The Review of the Autotomy of Agamid Lizards with Considerations about the Types of Autotomy and Regeneration
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Fredericq, L. Nouvelles recherches sur l’autotomie chez le crabbe. Bulletin de la Classe des Sciences. Académie Royale de Belgique 1891, XLVI. Available online: https://orbi.uliege.be/handle/2268/128917 (accessed on 17 June 2021).
- Bellairs, A.; Bryant, S.V. Autotomy and Regeneration in Reptiles. In Biology of the Reptilia; Development B; John Wiley & Sons: New York, NY, USA; Chichester, UK; Brisbane, Australia; Toronto, ON, Canada; Singapore, 1985; Volume 15, pp. 303–410. [Google Scholar]
- Dunoyer, L.A.; Seifert, A.W.; Cleve, J.V. Evolutionary Bedfellows: Reconstructing the Ancestral State of Autotomy and Regeneration. J. Exp. Zool. Part B Mol. Dev. Evol. 2021, 336, 94–115. [Google Scholar] [CrossRef]
- Hosotani, M.; Nakamura, T.; Ichii, O.; Irie, T.; Sunden, Y.; Elewa, Y.H.A.; Watanabe, T.; Ueda, H.; Mishima, T.; Kon, Y. Unique Histological Features of the Tail Skin of Cotton Rat (Sigmodon hispidus) Related to Caudal Autotomy. Biol. Open 2021, 10. [Google Scholar] [CrossRef]
- Arnold, E.N. Evolutionary Aspects of Tail Shedding in Lizards and Their Relatives. J. Nat. Hist. 1984, 18, 127–169. [Google Scholar] [CrossRef]
- Arnold, E.N. Caudal Autotomy as a Defense. In Biology of the Reptilia; Ecology B; Academic Press: London, UK; New York, NY, USA, 1988; Volume 16, pp. 235–273. [Google Scholar]
- Ananjeva, N.B.; Orlov, N.L. Caudal Autotomy in Colubrid Snake Xenochrophis Piscator from Vietnam. Russ. J. Herpetol. 1994, 1, 169–171. [Google Scholar] [CrossRef]
- Savage, J.M.; Slowinski, J.B. Evolution of Coloration, Urotomy and Coral Snake Mimicry in the Snake Genus Scaphiodontophis (Serpentes: Colubridae). Biol. J. Linn. Soc. 1996, 57, 129–194. [Google Scholar] [CrossRef]
- Bowen, K.D. Frequency of Tail Breakage of the Northern Watersnake, Nerodia Sipedon Sipedon. Can. Field Nat. 2004, 118, 435–437. [Google Scholar] [CrossRef] [Green Version]
- Alibardi, L. Regeneration in Reptiles and Its Position among Vertebrates. In Morphological and Cellular Aspects of Tail and Limb Regeneration in Lizards: A Model System With Implications for Tissue Regeneration in Mammals; Alibardi, L., Ed.; Advances in Anatomy, Embryology and Cell Biology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–49. [Google Scholar] [CrossRef]
- Costa, H.C.; Moura, M.R.; Feio, R.N. A Tale of Lost Tails: Pseudoautotomy in the Neotropical Snake Genus Drymoluber (Serpentes: Colubridae). Can. J. Zool. 2014, 92, 811–816. [Google Scholar] [CrossRef]
- Crnobrnja-Isailovic, J.; Jelena, Ć.; Halpern, B. Deliberate Tail Loss in Dolichophis Caspius and Natrix Tessellata (Serpentes: Colubridae) with a Brief Review of Pseudoautotomy in Contemporary Snake Families. North-West. J. Zool. 2016, 12, 367–372. [Google Scholar]
- Carlson, B.M. Principles of Regenerative Biology; Academic Press-Elsevier: Cambridge, MA, USA, 2007. [Google Scholar] [CrossRef]
- Xu, C.; Palade, J.; Fisher, R.E.; Smith, C.I.; Clark, A.R.; Sampson, S.; Bourgeois, R.; Rawls, A.; Elsey, R.M.; Wilson-Rawls, J.; et al. Anatomical and Histological Analyses Reveal That Tail Repair Is Coupled with Regrowth in Wild-Caught, Juvenile American Alligators (Alligator mississippiensis). Sci. Rep. 2020, 10, 20122. [Google Scholar] [CrossRef]
- Brazaitis, P. Maxillary Regeneration in a Marsh Crocodile, Crocodylus Palustris. J. Herpetol. 1981, 15, 360–362. [Google Scholar] [CrossRef]
- Seligmann, H.; Moravec, J.; Werner, Y.L. Morphological, Functional and Evolutionary Aspects of Tail Autotomy and Regeneration in the ‘Living Fossil’ Sphenodon (Reptilia: Rhynchocephalia). Biol. J. Linn. Soc. 2008, 93, 721–743. [Google Scholar] [CrossRef] [Green Version]
- Van Schingen, M.; Ha, Q.; Pham, C.; Le, T.; Nguyen, T.; Bonkowski, M.; Ziegler, T. Discovery of a New Crocodile Lizard Population in Vietnam: Population Trends, Future Prognoses and Identifi Cation of Key Habitats for Conservation. Rev. Suisse Zool. 2016, 132, 241–251. [Google Scholar] [CrossRef]
- Alibardi, L. Immunodetection of FGF1-2 and FGFR1-2 Indicates That These Proteins Disappear in the Wound Epidermis and Blastema of the Scarring Limb in Lizard. MOJ Biol. Med. 2017, 1, 00003. [Google Scholar] [CrossRef] [Green Version]
- Boulenger, G.A. On the Scaling of the Reproduced Tail in Lizards. Proc. Zool. Soc. Lond. 1888, 1, 351–353. [Google Scholar] [CrossRef]
- Gordeev, D.A.; Ananjeva, N.B.; Korost, D.V. Autotomy and Regeneration in Squamate Reptiles (Squamata, Reptilia): Defensive Behavior Strategies and Morphological Characteristics (Using Computer Microtomography Methods). Biol. Bull. 2020, 47, 389–398. [Google Scholar] [CrossRef]
- Slowinski, J.B.; Savage, J.M. Urotomy in Scaphiodontophis: Evidence for the Multiple Tail Break Hypothesis in Snakes. Herpetologica 1995, 51, 338–341. [Google Scholar]
- Koch, N.M.; Gauthier, J.A. Noise and Biases in Genomic Data May Underlie Radically Different Hypotheses for the Position of Iguania within Squamata. PLoS ONE 2018, 13, e0202729. [Google Scholar] [CrossRef]
- Schall, J.J.; Bromwich, C.R.; Werner, Y.L.; Midlege, J. Clubbed Regenerated Tails in Agama agama and Their Possible Use in Social Interactions. J. Herpetol. 1989, 23, 303–305. [Google Scholar] [CrossRef]
- Siebenrock, F. Das skelet der Agamidae; Sitzungsberichte der Mathematisch-Naturwissenschaftlichen Classe der Kaiserlichen Akademie der Wissenschaften; Verlag der Österreichischen Akademie der Wissenschaften: Vienna, Austria, 1895; Volume 104, pp. 1089–1196. [Google Scholar]
- Uetz, P.; Freed, P. The Reptile Database. Available online: http://www.reptile-database.org/ (accessed on 17 June 2021).
- Vidal, N.; Hedges, S.B. The Phylogeny of Squamate Reptiles (Lizards, Snakes, and Amphisbaenians) Inferred from Nine Nuclear Protein-Coding Genes. Comptes Rendus Biol. 2005, 328, 1000–1008. [Google Scholar] [CrossRef]
- Gauthier, J.; Kearney, M.; Maisano, J.; Rieppel, O.; Behlke, A. Assembling the Squamate Tree of Life: Perspectives from the Phenotype and the Fossil Record. Bull. Peabody Mus. Nat. Hist. 2012, 53, 3–308. [Google Scholar] [CrossRef]
- Losos, J.B.; Hillis, D.M.; Greene, H.W. Evolution. Who Speaks with a Forked Tongue? Science 2012, 338, 1428–1429. [Google Scholar] [CrossRef] [PubMed]
- Reeder, T.W.; Townsend, T.M.; Mulcahy, D.G.; Noonan, B.P.; Wood, P.L., Jr.; Sites, J.W., Jr.; Wiens, J.J. Integrated Analyses Resolve Conflicts over Squamate Reptile Phylogeny and Reveal Unexpected Placements for Fossil Taxa. PLoS ONE 2015, 10, e0118199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyron, R.A. Novel Approaches for Phylogenetic Inference from Morphological Data and Total-Evidence Dating in Squamate Reptiles (Lizards, Snakes, and Amphisbaenians). Syst. Biol. 2017, 66, 38–56. [Google Scholar] [CrossRef]
- Lin, Z.-H.; Qu, Y.-F.; Ji, X. Energetic and Locomotor Costs of Tail Loss in the Chinese Skink, Eumeces Chinensis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 143, 508–513. [Google Scholar] [CrossRef]
- Lovely, K.; Mahler, D.L.; Revell, L. The Rate and Pattern of Tail Autotomy in Five Species of Puerto Rican Anoles. Evol. Ecol. Res. 2010, 12, 67–88. [Google Scholar]
- Arribas, O. Autotomía Caudal En Las Lagartijas de Alta Montaña de Los Pirineos (Iberolacerta Arribas, 1997). Butll. Soc. Catalana Herpetol. 2014, 21, 115–126. [Google Scholar]
- Kaczmarski, M.; Ziemblińska, K.; Tryjanowski, P. Sand Lizards Lacerta Agilis with Higher Digit Ratios Are More Likely to Autotomy. J. Anat. 2020, 237, 1103–1113. [Google Scholar] [CrossRef]
- Barr, J.I.; Somaweera, R.; Godfrey, S.S.; Bateman, P.W. Increased Tail Length in the King’s Skink, Egernia Kingii (Reptilia: Scincidae): An Anti-Predation Tactic for Juveniles? Biol. J. Linn. Soc. 2019, 126, 268–275. [Google Scholar] [CrossRef]
- Barr, J.I.; Somaweera, R.; Godfrey, S.S.; Gardner, M.G.; Bateman, P.W. When One Tail Isn’t Enough: Abnormal Caudal Regeneration in Lepidosaurs and Its Potential Ecological Impacts. Biol. Rev. 2020, 95, 1479–1496. [Google Scholar] [CrossRef]
- Macey, J.R.; Schulte, J.A.; Larson, A.; Ananjeva, N.B.; Wang, Y.; Pethiyagoda, R.; Rastegar-Pouyani, N.; Papenfuss, T.J. Evaluating Trans-Tethys Migration: An Example Using Acrodont Lizard Phylogenetics. Syst. Biol. 2000, 49, 233–256. [Google Scholar] [CrossRef] [Green Version]
- Pyron, R.A.; Burbrink, F.T.; Wiens, J.J. A Phylogeny and Revised Classification of Squamata, Including 4161 Species of Lizards and Snakes. BMC Evol. Biol. 2013, 13, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, C.J.; Hardy, C.M. Tail Regeneration and Other Observations in a Species of Agamid Lizard. Aust. Zool. 1977, 19, 141–148. [Google Scholar]
- Ofori, B.; Martey, P.; Musah, Y.; Attuquayefio, D. Tail Bifurcation in the African Rainbow Lizard from Ghana, West Africa. Herpetol. Notes 2018, 11, 843–845. [Google Scholar]
- Ananjeva, N.B.; Danov, R.A. A Rare Case of Bifurcated Caudal Regeneration in the Caucasian Agama, Stellio Caucasius. Amphib. Reptil. 1991, 12, 343–349. [Google Scholar] [CrossRef]
- Brindley, H.H. Some Cases of Caudal Abnormality in Mabuya Carinata and Other Lizards. J. Bomb. Nat. Hist. Soc. 1898, 11, 680–689. [Google Scholar]
- Woodland, W.N.F. Some Observations on Caudal Autotomy and Regeneration in the Gecko {Hemidactylus Flaviviridis, Riippel), with Notes on the Tails of Sphenodon and Pygopus. Q. J. Microsc. Sci. 1921, 65, 63–100. [Google Scholar]
- Bergmann, P.J.; Hobbs, A.M.; Kavalench, M.L.; Russell, A.P. Modulated but Conserved Segmental Growth of the Original Tail in Callisaurus Draconoides (Phrynosomatidae) and Calotes Versicolor (Agamidae). Herpetologica 2004, 60, 62–74. [Google Scholar] [CrossRef]
- Ananjeva, N.B.; Orlov, N.L.; Nguyen, N.N. A New Species of Japalura (Agamidae: Lacertilia: Reptilia) from Central Highland, Vietnam. Asian Herpetol. Res. 2017, 8, 14–21. [Google Scholar] [CrossRef]
- Ananjeva, N.B.; Stuart, B.L. The Agamid Lizard Ptyctolaemus Phuwuanensis Manthey and Nabhitabhata, 1991 from Thailand and Laos Represents a New Genus. Russ. J. Herpetol. 2001, 8, 165–170. [Google Scholar] [CrossRef]
- Zheng, Y.; Wiens, J.J. Combining Phylogenomic and Supermatrix Approaches, and a Time-Calibrated Phylogeny for Squamate Reptiles (Lizards and Snakes) Based on 52 Genes and 4162 Species. Mol. Phylogenetics Evol. 2016, 94, 537–547. [Google Scholar] [CrossRef]
- Ananjeva, N.B. Current State of the Problems in the Phylogeny of Squamate Reptiles (Squamata, Reptilia). Biol. Bull. Rev. 2019, 9, 119–128. [Google Scholar] [CrossRef]
- Tatarinov, L.P. Essays on the Evolution of Reptiles; Proceedings of the Paleontological Institute; GEOS: Moscow, Russia, 2006; Volume 290. [Google Scholar]
- Townsend, T.; Larson, A.; Louis, E.; Macey, J.R. Molecular Phylogenetics of Squamata: The Position of Snakes, Amphisbaenians, and Dibamids, and the Root of the Squamate Tree. Syst. Biol. 2004, 53, 735–757. [Google Scholar] [CrossRef]
- Wiens, J.J.; Kuczynski, C.A.; Townsend, T.; Reeder, T.W.; Mulcahy, D.G.; Sites, J.W. Combining Phylogenomics and Fossils in Higher-Level Squamate Reptile Phylogeny: Molecular Data Change the Placement of Fossil Taxa. Syst. Biol. 2010, 59, 674–688. [Google Scholar] [CrossRef] [Green Version]
- Fry, B.G.; Vidal, N.; Norman, J.A.; Vonk, F.J.; Scheib, H.; Ramjan, S.F.R.; Kuruppu, S.; Fung, K.; Blair Hedges, S.; Richardson, M.K.; et al. Early Evolution of the Venom System in Lizards and Snakes. Nature 2006, 439, 584–588. [Google Scholar] [CrossRef]
- Kumazawa, Y. Mitochondrial Genomes from Major Lizard Families Suggest Their Phylogenetic Relationships and Ancient Radiations. Gene 2007, 388, 19–26. [Google Scholar] [CrossRef]
- Voronov, A.S.; Shibalev, D.V.; Kupriyanova, N.S. Evolutionary Relationships between Reptiles Inferred from the Comparison of Their ITS2 Sequences. Russ. J. Genet. 2011, 47, 864–873. [Google Scholar] [CrossRef]
- Douglas, M.E.; Douglas, M.R.; Schuett, G.W.; Beck, D.D.; Sullivan, B.K. Conservation Phylogenetics of Helodermatid Lizards Using Multiple Molecular Markers and a Supertree Approach. Mol. Phylogenet. Evol. 2010, 55, 153–167. [Google Scholar] [CrossRef]
- Albert, E.M.; San Mauro, D.; García-París, M.; Rüber, L.; Zardoya, R. Effect of Taxon Sampling on Recovering the Phylogeny of Squamate Reptiles Based on Complete Mitochondrial Genome and Nuclear Gene Sequence Data. Gene 2009, 441, 12–21. [Google Scholar] [CrossRef]
- McMahan, C.D.; Freeborn, L.R.; Wheeler, W.C.; Crother, B.I. Forked Tongues Revisited: Molecular Apomorphies Support Morphological Hypotheses of Squamate Evolution. Copeia 2015, 103, 525–529. [Google Scholar] [CrossRef]
- Harrington, S.M.; Leavitt, D.H.; Reeder, T.W. Squamate Phylogenetics, Molecular Branch Lengths, and Molecular Apomorphies: A Response to McMahan et al. Copeia 2016, 104, 702–707. [Google Scholar] [CrossRef]
- Ivanov, A.O.; Cherepanov, G.O. Fossil Lower Vertebrates; St. Petersburg State University: St Petersburg, Russia, 2007. [Google Scholar]
- Romer, A.S. Osteology of the Reptiles; Krieger Publishing Company: Malabar, FL, USA, 1997. [Google Scholar]
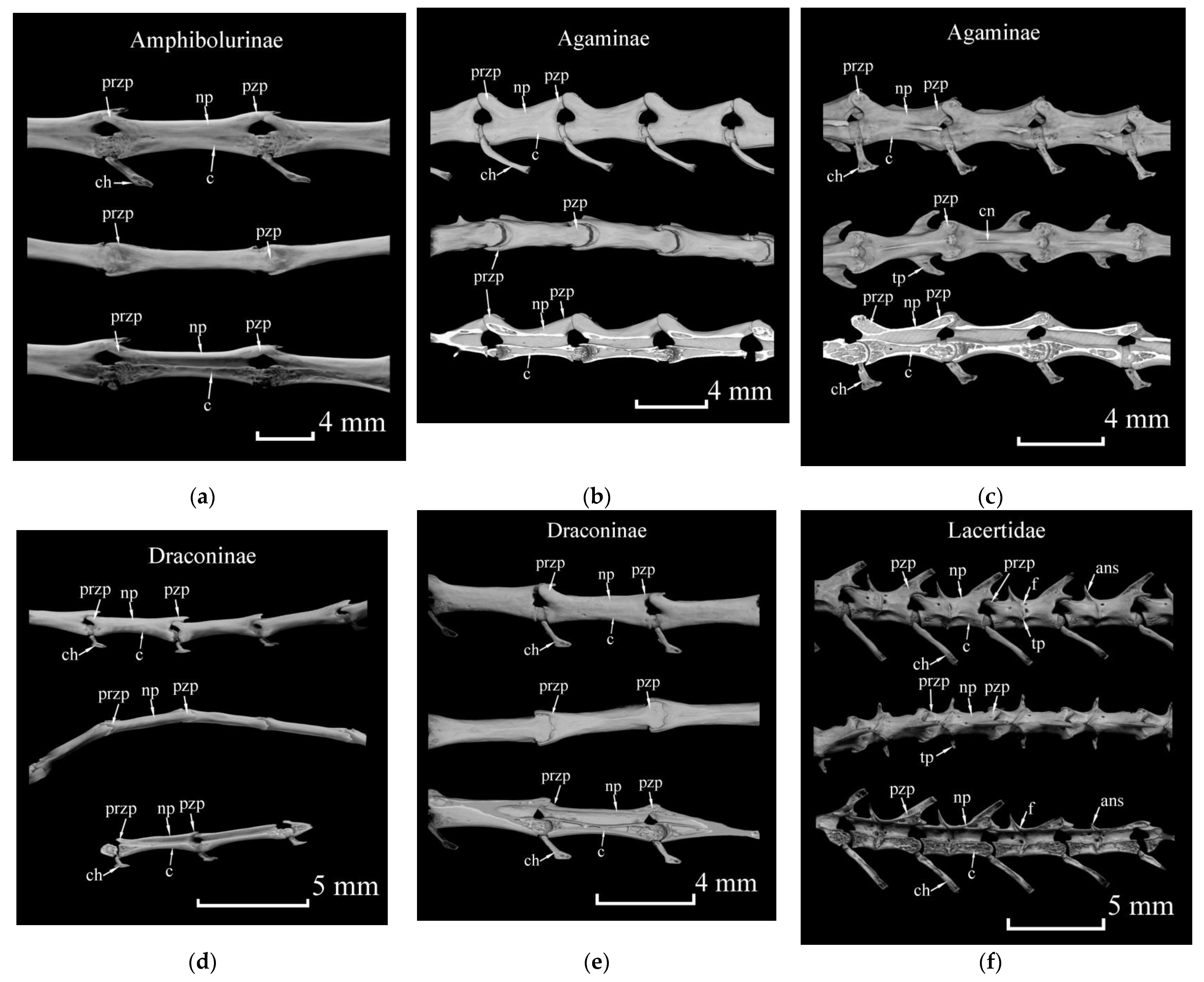
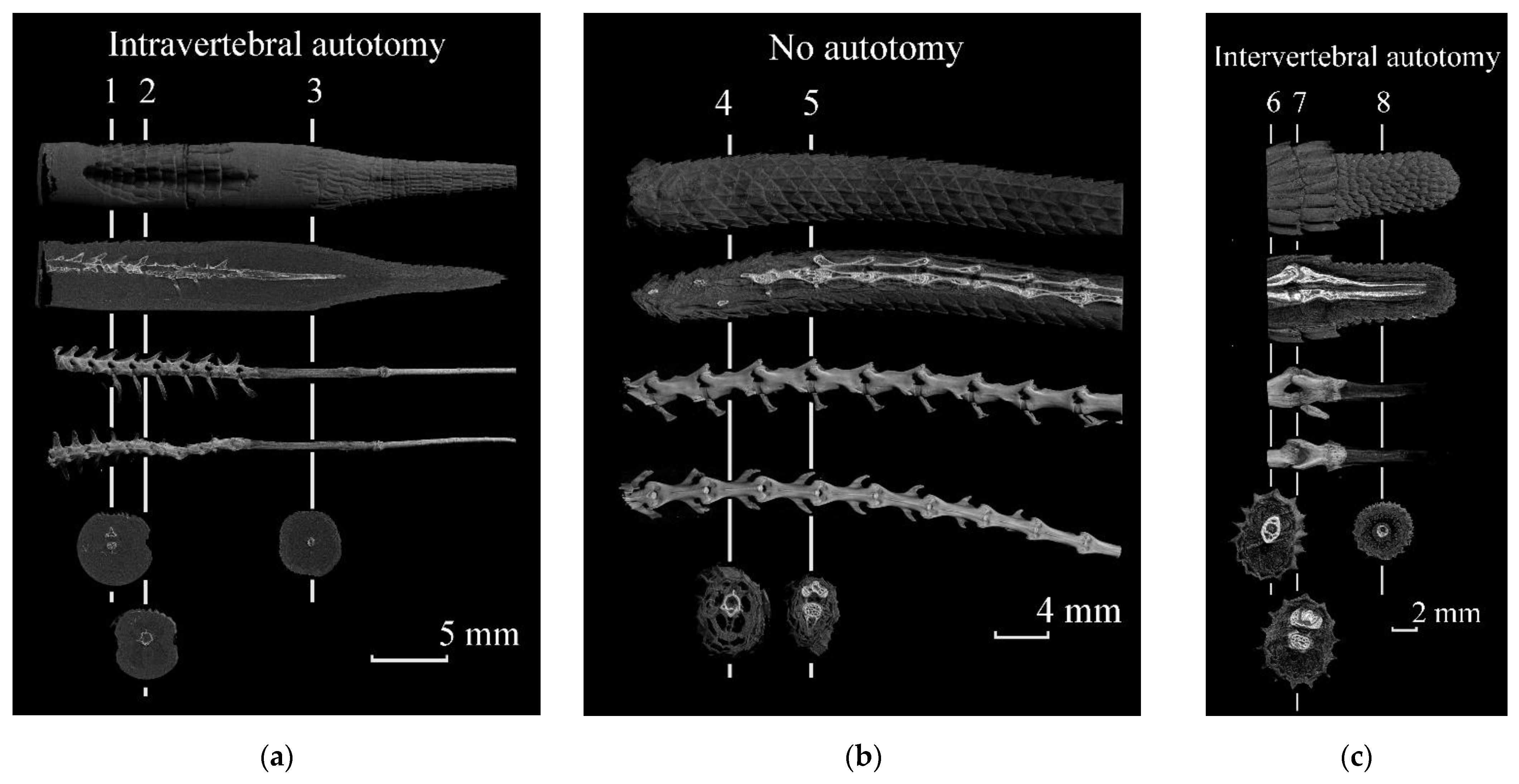



| Genus | Species | Total Number of Examined Specimens | Number of Specimens with Uncertain Cases of Broken Tails | Number of Specimens with Autotomized and Regenerated Tails |
|---|---|---|---|---|
| Leiolepidinae | ||||
| Leiolepis | L. belliana | 4 | 0 | 4 |
| L. guentherpetersi | 3 | 0 | 3 | |
| L. guttata | 10 | 2 | 8 | |
| L. reevesii | 8 | 2 | 6 | |
| Amphibolurinae | ||||
| Gowidon | G. longirostris | 1 | 0 | 1 |
| Hypsilurus | H. bruijnii | 1 | 0 | 1 |
| H. modestus | 5 | 0 | 5 | |
| Intellagama | I. lesueurii | 5 | 4 | 1 |
| Lophosaurus | L. spinipes | 3 | 0 | 3 |
| Physignathus | P. cocincinus | 6 | 0 | 6 |
| Pogona | P. barbata | 1 | 0 | 1 |
| P. vitticeps | 2 | 0 | 2 | |
| Agaminae | ||||
| Laudakia | L. nupta | 88 | 36 | 52 |
| L. tuberculata | 9 | 1 | 8 | |
| Paralaudakia | P. caucasia | 246 | 9 | 237 |
| P. erythrogaster | 32 | 4 | 28 | |
| P. himalayana | 57 | 12 | 45 | |
| P. lehmanni | 220 | 37 | 183 | |
| P. microlepis | 6 | 1 | 5 | |
| P. stoliczkana | 132 | 15 | 117 | |
| Stellagama | S. stellio | 67 | 1 | 66 |
| Draconinae | ||||
| Calotes | C. calotes | 8 | 1 | 7 |
| C. versicolor | 59 | 2 | 57 | |
| Gonocephalus | G. chamaeleontinus | 9 | 2 | 7 |
| G. liogaster | 6 | 0 | 6 | |
| G. sophiae | 1 | 0 | 1 | |
| Malayodracon | M. robinsonii | 2 | 0 | 2 |
| Mantheyus | M. phuwuanensis | 4 | 1 | 3 |
| Otocryptis | O. wiegmanni | 3 | 0 | 3 |
| Species | Sample Size | Intact Tails | Pseudoautotomy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| In Distal Third of Tail | In the Middle of Tail | In Proximal Third of Tail | |||||||
| n | % | n | % | n | % | n | % | ||
| Leiolepidinae | |||||||||
| Leiolepis: | |||||||||
| L. belliana | 4 | 2 | 50.0 | 1 | 25.0 | 0 | 0.0 | 1 | 25.0 |
| L. guentherpetersi | 3 | 2 | 66.7 | 1 | 33.3 | 0 | 0.0 | 0 | 0.0 |
| L. guttata | 8 | 4 | 50.0 | 1 | 12.5 | 3 | 37.5 | 0 | 0.0 |
| L. reevesii | 6 | 4 | 66.6 | 1 | 16.7 | 1 | 16.7 | 0 | 0.0 |
| Amphibolurinae | |||||||||
| Gowidon longirostris | 1 | 1 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Hypsilurus: | |||||||||
| H. bruijnii | 1 | 1 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| H. modestus | 5 | 5 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Intellagama lesueurii | 1 | 0 | 0.0 | 1 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| Lophosaurus spinipes | 3 | 2 | 66.7 | 1 | 33.3 | 0 | 0.0 | 0 | 0.0 |
| Physignathus cocincinus | 6 | 4 | 66.7 | 2 | 33.3 | 0 | 0.0 | 0 | 0.0 |
| Pogona: | |||||||||
| P. barbata | 1 | 1 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| P. vitticeps | 2 | 2 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Agaminae | |||||||||
| Laudakia: | |||||||||
| L. nupta | 52 | 34 | 65.4 | 11 | 21.2 | 6 | 11.5 | 1 | 1.9 |
| L. tuberculata | 8 | 4 | 50.0 | 2 | 25.0 | 1 | 12.5 | 1 | 12.5 |
| Paralaudakia: | |||||||||
| P. caucasia | 237 | 148 | 62.4 | 72 | 30.4 | 12 | 5.1 | 5 | 2.1 |
| P. erythrogaster | 28 | 15 | 53.6 | 10 | 35.7 | 1 | 3.6 | 2 | 7.1 |
| P. himalayana | 45 | 30 | 66.7 | 9 | 20.0 | 5 | 11.1 | 1 | 2.2 |
| P. lehmanni | 183 | 159 | 86.9 | 21 | 11.5 | 3 | 1.6 | 0 | 0.0 |
| P. microlepis | 5 | 2 | 40.0 | 3 | 60.0 | 0 | 0.0 | 0 | 0.0 |
| P. stoliczkana | 117 | 85 | 72.6 | 32 | 27.4 | 0 | 0.0 | 0 | 0.0 |
| Stellagama stellio | 66 | 43 | 65.2 | 21 | 31.8 | 2 | 3.0 | 0 | 0.0 |
| Draconinae | |||||||||
| Calotes: | |||||||||
| C. calotes | 7 | 6 | 85.7 | 1 | 14.3 | 0 | 0.0 | 0 | 0.0 |
| C. versicolor | 57 | 46 | 80.7 | 6 | 10.5 | 3 | 5.3 | 2 | 3.5 |
| Gonocephalus: | |||||||||
| G. chamaeleontinus | 7 | 3 | 42.9 | 4 | 57.1 | 0 | 0.0 | 0 | 0.0 |
| G. liogaster | 6 | 5 | 83.3 | 1 | 16.7 | 0 | 0.0 | 0 | 0.0 |
| G. sophiae | 1 | 1 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Malayodracon robinsonii | 2 | 2 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Mantheyus phuwuanensis | 3 | 0 | 0.0 | 1 | 33.3 | 1 | 33.3 | 1 | 33.3 |
| Otocryptis wiegmanni | 3 | 1 | 33.3 | 2 | 66.7 | 0 | 0.0 | 0 | 0.0 |
| Species | Presence of Autotomy and Regeneration 1 | Source |
|---|---|---|
| Uromastycinae | ||
| Genus Uromastyx | A+R? | [2] |
| Uromastyx aegyptia | A-R- | [5] |
| Uromastyx thomasi | A-R- | [5] |
| Leiolepidinae | ||
| Leiolepis belliana | A+R+ | (Figure A1a and Figure S7a) |
| Leiolepis guentherpetersi | A+R+ | (Figure A1b and Figure S7b) |
| Leiolepis guttata | A+R+ | Figure A1c and Figure S7c) |
| Leiolepis reevesii | A+R+ | (Figure A1d and Figure S7d) |
| Hydrosaurinae | ||
| Genus Hydrosaurus | A-R- | Probably |
| Amphibolurinae | ||
| Amphibolurus | A+R? | Some species of genus [5] |
| Genus Ctenophorus | ||
| Ctenophorus caudicinctus | A+R+ | [5] |
| Ctenophorus cristatus | A+R-? | [5] |
| Amphibolurus (Ctenophorus) decresii | A+R+ | [24] |
| Ctenophorus isolepis | A+R-? | [5] |
| Ctenophorus maculatus | A+R-? | [5] |
| Ctenophorus pictus | A+R-? | [5] |
| Genus Diporiphora | A+R- | [5,6] |
| Diporiphora bilineata | A+R- | [5] |
| Intellagama lesueurii | A+R+ | [5,39], (Figure A1e and Figure S7e) |
| Lophognathus gilberti | A+R- | [6] |
| Lophosaurus spinipes | A+R? | (Figure A1f and Figure S7f) |
| Physignathus cocincinus | A+R? | (Figure A1g and Figure S7g) |
| Tropicagama temporalis | A+R+ | [5,6] |
| Agaminae | ||
| Genus Acanthocercus | A+R+ | [5] |
| Genus Agama | A+R+ | [5] |
| Agama agama | A+R+ | [5,23] |
| Agama aculeata | A+R-? | [5] |
| Agama anchietae | A+R-? | [5] |
| Agama atra | A+R+ | [5] |
| Agama benueensis (=Agama doriae) | A+R+ | [5] |
| Agama boueti | A+R+ | [5] |
| Agama caudospinosa | A+R+ | [5] |
| Agama distanti (=Agama aculeata) | A+R-? | [5] |
| Agama doriae | A+R+ | [5] |
| Agama gracilimembris | A+R- | [5] |
| Agama hartmanni | A+R-? | [5] |
| Agama hispida | A+R-? | [5] |
| Agama kirkii | A+R-? | [5] |
| Agama mossambica | A+R-? | [5] |
| Agama mwanzae | A+R-? | [5] |
| Agama paragama | A+R+ | [5] |
| Agama persimilis | A+R-? | [5] |
| Agama picticauda | A+R+ | [40] |
| Agama planiceps | A+R+ | [5] |
| Agama rueppelli | A+R+ | [5] |
| Agama sankaranica | A+R+ | [5] |
| Agama spinosa | A+R+ | [5] |
| Agama sylvanus (=Agama africana) | A+R+ | [5] |
| Agama weidholzi | A+R-? | [5] |
| Acanthocercus adramitanus | A+R+ | [5] |
| Acanthocercus annectans | A+R+ | [5] |
| Acanthocercus atricollis | A+R+ | [5] |
| Acanthocercus cyanogaster | A+R+ | [5] |
| Acanthocercus phillipsii | A+R+ | [5] |
| Acanthocercus yemensis | [5] | |
| Laudakia agrorensis | A+R+ | [5] |
| Laudakia melanura | A+R+ | [5] |
| Laudakia nupta | A+R+ | [5,20], (Figure A1h and Figure S7h) |
| Laudakia tuberculata | A+R+ | [5,24], (Figure A1i and Figure S7i) |
| Paralaudakia caucasia | A+R+ | [5,20,41], (Figure A1j and Figure S7j) |
| Paralaudakia erythrogaster | A+R+ | [5,20], (Figure A1k and Figure S7k) |
| Paralaudakia himalayana | A+R+ | [5,20,24], (Figure A1l and Figure S7l) |
| Paralaudakia lehmanni | A+R+ | [5,20], (Figure A1m and Figure S7m) |
| Paralaudakia microlepis | A+R+ | [5,20], (Figure A1n and Figure S7n) |
| Paralaudakia stoliczkana | A+R+ | [5,20], (Figure A1o and Figure S7o) |
| Phrynocephalus | A-R- | [5] |
| Pseudotrapelus | A-R- | [5] |
| Pseudotrapelus sinaitus | A+R+ | [5] |
| Stellagama stellio | A+R+ | [5,6,10,23], (Figure A1p and Figure S7p) |
| Trapelus | A-R- | [5,6] |
| Xenagama batillifera | A+R- | [2,5] |
| Draconinae | ||
| Bronchocela cristatella | A+R+ | [42] |
| Calotes calotes | A+R-A-R- | [43,44], (Figure A1q and Figure S7q) |
| C. ophiomachus (C.calotes) | A+R+ | [24] |
| C. mystaceus | A+R | [24] |
| C. versicolor | A+R- | [10], (Figure A1r and Figure S7r) |
| Genus Diploderma | [5] | |
| Diploderma ngoclinense | A+R? | [45] |
| Genus Gonyocephalus | [5] | |
| Gonyocephalus subcristatus (=Coryphophylax subcristatus) | A+R+ | [2,24,42] |
| Gonocephalus chamaeleontinus | A+R-? | (Figure A1s and Figure S7s) |
| Gonocephalus liogaster | A+R? | (Figure A1t and Figure S7t) |
| Mantheyus phuwuanensis | A+R+ | [46], (Figure A1u and Figure S7u) |
| Otocryptis | A+R- | [5] |
| Otocryptis wiegmanni | A+R- | [5], (Figure A1v and Figure S7v) |
| Pelturagonia nigrilabris | A+R-? | (Figure A1w and Figure S7w) |
| Psammophilus | A+R- | [6] |
| Psammophilus dorsalis | A+R-? | [5] |
| Charasia (=Psammophilus) blanfordiana | A+R+ | [24] |
| Sitana | A+R- | [6] |
| Sitana ponticeriana | A+R- | [5,6] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ananjeva, N.B.; Gordeev, D.A.; Korost, D.V. The Review of the Autotomy of Agamid Lizards with Considerations about the Types of Autotomy and Regeneration. J. Dev. Biol. 2021, 9, 32. https://doi.org/10.3390/jdb9030032
Ananjeva NB, Gordeev DA, Korost DV. The Review of the Autotomy of Agamid Lizards with Considerations about the Types of Autotomy and Regeneration. Journal of Developmental Biology. 2021; 9(3):32. https://doi.org/10.3390/jdb9030032
Chicago/Turabian StyleAnanjeva, Natalia Borisovna, Dmitry Anatolyevich Gordeev, and Dmitry Vyacheslavovich Korost. 2021. "The Review of the Autotomy of Agamid Lizards with Considerations about the Types of Autotomy and Regeneration" Journal of Developmental Biology 9, no. 3: 32. https://doi.org/10.3390/jdb9030032
APA StyleAnanjeva, N. B., Gordeev, D. A., & Korost, D. V. (2021). The Review of the Autotomy of Agamid Lizards with Considerations about the Types of Autotomy and Regeneration. Journal of Developmental Biology, 9(3), 32. https://doi.org/10.3390/jdb9030032







