl loci. Development 1991, 113, 1207–1221. [Google Scholar] [PubMed]
- Wisden, W.; Seeburg, P.H. A complex mosaic of high-affinity kainate receptors in rat brain. J. Neurosci. Off. J. Soc. Neurosci. 1993, 13, 3582–3598. [Google Scholar] [CrossRef] [Green Version]
- Tole, S.; Goudreau, G.; Assimacopoulos, S.; Grove, E.A. Emx2 is required for growth of the hippocampus but not for hippocampal field specification. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 2618–2625. [Google Scholar] [CrossRef]
- Danesin, C.; Peres, J.N.; Johansson, M.; Snowden, V.; Cording, A.; Papalopulu, N.; Houart, C. Integration of telencephalic Wnt and hedgehog signaling center activities by Foxg1. Dev. Cell 2009, 16, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Bielle, F.; Griveau, A.; Narboux-Neme, N.; Vigneau, S.; Sigrist, M.; Arber, S.; Wassef, M.; Pierani, A. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat. Neurosci. 2005, 8, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Hanashima, C.; Shen, L.; Li, S.C.; Lai, E. Brain factor-1 controls the proliferation and differentiation of neocortical progenitor cells through independent mechanisms. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 6526–6536. [Google Scholar] [CrossRef]
- Hanashima, C.; Li, S.C.; Shen, L.; Lai, E.; Fishell, G. Foxg1 suppresses early cortical cell fate. Science 2004, 303, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, G.; Fishell, G. Dynamic FoxG1 expression coordinates the integration of multipolar pyramidal neuron precursors into the cortical plate. Neuron 2012, 74, 1045–1058. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Xiao, H.; Zhao, C. Forced Expression of Foxg1 in the Cortical Hem Leads to the Transformation of Cajal-Retzius Cells into Dentate Granule Neurons. J. Dev. Biol. 2018, 6, 16. https://doi.org/10.3390/jdb6030016
Liu B, Xiao H, Zhao C. Forced Expression of Foxg1 in the Cortical Hem Leads to the Transformation of Cajal-Retzius Cells into Dentate Granule Neurons. Journal of Developmental Biology. 2018; 6(3):16. https://doi.org/10.3390/jdb6030016
Chicago/Turabian StyleLiu, Bin, Hongmei Xiao, and Chunjie Zhao. 2018. "Forced Expression of Foxg1 in the Cortical Hem Leads to the Transformation of Cajal-Retzius Cells into Dentate Granule Neurons" Journal of Developmental Biology 6, no. 3: 16. https://doi.org/10.3390/jdb6030016
APA StyleLiu, B., Xiao, H., & Zhao, C. (2018). Forced Expression of Foxg1 in the Cortical Hem Leads to the Transformation of Cajal-Retzius Cells into Dentate Granule Neurons. Journal of Developmental Biology, 6(3), 16. https://doi.org/10.3390/jdb6030016



