Gbx1 and Gbx2 Are Essential for Normal Patterning and Development of Interneurons and Motor Neurons in the Embryonic Spinal Cord
Abstract
:1. Introduction
2. Materials and Methods
2.1. Generation of Gbx1−/−/Gbx2−/− Mice
2.2. Immunohistochemistry
2.3. Islet1 and PAX2
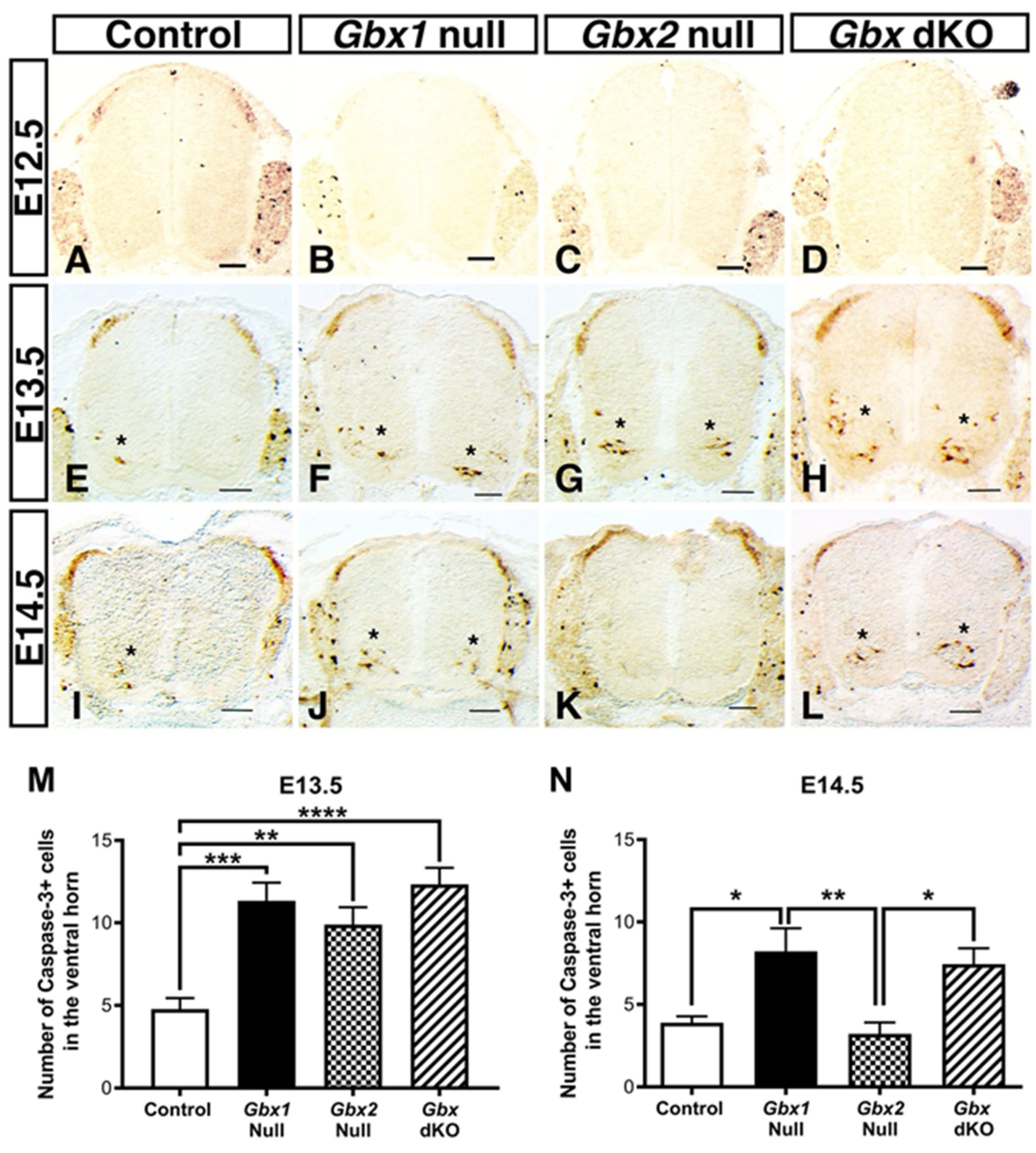
2.4. Caspase-3 Staining and Phosphorylated Histone H3
2.5. Section In Situ Hybridization
2.6. Microscopy
2.7. Statistical Analysis
2.8. Animal Ethics Statement
3. Results
3.1. Increase in Embryonic Spinal Cord Expression of Gbx1 and Gbx2 in Homozygous Null Counterparts
3.2. Loss of Gbx1 and Gbx2 Results in Abnormal Development of PAX2+ Dorsal Spinal Cord Neurons
3.3. Loss of Gbx Transcription Factor Function Results in Increased Apoptosis in the Ventral Spinal Cord
4. Discussion
4.1. Gbx1 and Gbx2 Are Required for Normal Development of Late-Born Inhibitory Interneurons in the Dorsal Spinal Cord
4.2. Gbx Family Members Act to Maintain Ventral Motor Neuron Populations through a Shared Molecular Mechanism
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jessell, T.M. Neuronal specification in the spinal cord: Inductive signals and transcriptional codes. Nat. Rev. Genet. 2000, 1, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Goulding, M.; Lanuza, G.; Sapir, T.; Narayan, S. The formation of sensorimotor circuits. Curr. Opin. Neurobiol. 2002, 12, 508–515. [Google Scholar] [CrossRef]
- Briscoe, J.; Pierani, A.; Jessell, T.M.; Ericson, J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell 2000, 101, 435–445. [Google Scholar] [CrossRef] [Green Version]
- Caspary, T.; Anderson, K.V. Patterning cell types in the dorsal spinal cord: What the mouse mutants say. Nat. Rev. Neurosci. 2003, 4, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.K.; Dottori, M.; Goulding, M. Lbx1 specifies somatosensory association interneurons in the dorsal spinal cord. Neuron 2002, 34, 535–549. [Google Scholar] [CrossRef] [Green Version]
- Müller, T.; Brohmann, H.; Pierani, A.; Heppenstall, P.A.; Lewin, G.R.; Jessell, T.M.; Birchmeier, C. The homeodomain factor lbx1 distinguishes two major programs of neuronal differentiation in the dorsal spinal cord. Neuron 2002, 34, 551–562. [Google Scholar] [CrossRef] [Green Version]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef]
- Gillespie, P.G.; Walker, R.G. Molecular basis of mechanosensory transduction. Nature 2001, 413, 194–202. [Google Scholar] [CrossRef]
- Helms, A.W.; Johnson, J.E. Specification of dorsal spinal cord interneurons. Curr. Opin. Neurobiol. 2003, 13, 42–49. [Google Scholar] [CrossRef]
- Pillai, A.; Mansouri, A.; Behringer, R.; Westphal, H.; Goulding, M. Lhx1 and Lhx5 maintain the inhibitory-neurotransmitter status of interneurons in the dorsal spinal cord. Development 2007, 134, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Arata, A.; Mizuguchi, R.; Qian, Y.; Karunaratne, A.; Gray, P.A.; Arata, S.; Shirasawa, S.; Bouchard, M.; Luo, P.; et al. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat. Neurosci. 2004, 7, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, R.; Kriks, S.; Cordes, R.; Gossler, A.; Ma, Q.; Goulding, M. Ascl1 and Gsh1/2 control inhibitory and excitatory cell fate in spinal sensory interneurons. Nat. Neurosci. 2006, 9, 770–778. [Google Scholar] [CrossRef]
- Glasgow, S.M.; Henke, R.M.; MacDonald, R.J.; Wright, C.V.; Johnson, J.E. Ptf1a determines GABAergic over glutamatergic neuronal cell fate in the spinal cord dorsal horn. Development 2005, 132, 5461–5469. [Google Scholar] [CrossRef] [Green Version]
- Wildner, H.; Gupta, R.D.; Bröhl, D.; Heppenstall, P.A.; Zeilhofer, H.U.; Birchmeier, C. Genome-wide expression analysis of Ptf1a- and Ascl1-deficient mice reveals new markers for distinct dorsal horn interneuron populations contributing to nociceptive reflex plasticity. J. Neurosci. 2013, 33, 7299–7307. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, F.; Briscoe, J. Morphogens and the control of cell proliferation and patterning in the spinal cord. Cell Cycle 2007, 6, 2640–2649. [Google Scholar] [CrossRef] [PubMed]
- Goulding, M. Circuits controlling vertebrate locomotion: Moving in a new direction. Nat. Rev. Neurosci. 2009, 10, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Arber, S. Motor circuits in action: Specification, connectivity, and function. Neuron 2012, 74, 975–989. [Google Scholar] [CrossRef] [Green Version]
- Ericson, J.; Rashbass, P.; Schedl, A.; Brenner-Morton, S.K.A.W.A.K.A.M.I.; Kawakami, A.; Van Heyningen, V.; Jessell, T.M.; Briscoe, J. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell 1997, 90, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Waters, S.T.; Lewandoski, M. A threshold requirement for Gbx2 levels in hindbrain development. Development 2006, 133, 1991–2000. [Google Scholar] [CrossRef] [Green Version]
- Wassarman, K.M.; Lewandoski, M.; Campbell, K.; Joyner, A.L.; Rubenstein, J.L.; Martinez, S.; Martin, G.R. Specification of the anterior hindbrain and establishment of a normal mid/hindbrain organizer is dependent on Gbx2 gene function. Development 1997, 124, 2923–2934. [Google Scholar]
- Luu, B.; Ellisor, D.; Zervas, M. The lineage contribution and role of Gbx2 in spinal cord development. PLoS ONE 2011, 6, e20940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.; Joyner, A.L. EN and GBX2 play essential roles downstream of FGF8 in patterning the mouse mid/hindbrain region. Development 2001, 128, 181–191. [Google Scholar] [PubMed]
- Li, J.Y.; Lao, Z.; Joyner, A.L. Changing requirements for Gbx2 in development of the cerebellum and maintenance of the mid/hindbrain organizer. Neuron 2002, 36, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Burroughs-Garcia, J.; Sittaramane, V.; Chandrasekhar, A.; Waters, S.T. Evolutionarily conserved function of Gbx2 in anterior hindbrain development. Dev. Dyn. 2011, 240, 828–838. [Google Scholar] [CrossRef]
- Buckley, D.M.; Burroughs-Garcia, J.; Lewandoski, M.; Waters, S.T. Characterization of the Gbx1−/− mouse mutant: A requirement for Gbx1 in normal locomotion and sensorimotor circuit development. PLoS ONE 2013, 8, e56214. [Google Scholar] [CrossRef] [Green Version]
- Del Barrio, M.G.; Bourane, S.; Grossmann, K.; Schüle, R.; Britsch, S.; O’Leary, D.D.; Goulding, M. A transcription factor code defines nine sensory interneuron subtypes in the mechanosensory area of the spinal cord. PLoS ONE 2013, 8, e77928. [Google Scholar] [CrossRef]
- Su, C.Y.; Kemp, H.A.; Moens, C.B. Cerebellar development in the absence of Gbx function in zebrafish. Dev. Biol. 2014, 386, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Meziane, H.; Fraulob, V.; Riet, F.; Krezel, W.; Selloum, M.; Geffarth, M.; Acampora, D.; Hérault, Y.; Simeone, A.; Brand, M.; et al. The homeodomain factor Gbx1 is required for locomotion and cell specification in the dorsal spinal cord. PeerJ 2013, 1, e142. [Google Scholar] [CrossRef]
- Waters, S.T.; Wilson, C.P.; Lewandoski, M. Cloning and embryonic expression analysis of the mouse Gbx1 gene. Gene Expr. Patterns 2003, 3, 313–317. [Google Scholar] [CrossRef]
- John, A.; Wildner, H.; Britsch, S. The homeodomain transcription factor Gbx1 identifies a subpopulation of late-born GABAergic interneurons in the developing dorsal spinal cord. Dev. Dyn. 2005, 234, 767–771. [Google Scholar] [CrossRef]
- Tourtellotte, W.G.; Nagarajan, R.; Bartke, A.; Milbrandt, J. Functional compensation by Egr4 in Egr1-dependent luteinizing hormone regulation and Leydig cell steroidogenesis. Mol. Cell Biol. 2000, 20, 5261–5268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Relaix, F.; Rocancourt, D.; Mansouri, A.; Buckingham, M. Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes Dev. 2004, 18, 1088–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanks, M.; Wurst, W.; Anson-Cartwright, L.; Auerbach, A.B.; Joyner, A.L. Rescue of the En-1 mutant phenotype by replacement of En-1 with En-2. Science 1995, 269, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Urbánek, P.; Fetka, I.; Meisler, M.H.; Busslinger, M. Cooperation of Pax2 and Pax5 in midbrain and cerebellum development. Proc. Natl. Acad. Sci. USA 1997, 94, 5703–5708. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Cantos, R.; Patente, M.; Wu, D.K. Gbx2 is required for the morphogenesis of the mouse inner ear: A downstream candidate of hindbrain signaling. Development 2005, 132, 2309–2318. [Google Scholar] [CrossRef] [Green Version]
- Byrd, N.A.; Meyers, E.N. Loss of Gbx2 results in neural crest cell patterning and pharyngeal arch artery defects in the mouse embryo. Dev. Biol. 2005, 284, 233–245. [Google Scholar] [CrossRef] [Green Version]
- Villalon, E.; Schulz, D.J.; Waters, S.T. Real-time PCR quantification of gene expression in embryonic mouse tissue. Methods Mol. Biol. 2014, 1092, 81–94. [Google Scholar]
- Smart, I.H. Proliferative characteristics of the ependymal layer during the early development of the mouse diencephalon, as revealed by recording the number, location, and plane of cleavage of mitotic figures. J. Anat. 1972, 113 Pt 1, 109–129. [Google Scholar]
- Alnemri, E.S.; Livingston, D.J.; Nicholson, D.W.; Salvesen, G.; Thornberry, N.A.; Wong, W.W.; Yuan, J. Human ICE/CED-3 protease nomenclature. Cell 1996, 87, 171. [Google Scholar] [CrossRef] [Green Version]
- Wolpert, L. Positional information and the spatial pattern of cellular differentiation. J. Theor. Biol. 1969, 25, 1–47. [Google Scholar] [CrossRef]
- Liu, F.; Morrison, A.H.; Gregor, T. Dynamic interpretation of maternal inputs by the Drosophila segmentation gene network. Proc. Natl. Acad. Sci. USA 2013, 110, 6724–6729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, T.; Wang, X.; Gray, P.A.; Weiner, J.A. A differential developmental pattern of spinal interneuron apoptosis during synaptogenesis: Insights from genetic analyses of the protocadherin-gamma gene cluster. Development 2008, 135, 4153–4164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowrie, M.B.; Lawson, S.J. Cell death of spinal interneurones. Prog. Neurobiol. 2000, 61, 543–555. [Google Scholar] [CrossRef]
- Oppenheim, R.W. Cell death during development of the nervous system. Annu. Rev. Neurosci. 1991, 14, 453–501. [Google Scholar] [CrossRef] [PubMed]
- Mennerick, S.; Zorumski, C.F. Neural activity and survival in the developing nervous system. Mol. Neurobiol. 2000, 22, 41–54. [Google Scholar]
- Rhinn, M.; Lun, K.; Amores, A.; Yan, Y.L.; Postlethwait, J.H.; Brand, M. Cloning, expression and relationship of zebrafish gbx1 and gbx2 genes to Fgf signaling. Mech. Dev. 2003, 120, 919–936. [Google Scholar] [CrossRef]
- Kikuta, H.; Kanai, M.; Ito, Y.; Yamasu, K. gbx2 Homeobox gene is required for the maintenance of the isthmic region in the zebrafish embryonic brain. Dev. Dyn. 2003, 228, 433–450. [Google Scholar] [CrossRef]
- Sugiyama, S.; Di Nardo, A.A.; Aizawa, S.; Matsuo, I.; Volovitch, M.; Prochiantz, A.; Hensch, T.K. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 2008, 134, 508–520. [Google Scholar] [CrossRef] [Green Version]
- Lesaffre, B.; Joliot, A.; Prochiantz, A.; Volovitch, M. Direct non-cell autonomous Pax6 activity regulates eye development in the zebrafish. Neural Dev. 2007, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Brunet, I.; Weinl, C.; Piper, M.; Trembleau, A.; Volovitch, M.; Harris, W.; Prochiantz, A.; Holt, C. The transcription factor Engrailed-2 guides retinal axons. Nature 2005, 438, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Han, D.Y.; Kobayashi, M.; Nakano, M.; Atobe, Y.; Kadota, T.; Funakoshi, K. Differential Islet-1 expression among lumbosacral spinal motor neurons in prenatal mouse. Brain Res. 2009, 1265, 30–36. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buckley, D.M.; Burroughs-Garcia, J.; Kriks, S.; Lewandoski, M.; Waters, S.T. Gbx1 and Gbx2 Are Essential for Normal Patterning and Development of Interneurons and Motor Neurons in the Embryonic Spinal Cord. J. Dev. Biol. 2020, 8, 9. https://doi.org/10.3390/jdb8020009
Buckley DM, Burroughs-Garcia J, Kriks S, Lewandoski M, Waters ST. Gbx1 and Gbx2 Are Essential for Normal Patterning and Development of Interneurons and Motor Neurons in the Embryonic Spinal Cord. Journal of Developmental Biology. 2020; 8(2):9. https://doi.org/10.3390/jdb8020009
Chicago/Turabian StyleBuckley, Desirè M., Jessica Burroughs-Garcia, Sonja Kriks, Mark Lewandoski, and Samuel T. Waters. 2020. "Gbx1 and Gbx2 Are Essential for Normal Patterning and Development of Interneurons and Motor Neurons in the Embryonic Spinal Cord" Journal of Developmental Biology 8, no. 2: 9. https://doi.org/10.3390/jdb8020009
APA StyleBuckley, D. M., Burroughs-Garcia, J., Kriks, S., Lewandoski, M., & Waters, S. T. (2020). Gbx1 and Gbx2 Are Essential for Normal Patterning and Development of Interneurons and Motor Neurons in the Embryonic Spinal Cord. Journal of Developmental Biology, 8(2), 9. https://doi.org/10.3390/jdb8020009




