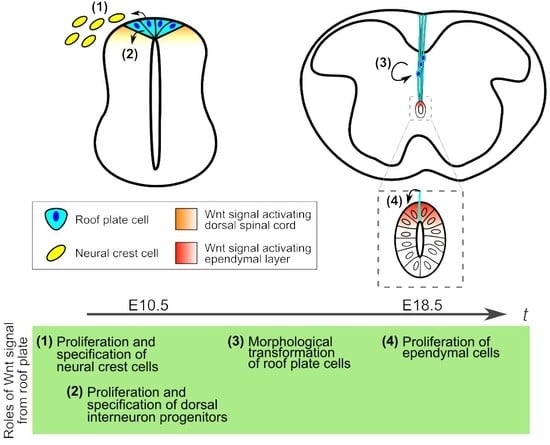
Morphological and Functional Changes of Roof Plate Cells in Spinal Cord Development
Abstract
:1. Roof Plate Functions in the Early Developmental Stage of Spinal Cord
1.1. Generation of Trunk Neural Crest Cells
1.2. Specification of Dorsal Interneurons
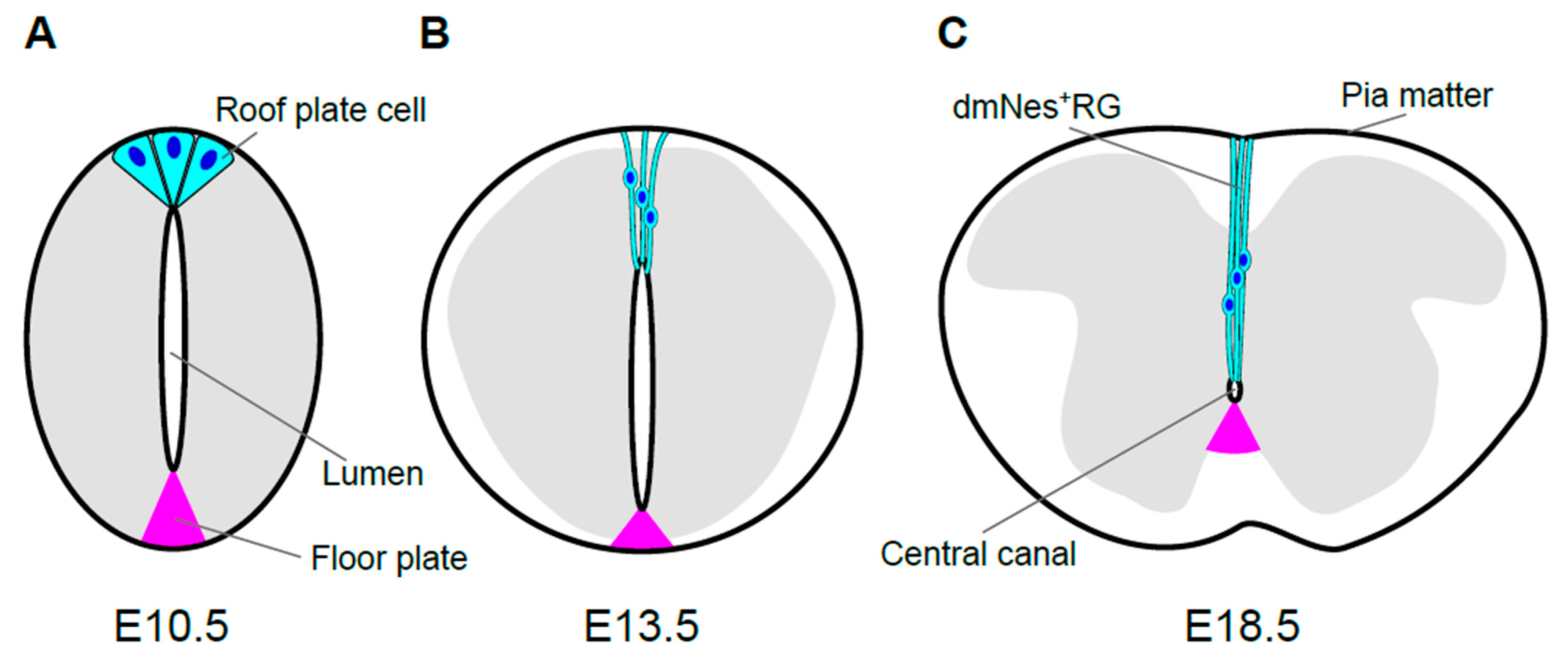
2. Morphological Transformation of the Spinal Cord and Central Canal Formation in Mouse Development
2.1. Morphological Transformation of the Spinal Cord
2.2. Morphological Transformation of the Lumen
2.3. Origin of Ependymal Layer
2.4. Regulatory Mechanisms Governing Lumen Reduction
3. Development of Roof Plate Cells in Formation of the Central Canal
3.1. Formation of dmNes+RGs
3.2. Morphology and Roles of dmNes+RGs
4. Wnt Signaling in Morphological Transformation of Roof Plate Cells
4.1. Expression of Wnt Ligands and Activation of Wnt Signaling
4.2. Functions of Wnt Ligands Secreted by dmNes+RGs
5. Wnt Signaling in Development of Cells Surrounding the Central Canal
5.1. Wnt Signaling in Ependymal Cells
5.2. Proliferation of Ependymal Cells
5.3. Adult Spinal Cord
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Copp, A.J.; Greene, N.D.E.; Murdoch, J.N. The Genetic Basis of Mammalian Neurulation. Nat. Rev. Genet. 2003, 4, 784–793. [Google Scholar] [CrossRef]
- Chizhikov, V.V.; Millen, K.J. Mechanisms of Roof Plate Formation in the Vertebrate CNS. Nat. Rev. Neurosci. 2004, 5, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Serbedzija, G.N.; Fraser, S.E.; Bronner-Fraser, M. Pathways of Trunk Neural Crest Cell Migration in the Mouse Embryo as Revealed by Vital Dye Labelling. Development 1990, 108, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Serbedzija, G.N.; Bronner-Fraser, M.; Fraser, S.E. Developmental Potential of Trunk Neural Crest Cells in the Mouse. Development 1994, 120, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Wilson, Y.M.; Richards, K.L.; Ford-Perriss, M.L.; Panthier, J.-J.; Murphy, M. Neural Crest Cell Lineage Segregation in the Mouse Neural Tube. Development 2004, 131, 6153–6162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baggiolini, A.; Varum, S.; Mateos, J.M.; Bettosini, D.; John, N.; Bonalli, M.; Ziegler, U.; Dimou, L.; Clevers, H.; Furrer, R.; et al. Premigratory and Migratory Neural Crest Cells Are Multipotent In Vivo. Cell Stem Cell 2015, 16, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Martik, M.L.; Bronner, M.E. Regulatory Logic Underlying Diversification of the Neural Crest. Trends Genet. 2017, 33, 715–727. [Google Scholar] [CrossRef] [Green Version]
- Krispin, S.; Nitzan, E.; Kassem, Y.; Kalcheim, C. Evidence for a Dynamic Spatiotemporal Fate Map and Early Fate Restrictions of Premigratory Avian Neural Crest. Development 2010, 137, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Nitzan, E.; Avraham, O.; Kahane, N.; Ofek, S.; Kumar, D.; Kalcheim, C. Dynamics of BMP and Hes1/Hairy1 Signaling in the Dorsal Neural Tube Underlies the Transition from Neural Crest to Definitive Roof Plate. BMC Biol. 2016, 14, 23. [Google Scholar] [CrossRef] [Green Version]
- Rekler, D.; Kalcheim, C. From Neural Crest to Definitive Roof Plate: The Dynamic Behavior of the Dorsal Neural Tube. Int. J. Mol. Sci. 2021, 22, 3911. [Google Scholar] [CrossRef]
- Caspary, T.; Anderson, K.V. Patterning Cell Types in the Dorsal Spinal Cord: What the Mouse Mutants Say. Nat. Rev. Neurosci. 2003, 4, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Chizhikov, V.V.; Millen, K.J. Roof Plate-Dependent Patterning of the Vertebrate Dorsal Central Nervous System. Dev. Biol. 2005, 277, 287–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, D.G.; Bailes, J.A.; McMahon, A.P. Expression of the Proto-Oncogene Int-1 Is Restricted to Specific Neural Cells in the Developing Mouse Embryo. Cell 1987, 50, 79–88. [Google Scholar] [CrossRef]
- McMahon, A.P.; Joyner, A.L.; Bradley, A.; McMahon, J.A. The Midbrain-Hindbrain Phenotype of Wnt-1-/Wnt-1- Mice Results from Stepwise Deletion of Engrailed-Expressing Cells by 9.5 Days Postcoitum. Cell 1992, 69, 581–595. [Google Scholar] [CrossRef]
- Parr, B.A.; Shea, M.J.; Vassileva, G.; McMahon, A.P. Mouse Wnt Genes Exhibit Discrete Domains of Expression in the Early Embryonic CNS and Limb Buds. Development 1993, 119, 247–261. [Google Scholar] [CrossRef]
- McMahon, A.P.; Bradley, A. The Wnt-1 (Int-1) Proto-Oncogene Is Required for Development of a Large Region of the Mouse Brain. Cell 1990, 62, 1073–1085. [Google Scholar] [CrossRef]
- Thomas, K.R.; Capecchi, M.R. Targeted Disruption of the Murine Int-1 Proto-Oncogene Resulting in Severe Abnormalities in Midbrain and Cerebellar Development. Nature 1990, 346, 847–850. [Google Scholar] [CrossRef]
- Takada, S.; Stark, K.L.; Shea, M.J.; Vassileva, G.; McMahon, J.A.; McMahon, A.P. Wnt-3a Regulates Somite and Tailbud Formation in the Mouse Embryo. Genes. Dev. 1994, 8, 174–189. [Google Scholar] [CrossRef] [Green Version]
- Ikeya, M.; Lee, S.M.K.; Johnson, J.E.; McMahon, A.P.; Takada, S. Wnt Signalling Required for Expansion of Neural Crest and CNS Progenitors. Nature 1997, 389, 966–970. [Google Scholar] [CrossRef]
- LaBonne, C.; Bronner-Fraser, M. Neural Crest Induction in Xenopus: Evidence for a Two-Signal Model. Development 1998, 125, 2403–2414. [Google Scholar] [CrossRef]
- Deardorff, M.A.; Tan, C.; Saint-Jeannet, J.P.; Klein, P.S. A Role for Frizzled 3 in Neural Crest Development. Development 2001, 128, 3655–3663. [Google Scholar] [CrossRef]
- García-Castro, M.I.; Marcelle, C.; Bronner-Fraser, M. Ectodermal Wnt Function as a Neural Crest Inducer. Science 2002, 297, 848–851. [Google Scholar]
- Wu, J.; Yang, J.; Klein, P.S. Neural Crest Induction by the Canonical Wnt Pathway Can Be Dissociated from Anterior–Posterior Neural Patterning in Xenopus. Dev. Biol. 2005, 279, 220–232. [Google Scholar] [CrossRef] [Green Version]
- Hassler, C.; Cruciat, C.-M.; Huang, Y.-L.; Kuriyama, S.; Mayor, R.; Niehrs, C. Kremen Is Required for Neural Crest Induction in Xenopus and Promotes LRP6-Mediated Wnt Signaling. Development 2007, 134, 4255–4263. [Google Scholar] [CrossRef] [Green Version]
- Dorsky, R.I.; Moon, R.T.; Raible, D.W. Control of Neural Crest Cell Fate by the Wnt Signalling Pathway. Nature 1998, 396, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Hari, L.; Brault, V.; Kléber, M.; Lee, H.-Y.; Ille, F.; Leimeroth, R.; Paratore, C.; Suter, U.; Kemler, R.; Sommer, L. Lineage-Specific Requirements of Beta-Catenin in Neural Crest Development. J. Cell Biol. 2002, 159, 867–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.-Y.; Kléber, M.; Hari, L.; Brault, V.; Suter, U.; Taketo, M.M.; Kemler, R.; Sommer, L. Instructive Role of Wnt/Beta-Catenin in Sensory Fate Specification in Neural Crest Stem Cells. Science 2004, 303, 1020–1023. [Google Scholar] [CrossRef] [Green Version]
- Sommer, L. Generation of Melanocytes from Neural Crest Cells: Generation of Melanocytes from Neural Crest Cells. Pigment Cell Melanoma Res. 2011, 24, 411–421. [Google Scholar] [CrossRef]
- Hari, L.; Miescher, I.; Shakhova, O.; Suter, U.; Chin, L.; Taketo, M.; Richardson, W.D.; Kessaris, N.; Sommer, L. Temporal Control of Neural Crest Lineage Generation by Wnt/β-Catenin Signaling. Development 2012, 139, 2107–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, L.; Dormand, E.L.; Anderson, D.J. Late-Emigrating Neural Crest Cells in the Roof Plate Are Restricted to a Sensory Fate by GDF7. Proc. Natl. Acad. Sci. USA 2005, 102, 7192–7197. [Google Scholar] [CrossRef] [Green Version]
- Kléber, M.; Lee, H.-Y.; Wurdak, H.; Buchstaller, J.; Riccomagno, M.M.; Ittner, L.M.; Suter, U.; Epstein, D.J.; Sommer, L. Neural Crest Stem Cell Maintenance by Combinatorial Wnt and BMP Signaling. J. Cell Biol. 2005, 169, 309–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helms, A.W.; Johnson, J.E. Specification of Dorsal Spinal Cord Interneurons. Curr. Opin. Neurobiol. 2003, 13, 42–49. [Google Scholar] [CrossRef]
- Lee, K.J.; Dietrich, P.; Jessell, T.M. Genetic Ablation Reveals That the Roof Plate Is Essential for Dorsal Interneuron Specification. Nature 2000, 403, 734–740. [Google Scholar] [CrossRef]
- Muroyama, Y.; Fujihara, M.; Ikeya, M.; Kondoh, H.; Takada, S. Wnt Signaling Plays an Essential Role in Neuronal Specification of the Dorsal Spinal Cord. Genes Dev. 2002, 16, 548–553. [Google Scholar] [CrossRef] [Green Version]
- Shinozuka, T.; Takada, R.; Yoshida, S.; Yonemura, S.; Takada, S. Wnt Produced by Stretched Roof-Plate Cells Is Required for the Promotion of Cell Proliferation around the Central Canal of the Spinal Cord. Development 2019, 146, dev159343. [Google Scholar] [CrossRef] [Green Version]
- Zechner, D.; Müller, T.; Wende, H.; Walther, I.; Taketo, M.M.; Crenshaw, E.B.; Treier, M.; Birchmeier, W.; Birchmeier, C. Bmp and Wnt/β-Catenin Signals Control Expression of the Transcription Factor Olig3 and the Specification of Spinal Cord Neurons. Dev. Biol. 2007, 303, 181–190. [Google Scholar] [CrossRef]
- Dickinson, M.E.; Krumlauf, R.; McMahon, A.P. Evidence for a Mitogenic Effect of Wnt-1 in the Developing Mammalian Central Nervous System. Development 1994, 120, 1453–1471. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, T.; Wend, P.; Klaus, A.; Birchmeier, W. Deciphering the Function of Canonical Wnt Signals in Development and Disease: Conditional Loss- and Gain-of-Function Mutations of Beta-Catenin in Mice. Genes Dev. 2008, 22, 2308–2341. [Google Scholar] [CrossRef] [Green Version]
- Megason, S.G.; McMahon, A.P. A Mitogen Gradient of Dorsal Midline Wnts Organizes Growth in the CNS. Development 2002, 129, 2087–2098. [Google Scholar] [CrossRef] [PubMed]
- Winnier, G.; Blessing, M.; Labosky, P.A.; Hogan, B.L. Bone Morphogenetic Protein-4 Is Required for Mesoderm Formation and Patterning in the Mouse. Genes Dev. 1995, 9, 2105–2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudley, A.T.; Robertson, E.J. Overlapping Expression Domains of Bone Morphogenetic Protein Family Members Potentially Account for Limited Tissue Defects in BMP7 Deficient Embryos. Dev. Dyn. 1997, 208, 349–362. [Google Scholar] [CrossRef]
- Lee, K.J.; Mendelsohn, M.; Jessell, T.M. Neuronal Patterning by BMPs: A Requirement for GDF7 in the Generation of a Discrete Class of Commissural Interneurons in the Mouse Spinal Cord. Genes Dev. 1998, 12, 3394–3407. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Ueno, N.; Behringer, R.R. Restriction of BMP4 Activity Domains in the Developing Neural Tube of the Mouse Embryo. EMBO Rep. 2004, 5, 734–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Dréau, G.; Martí, E. The Multiple Activities of BMPs during Spinal Cord Development. Cell. Mol. Life Sci. 2013, 70, 4293–4305. [Google Scholar] [CrossRef]
- Liem, K.F.; Tremml, G.; Roelink, H.; Jessell, T.M. Dorsal Differentiation of Neural Plate Cells Induced by BMP-Mediated Signals from Epidermal Ectoderm. Cell 1995, 82, 969–979. [Google Scholar] [CrossRef] [Green Version]
- Liem, K.F.; Tremml, G.; Jessell, T.M. A Role for the Roof Plate and Its Resident TGFbeta-Related Proteins in Neuronal Patterning in the Dorsal Spinal Cord. Cell 1997, 91, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Le Dréau, G.; Garcia-Campmany, L.; Rabadán, M.A.; Ferronha, T.; Tozer, S.; Briscoe, J.; Martí, E. Canonical BMP7 Activity Is Required for the Generation of Discrete Neuronal Populations in the Dorsal Spinal Cord. Development 2012, 139, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Hazen, V.M.; Phan, K.D.; Hudiburgh, S.; Butler, S.J. Inhibitory Smads Differentially Regulate Cell Fate Specification and Axon Dynamics in the Dorsal Spinal Cord. Dev. Biol. 2011, 356, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Butler, S.J.; Dodd, J. A Role for BMP Heterodimers in Roof Plate-Mediated Repulsion of Commissural Axons. Neuron 2003, 38, 389–401. [Google Scholar] [CrossRef] [Green Version]
- Ille, F.; Atanasoski, S.; Falk, S.; Ittner, L.M.; Märki, D.; Büchmann-Møller, S.; Wurdak, H.; Suter, U.; Taketo, M.M.; Sommer, L. Wnt/BMP Signal Integration Regulates the Balance between Proliferation and Differentiation of Neuroepithelial Cells in the Dorsal Spinal Cord. Dev. Biol. 2007, 304, 394–408. [Google Scholar] [CrossRef] [Green Version]
- Ofek, S.; Wiszniak, S.; Kagan, S.; Tondl, M.; Schwarz, Q.; Kalcheim, C. Notch Signaling Is a Critical Initiator of Roof Plate Formation as Revealed by the Use of RNA Profiling of the Dorsal Neural Tube. BMC Biol. 2021, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, K.; Jeong, J. Developmental Biology of the Meninges. Genesis 2019, 57, e23288. [Google Scholar] [CrossRef] [PubMed]
- Sturrock, R.R. An Electron Microscopic Study of the Development of the Ependyma of the Central Canal of the Mouse Spinal Cord. J. Anat. 1981, 132, 119–136. [Google Scholar]
- Cañizares, M.A.; Albors, A.R.; Singer, G.; Suttie, N.; Gorkic, M.; Felts, P.; Storey, K.G. Multiple Steps Characterise Ventricular Layer Attrition to Form the Ependymal Cell Lining of the Adult Mouse Spinal Cord Central Canal. J. Anat. 2020, 236, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Meletis, K.; Barnabé-Heider, F.; Carlén, M.; Evergren, E.; Tomilin, N.; Shupliakov, O.; Frisén, J. Spinal Cord Injury Reveals Multilineage Differentiation of Ependymal Cells. PLoS Biol. 2008, 6, e182. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, L.K.; Truong, M.K.V.; Bednarczyk, M.R.; Aumont, A.; Fernandes, K.J.L. Cellular Organization of the Central Canal Ependymal Zone, a Niche of Latent Neural Stem Cells in the Adult Mammalian Spinal Cord. Neuroscience 2009, 164, 1044–1056. [Google Scholar] [CrossRef] [PubMed]
- Sabourin, J.-C.; Ackema, K.B.; Ohayon, D.; Guichet, P.-O.; Perrin, F.E.; Garces, A.; Ripoll, C.; Charité, J.; Simonneau, L.; Kettenmann, H.; et al. A Mesenchymal-like ZEB1(+) Niche Harbors Dorsal Radial Glial Fibrillary Acidic Protein-Positive Stem Cells in the Spinal Cord. Stem Cells 2009, 27, 2722–2733. [Google Scholar] [CrossRef] [PubMed]
- Hugnot, J.P.; Franzen, R. The Spinal Cord Ependymal Region: A Stem Cell Niche in the Caudal Central Nervous System. Front. Biosci. 2011, 16, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Del Bigio, M.R. The Ependyma: A Protective Barrier between Brain and Cerebrospinal Fluid. Glia 1995, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bruni, J.E. Ependymal Development, Proliferation, and Functions: A Review. Microsc. Res. Tech. 1998, 41, 2–13. [Google Scholar] [CrossRef]
- Johansson, C.B.; Momma, S.; Clarke, D.L.; Risling, M.; Lendahl, U.; Frisén, J. Identification of a Neural Stem Cell in the Adult Mammalian Central Nervous System. Cell 1999, 96, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Shechter, R.; Ziv, Y.; Schwartz, M. New GABAergic Interneurons Supported by Myelin-Specific T Cells Are Formed in Intact Adult Spinal Cord. Stem Cells 2007, 25, 2277–2282. [Google Scholar] [CrossRef]
- Sevc, J.; Daxnerová, Z.; Miklosová, M. Role of Radial Glia in Transformation of the Primitive Lumen to the Central Canal in the Developing Rat Spinal Cord. Cell Mol. Neurobiol. 2009, 29, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Ghazale, H.; Ripoll, C.; Leventoux, N.; Jacob, L.; Azar, S.; Mamaeva, D.; Glasson, Y.; Calvo, C.-F.; Thomas, J.-L.; Meneceur, S.; et al. RNA Profiling of the Human and Mouse Spinal Cord Stem Cell Niches Reveals an Embryonic-like Regionalization with MSX1+ Roof-Plate-Derived Cells. Stem Cell Rep. 2019, 12, 1159–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tait, C.M.; Chinnaiya, K.; Manning, E.; Murtaza, M.; Ashton, J.-P.; Furley, N.; Hill, C.J.; Alves, C.H.; Wijnholds, J.; Erdmann, K.S.; et al. Crumbs2 Mediates Ventricular Layer Remodelling to Form the Spinal Cord Central Canal. PLoS Biol. 2020, 18, e3000470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leber, S.M.; Breedlove, S.M.; Sanes, J.R. Lineage, Arrangement, and Death of Clonally Related Motoneurons in Chick Spinal Cord. J. Neurosci. 1990, 10, 2451–2462. [Google Scholar] [CrossRef]
- Fu, H.; Qi, Y.; Tan, M.; Cai, J.; Hu, X.; Liu, Z.; Jensen, J.; Qiu, M. Molecular Mapping of the Origin of Postnatal Spinal Cord Ependymal Cells: Evidence That Adult Ependymal Cells Are Derived from Nkx6.1+ Ventral Neural Progenitor Cells. J. Comp. Neurol. 2003, 456, 237–244. [Google Scholar] [CrossRef]
- Masahira, N.; Takebayashi, H.; Ono, K.; Watanabe, K.; Ding, L.; Furusho, M.; Ogawa, Y.; Nabeshima, Y.; Alvarez-Buylla, A.; Shimizu, K.; et al. Olig2-Positive Progenitors in the Embryonic Spinal Cord Give Rise Not Only to Motoneurons and Oligodendrocytes, but Also to a Subset of Astrocytes and Ependymal Cells. Dev. Biol. 2006, 293, 358–369. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; McGlynn, S.; Matise, M.P. Floor Plate-Derived Sonic Hedgehog Regulates Glial and Ependymal Cell Fates in the Developing Spinal Cord. Development 2013, 140, 1594–1604. [Google Scholar] [CrossRef] [Green Version]
- Xing, L.; Anbarchian, T.; Tsai, J.M.; Plant, G.W.; Nusse, R. Wnt/β-Catenin Signaling Regulates Ependymal Cell Development and Adult Homeostasis. Proc. Natl. Acad. Sci. USA 2018, 115, E5954–E5962. [Google Scholar] [CrossRef] [Green Version]
- Munson, C.; Huisken, J.; Bit-Avragim, N.; Kuo, T.; Dong, P.D.; Ober, E.A.; Verkade, H.; Abdelilah-Seyfried, S.; Stainier, D.Y.R. Regulation of Neurocoel Morphogenesis by Pard6γb. Dev. Biol. 2008, 324, 41–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondrychyn, I.; Teh, C.; Sin, M.; Korzh, V. Stretching Morphogenesis of the Roof Plate and Formation of the Central Canal. PLoS ONE 2013, 8, e56219. [Google Scholar] [CrossRef] [Green Version]
- Böhme, G. Formation of the Central Canal and Dorsal Glial Septum in the Spinal Cord of the Domestic Cat. J. Anat. 1988, 159, 37–47. [Google Scholar]
- Korzh, V. Stretching Cell Morphogenesis during Late Neurulation and Mild Neural Tube Defects. Dev. Growth Differ. 2014, 56, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Snow, D.M.; Steindler, D.A.; Silver, J. Molecular and Cellular Characterization of the Glial Roof Plate of the Spinal Cord and Optic Tectum: A Possible Role for a Proteoglycan in the Development of an Axon Barrier. Dev. Biol. 1990, 138, 359–376. [Google Scholar] [CrossRef]
- Sarnat, H.B. Role of Human Fetal Ependyma. Pediatr. Neurol. 1992, 8, 163–178. [Google Scholar] [CrossRef]
- Millonig, J.H.; Millen, K.J.; Hatten, M.E. The Mouse Dreher Gene Lmx1a Controls Formation of the Roof Plate in the Vertebrate CNS. Nature 2000, 403, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Kridsada, K.; Niu, J.; Haldipur, P.; Wang, Z.; Ding, L.; Li, J.J.; Lindgren, A.G.; Herrera, E.; Thomas, G.M.; Chizhikov, V.V.; et al. Roof Plate-Derived Radial Glial-like Cells Support Developmental Growth of Rapidly Adapting Mechanoreceptor Ascending Axons. Cell Rep. 2018, 23, 2928–2941. [Google Scholar] [CrossRef]
- Shimizu, T.; Kagawa, T.; Wada, T.; Muroyama, Y.; Takada, S.; Ikenaka, K. Wnt Signaling Controls the Timing of Oligodendrocyte Development in the Spinal Cord. Dev. Biol. 2005, 282, 397–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bänziger, C.; Soldini, D.; Schütt, C.; Zipperlen, P.; Hausmann, G.; Basler, K. Wntless, a Conserved Membrane Protein Dedicated to the Secretion of Wnt Proteins from Signaling Cells. Cell 2006, 125, 509–522. [Google Scholar] [CrossRef] [Green Version]
- Bartscherer, K.; Pelte, N.; Ingelfinger, D.; Boutros, M. Secretion of Wnt Ligands Requires Evi, a Conserved Transmembrane Protein. Cell 2006, 125, 523–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, R.M.; Thombre, S.; Firtina, Z.; Gray, D.; Betts, D.; Roebuck, J.; Spana, E.P.; Selva, E.M. Sprinter: A Novel Transmembrane Protein Required for Wg Secretion and Signaling. Development 2006, 133, 4901–4911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GENSAT Brain Atlas of Gene Expression in EGFP Transgenic Mice. Available online: http://www.gensat.org/index.html (accessed on 29 July 2021).
- Horner, P.J.; Power, A.E.; Kempermann, G.; Kuhn, H.G.; Palmer, T.D.; Winkler, J.; Thal, L.J.; Gage, F.H. Proliferation and Differentiation of Progenitor Cells Throughout the Intact Adult Rat Spinal Cord. J. Neurosci. 2000, 20, 2218–2228. [Google Scholar] [CrossRef] [PubMed]
- Lie, D.-C.; Colamarino, S.A.; Song, H.-J.; Désiré, L.; Mira, H.; Consiglio, A.; Lein, E.S.; Jessberger, S.; Lansford, H.; Dearie, A.R.; et al. Wnt Signalling Regulates Adult Hippocampal Neurogenesis. Nature 2005, 437, 1370–1375. [Google Scholar] [CrossRef]
- Adachi, K.; Mirzadeh, Z.; Sakaguchi, M.; Yamashita, T.; Nikolcheva, T.; Gotoh, Y.; Peltz, G.; Gong, L.; Kawase, T.; Alvarez-Buylla, A.; et al. β-Catenin Signaling Promotes Proliferation of Progenitor Cells in the Adult Mouse Subventricular Zone. Stem Cells 2007, 25, 2827–2836. [Google Scholar] [CrossRef]
- Varela-Nallar, L.; Inestrosa, N.C. Wnt Signaling in the Regulation of Adult Hippocampal Neurogenesis. Front. Cell. Neurosci. 2013, 7, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinozuka, T.; Takada, S. Morphological and Functional Changes of Roof Plate Cells in Spinal Cord Development. J. Dev. Biol. 2021, 9, 30. https://doi.org/10.3390/jdb9030030
Shinozuka T, Takada S. Morphological and Functional Changes of Roof Plate Cells in Spinal Cord Development. Journal of Developmental Biology. 2021; 9(3):30. https://doi.org/10.3390/jdb9030030
Chicago/Turabian StyleShinozuka, Takuma, and Shinji Takada. 2021. "Morphological and Functional Changes of Roof Plate Cells in Spinal Cord Development" Journal of Developmental Biology 9, no. 3: 30. https://doi.org/10.3390/jdb9030030
APA StyleShinozuka, T., & Takada, S. (2021). Morphological and Functional Changes of Roof Plate Cells in Spinal Cord Development. Journal of Developmental Biology, 9(3), 30. https://doi.org/10.3390/jdb9030030