Revealing the Complex Interplay of Biostimulant Applications
Abstract
1. Introduction
2. Results
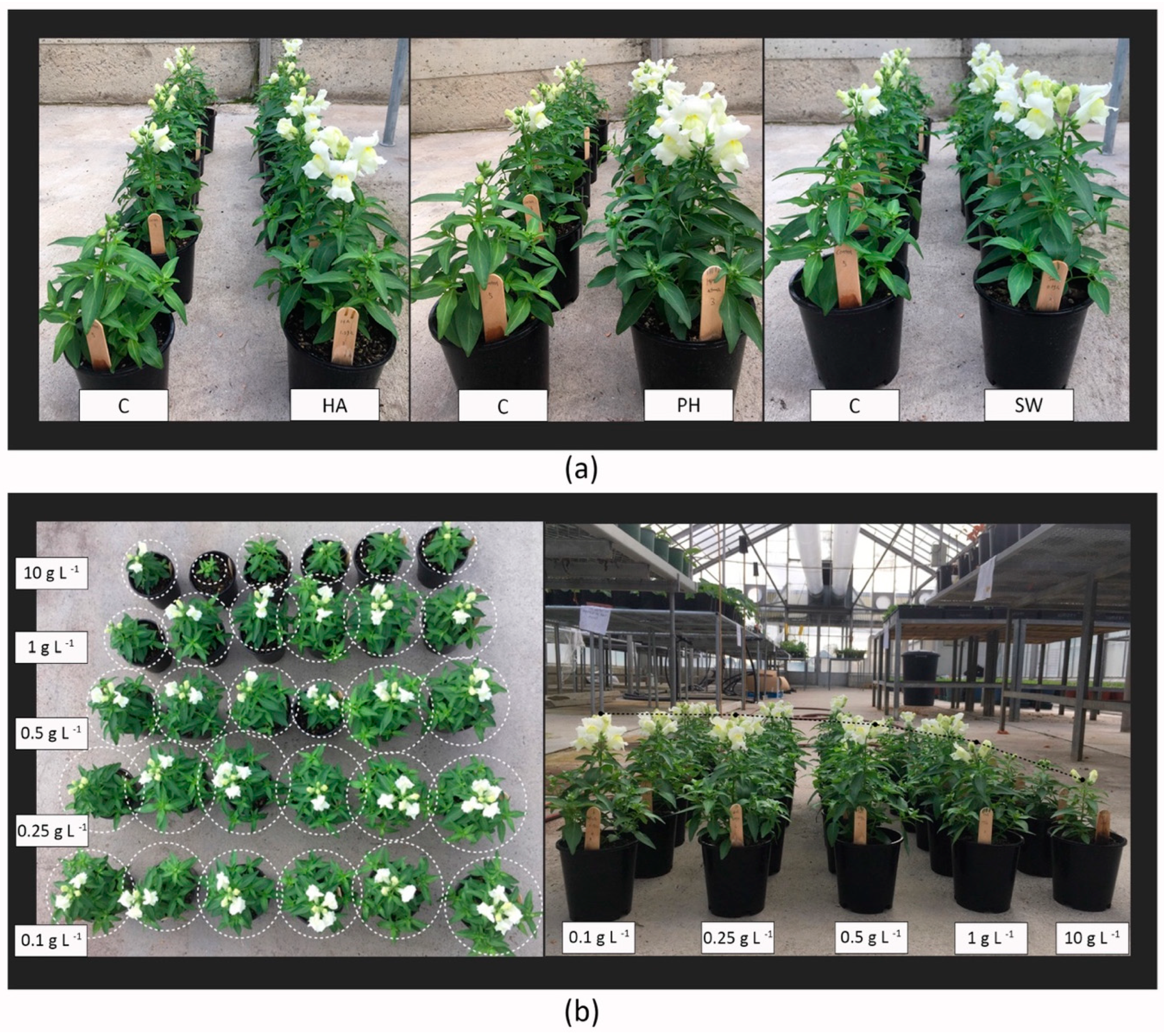
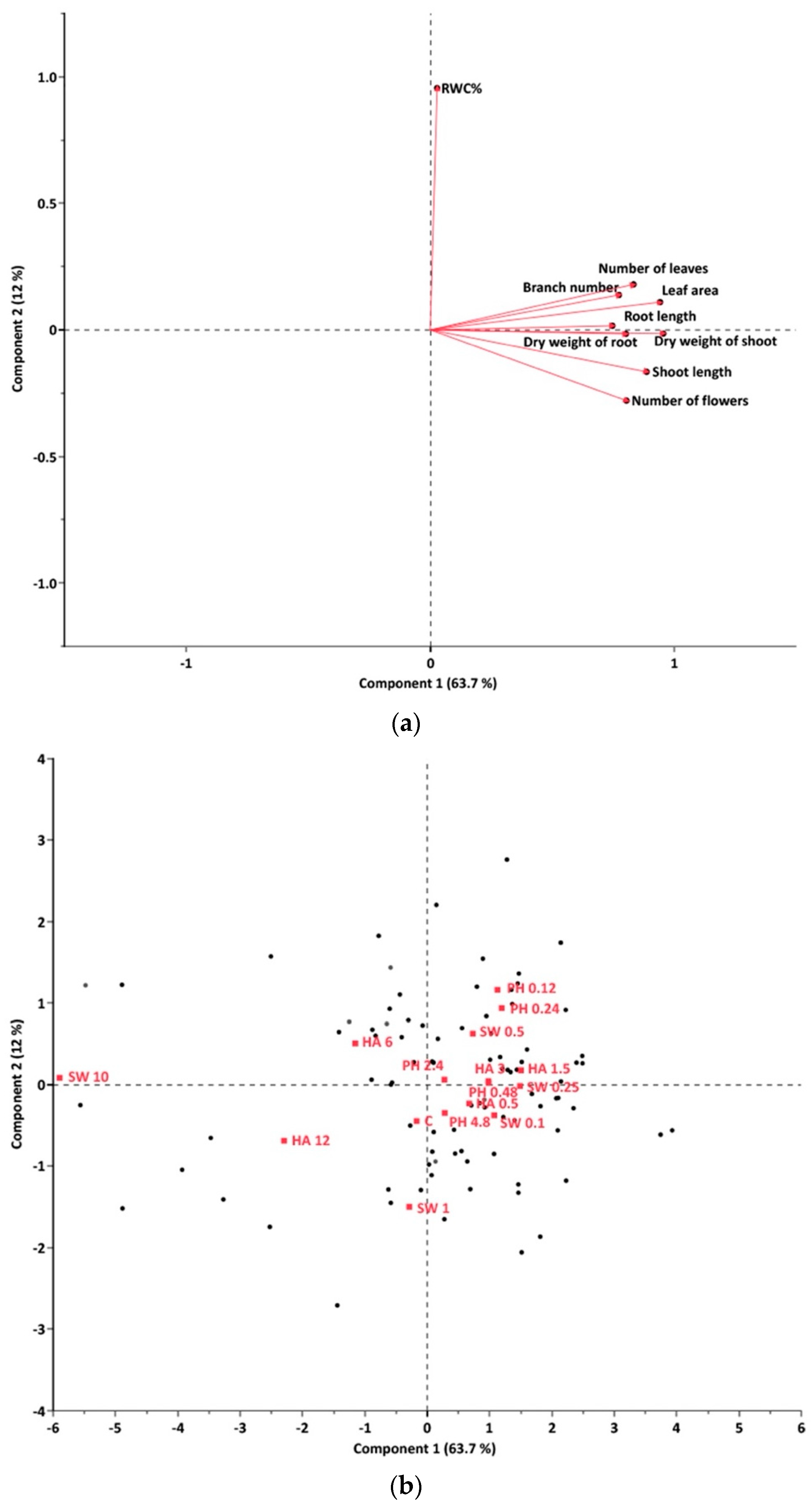
2.1. Dosage Effects of Greenhouse Conditions
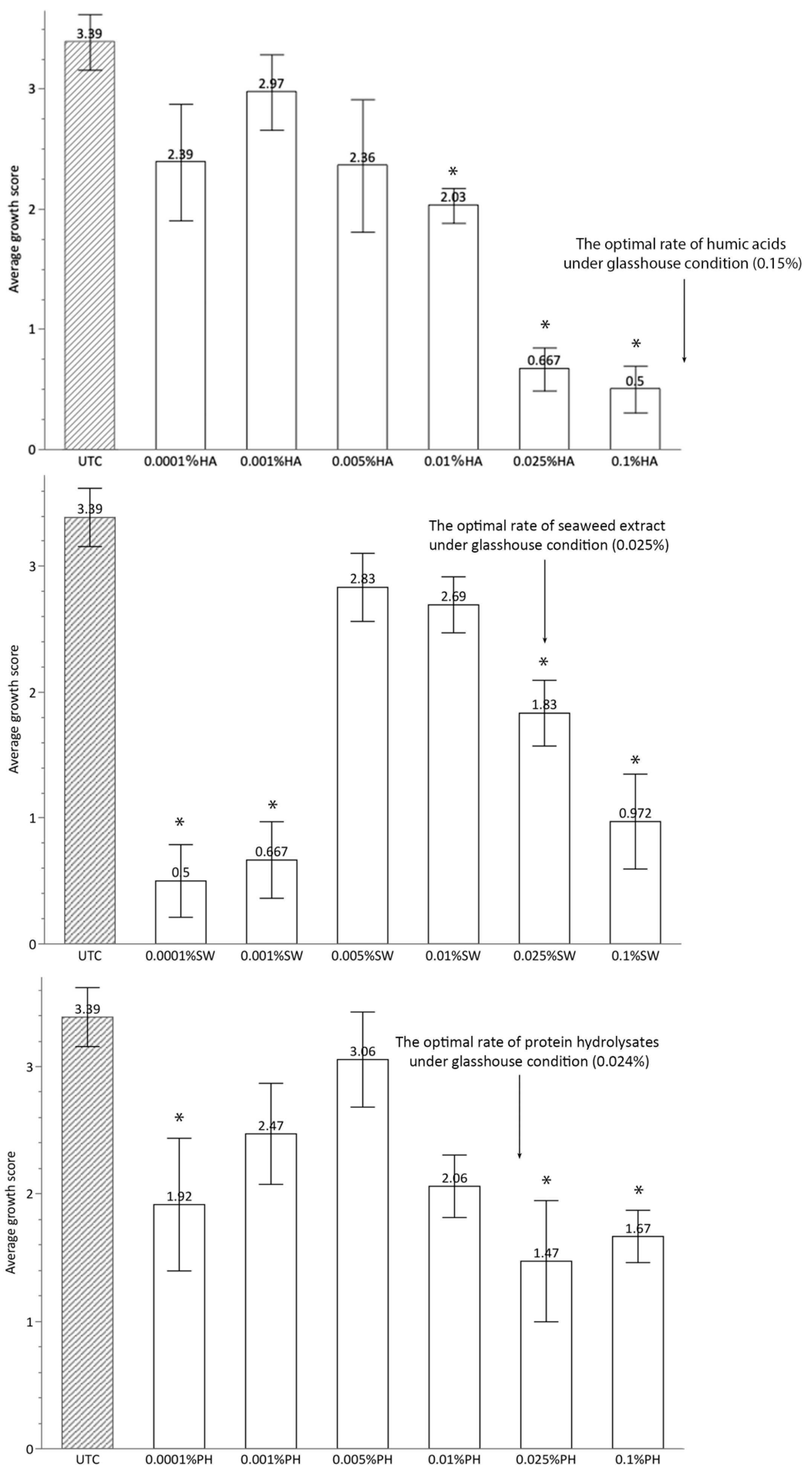
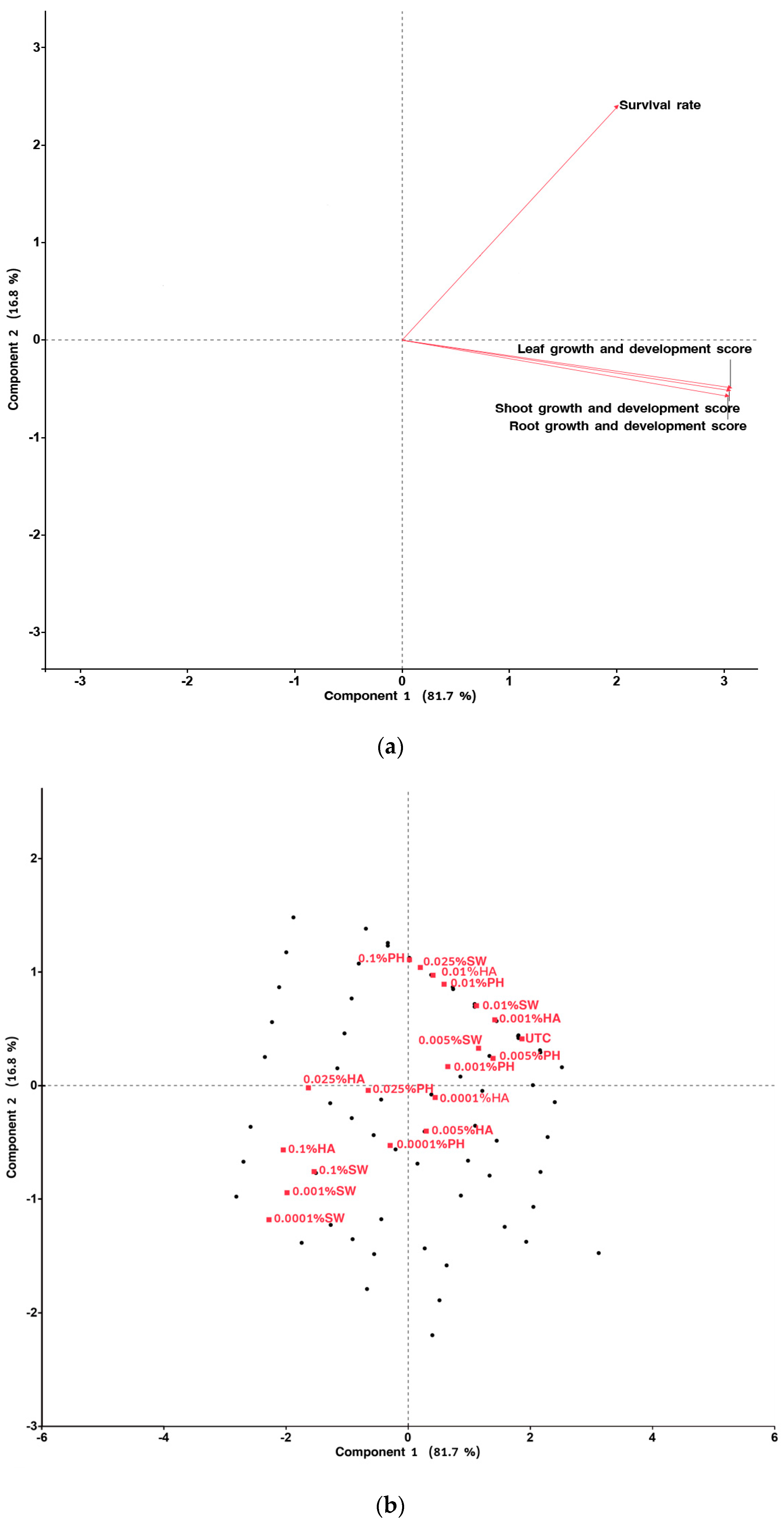
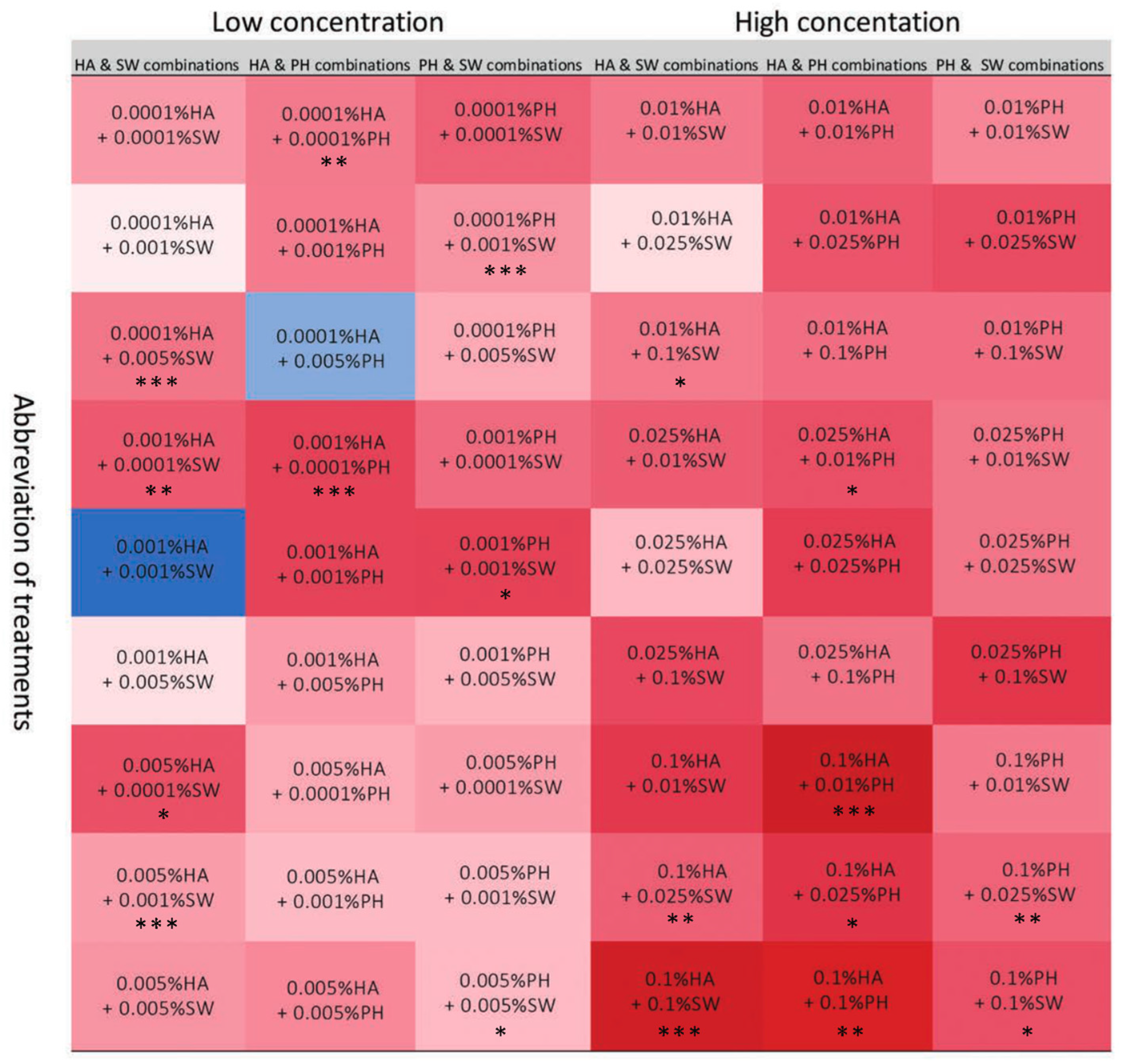
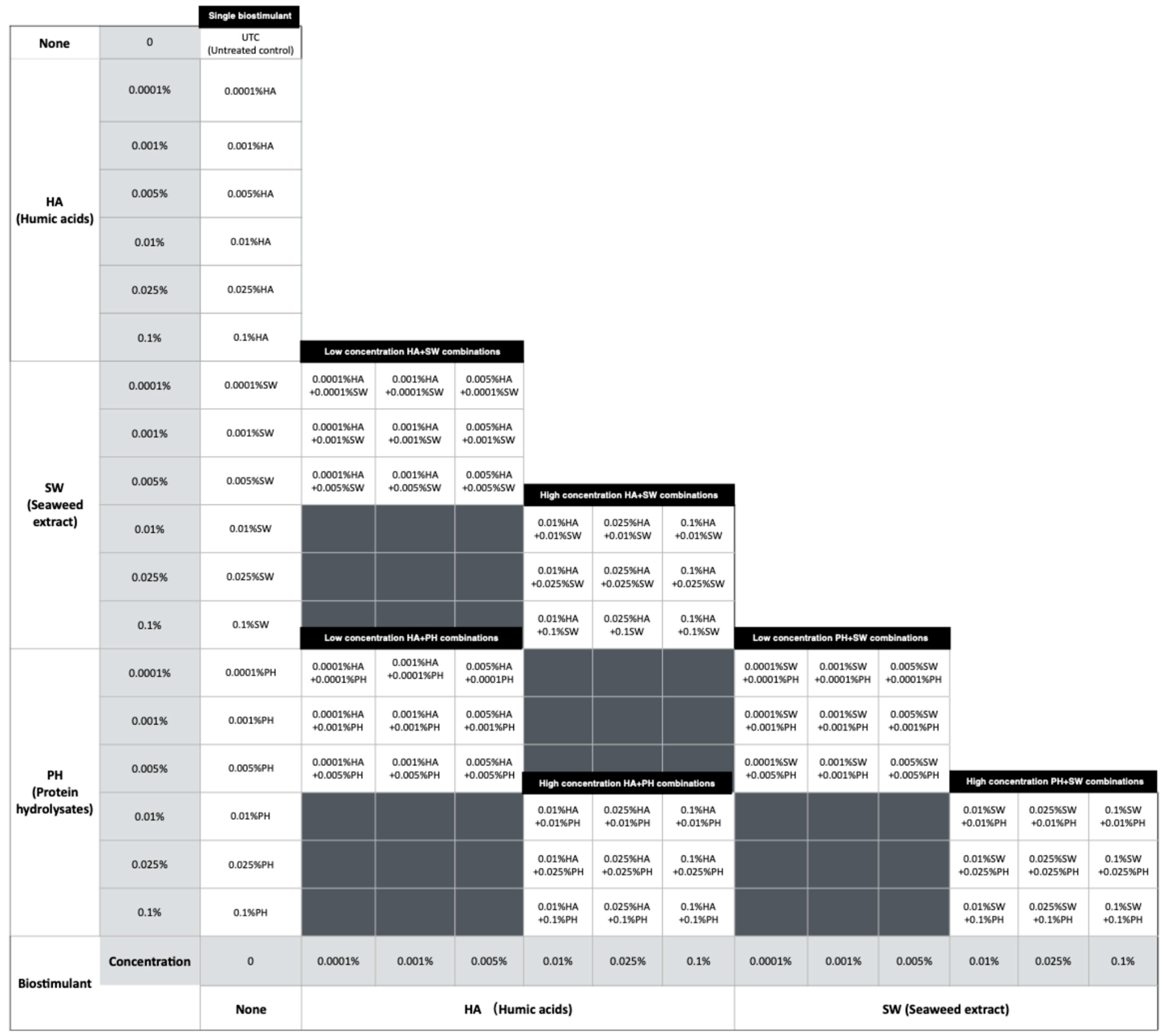
2.2. In Vitro Experiment
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Constitution of Biostimulants
4.2. Glasshouse Experiment
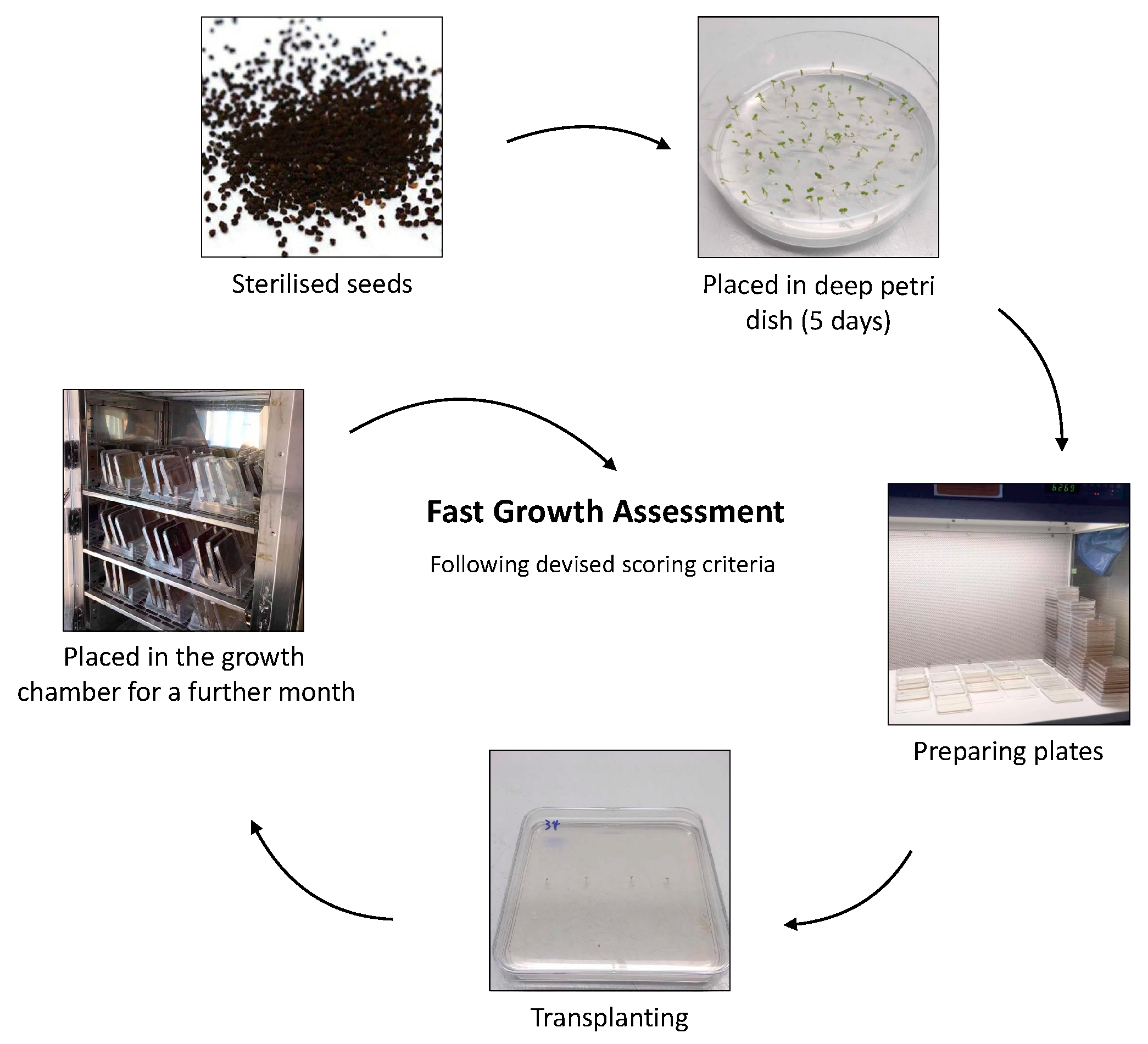
4.3. In Vitro Experiment
4.4. Statistics Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, S.C.; Sharma, K. Biostimulant: An innovative approach for sustainable crop production. Curr. Sci. 2023, 125, 377–382. [Google Scholar]
- Ricca, M.; Tilbury, L.; Daridon, B.; Sukalac, K. General principles to justify plant biostimulant claims. Front. Plant Sci. 2019, 10, 494. [Google Scholar]
- Caradonia, F.; Battaglia, V.; Righi, L.; Pascali, G.; Torre, A.L. Plant biostimulant regulatory framework: Prospects in Europe and current situation at international level. J. Plant Growth Regul. 2019, 38, 438–448. [Google Scholar] [CrossRef]
- Li, J.; Gerrewey, T.V.; Geelen, D. A meta-analysis of biostimulant yield effectiveness in field trials. Front. Plant Sci. 2022, 13, 836702. [Google Scholar] [CrossRef] [PubMed]
- du Jardin, P. Plant biostimulant: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Malecange, M.; Sergheraert, R.; Teulat, B.; Mounier, E.; Lothier, J.; Sakr, S. Biostimulant properties of protein hydrolysayes: Recent advances and future challenges. Int. J. Mol. Sci. 2023, 24, 9714. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. A meta-analysis and review of plant-growth response to humic substances: Practical implications for agriculture. Adv. Agron. 2014, 124, 37–89. [Google Scholar]
- Boukhari, M.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulant and crop responses: A review. Biol Agric Hortic. 2014, 31, 1–17. [Google Scholar] [CrossRef]
- Hudson, A.; Critchley, J.; Erasmus, Y. The Genus Antirrhinum (Snapdragon): A flowering plant model for evolution and development. Cold Spring Harb. 2008, 3, 1–7. [Google Scholar] [CrossRef]
- Cristiano, G.; Pallozzi, E.; Conversa, G.; Tufarelli, V.; Lucia, B.D. Effects of an animal-derived biostimulant on the growth and physiological parameters of potted snapdragon (Antirrhinum majus L.). Front. Plant Sci. 2018, 9, 861. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.; Noor, F.; Uddin, S.; Mukhtar, H.; Ramzan, M.; Raheel, M.; Hussain, Z.; Wisal, S. Exogenous application of moringa leaf extract influences growth, flowering, and vase life of snap dragon (Antirrhinum majus) cultivars. Pure Appl. Biol. 2022, 11, 459–467. [Google Scholar] [CrossRef]
- Rouphael, Y.; Spichal, L.; Panzarova, K.; Casa, R.; Colla, G. High-throughput plant phenotyping for developing novel biostimulants: From lab to field or from field to lab? Front. Plant Sci. 2018, 9, 1197. [Google Scholar] [CrossRef]
- Diordano, M.; El-Nakhel, C.; Caruso, G.; Cozzolino, E.; Pascale, S.D.; Kyriacou, M.C.; Colla, G.; Rouphael, Y. Stand-Alone and combinatorial effects of plant-based biostimulants on the production and leaf quality of perennial wall rocket. Plants 2020, 9, 922. [Google Scholar] [CrossRef]
- Wise, K.; Selby-Pham, J.; Chai, X.; Simovich, T.; Gupta, S.; Gill, H. Fertiliser supplementation with a biostimulant complex of fish hydrolysate, Aloe vera extract, and kelp alters cannabis root architecture to enhance nutrient uptake. Sci. Hortic. 2024, 323, 112483. [Google Scholar] [CrossRef]
- Kumar, H.D.; Aloke, P. Role of biostimulant formulations in crop production: An overview. Int. J. Agric. Sci. 2020, 8, 38–46. [Google Scholar]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. Hortic. Sci. 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Nunez, G.H.; Buzzi, G.; Heller, C.R. Southern highbush blueberry responses to humic acid application in soilless substrates. Sci. Hortic. 2023, 308, 111541. [Google Scholar] [CrossRef]
- Drocco, C.; Habteselassie, M.; Itle, R.A.; Pennisi, S.V. Commercial biostimulants had minimal effects on greenhouse grown blueberry vegetative growth. Sci. Hortic. 2024, 336, 113433. [Google Scholar] [CrossRef]
- Silva, T.S.; Silva, A.P.S.; Santos, A.A.; Ribeiro, K.G.; Souza, D.C.; Bueno, P.A.A.; Marques, M.M.M.; Almeida, P.M.; Peron, A.P. Cytotoxicity, genotoxicity, and toxicity of plant biostimulants produced in Brazil: Subsidies for determining environmental risk to non-target species. Water Air Soil Pollut. 2020, 231, 233. [Google Scholar] [CrossRef]
- Kisvarga, S.; Farkas, D.; Boronkay, G.; Nemenyi, A.; Orloci, L. Effects of biostimulants in horticulture, with emphasis on ornamental plant production. Agronomy 2022, 12, 1043. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Braganca, R. Recent advances in the molecular effects of biostimulants in plants: An overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Rupiasih, N.I.; Vidyasagar, P. A review: Compositions, structures, properties and applications of humic substances. JAST. 2005, 8, 16–25. [Google Scholar]
- Mahler, C.F.; Svierzoski, N.D.S.; Bernardino, C.A.R. Chemical characteristics of humic substances in nature. In Humic Substance; BoD—Books on Demand: Norderstedt, Germany, 2021. [Google Scholar]
- Colla, G.; Hoahland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unravelling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Pascale, S.D.; Cozzolino, E.; Giordano, M.; El-Nakhel, C.; Cuciniello, A.; Cenvinzo, E.; Colla, G. Protein hydrolysate or plant extract-based biostimulants enhanced yield and quality performances of greenhouse perennial wall rocket grown in different seasons. Plants 2019, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Blunden, G.; Morse, P.F.; Mathe, I.; Hohmann, J.; Critchley, A.T.; Morrell, S. Betaine yields from marine algal species utilized in the preparation of seaweed extracts used in agriculture. Nat. Prod. Commun. 2010, 5, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Novak, O.; Hradecka, V.; Pencik, A.; Rolcik, J.; Strnad, M.; Staden, J.V. Endogenous cytokinins, auxins and abscisic acid in Ulva fasciata (Chlorophyta) and Dictyota humifusa (Phaeophyta): Towards understanding their biosynthesis and homoeostasis. Eur. J. Phycol. 2009, 44, 231–240. [Google Scholar] [CrossRef]
- Graigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar]
- Khan, W.; Hilta, D.; Critchley, A.T.; Prithiviraj, B. Bioassay to detect Ascophyllum nodosum extract-induced cytokinin-like activity in Arabidopsis thaliana. J. Appl. Phycol. 2011, 23, 409–414. [Google Scholar] [CrossRef]
- Vallini, G.; Pera, A.; Avio, L.; Valdrighi, M.; Giovannetti, M. Influence of humic acids on laurel growth, associated rhizospheric microorganisms and mycorrhizal fungi. Biol. Fertil. Soils 1993, 16, 1–4. [Google Scholar] [CrossRef]
- Atiyeh, R.M.; Lee, S.; Edwards, C.A.; Arancon, N.Q.; Metzger, J.D. The influence of humic acids derived from earthworm-processed organic wastes on plant growth. Bioresour. Technol. 2002, 84, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Asli, S.; Neumann, P.M. Rhizosphere humic acid interacts with root cell walls to reduce hydraulic conductivity and plant development. Plant Soil 2010, 336, 313–322. [Google Scholar] [CrossRef]
- Jannin, L.; Arkoun, M.; Ourry, A.; Laine, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F. Microarray analysis of humic acid effects on Brassica napus growth: Involvement of N, C and S metabolisms. Plant Soil. 2012, 359, 297–319. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Nardi, S. Transcriptome-wide identification of differentially expressed genes in Solanum lycopersicon L. in response to an alfalfa-protein hydrolysate using microarrays. Front. Plant Sci. 2017, 8, 1159. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.; Zamboni, A.; Varanini, Z.; Pandolfini, T. Growth stimulatory effects and genome-wide transcriptional changes produced by protein hydrolysates in maize seedlings. Front. Plant Sci. 2017, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Langowski, L.; Goni, O.; Ikuyinminu, E.; Feeney, E.; O’Connell, S. Investigation of the direct effect of a precision Ascophyllum nodosum biostimulant on nitrogen use efficiency in wheat seedlings. Plant Physiol. Bioch. 2022, 179, 44–57. [Google Scholar] [CrossRef]
- Akladious, S.A.; Mohamed, H.I. Ameliorative effects of calcium nitrate and humic acid on the growth, yield component and biochemical attribute of pepper (Capsicum annuum) plants grown under salt stress. Sci. Hortic. 2018, 236, 244–250. [Google Scholar] [CrossRef]
- Garnica-vergara, A.; Barrera-Ortiz, S.; Muñoz-Parra, E.; Raya-González, J.; Méndez-Bravo, A.; Macías-Rodríguez, L.; López-Bucio, J. The volatile 6-pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ethylene insensitive 2 functioning. New Phytol. 2015, 209, 1496–1512. [Google Scholar] [CrossRef]
- Delaplace, P.; Delory, B.M.; Baudson, C.; Cazenave, M.M.D.; Spaepen, S.; Varin, S.; Varin, S.; du Jardin, P. Influence of rhizobacterial volatiles on the root system architecture and the production and allocation of biomass in the model grass Brachypodium distachyon (L.) P. Beauv. BMC Plant Biol. 2015, 15, 195. [Google Scholar] [CrossRef] [PubMed]



| Component | Humic Acids (HA) | Protein Hydrolysates (PH) | Seaweed Extract (SW) |
|---|---|---|---|
| Carbon (C) % | 23.36 | 11.34 | 29.97 |
| Nitrogen (N) % | 1.22 | 4.14 | 5.41 |
| Phosphorus (P) µg g−1 | 334 | 15,000 | 12,500 |
| Potassium (K) µg g−1 | 3970 | 4100 | 107,100 |
| Calcium (Ca) µg g−1 | 3940 | 772 | 10,800 |
| Magnesium (Mg) µg g−1 | 1600 | 269 | 936 |
| Sulphur (S) µg g−1 | 4990 | 1730 | 12,500 |
| Iron (Fe) µg g−1 | 8470 | 17 | 3310 |
| Manganese (Mn) µg g−1 | 49 | 142 | 57 |
| Zinc (Zn) µg g−1 | 32 | 12 | 558 |
| Copper (Cu) µg g−1 | 22 | 1 | 523 |
| Boron (B) µg g−1 | 22 | 4 | 944 |
| Molybdenum (Mo) µg g−1 | 5 | nd | 8 |
| Amino Acids | Content mM | Amino Acids | Content mM |
|---|---|---|---|
| Alanine (Ala) | 58.31 | Lysine (Lys) | 52.84 |
| Arginine (Arg) | 4.46 | Methionine (Met) | 21.10 |
| Asparagine (Asn) | 18.51 | Phenylalanine (Phe) | 34.67 |
| Aspartic Acid (Asp) | 39.02 | Proline (Pro) | 6.43 |
| Glutamic acid (Glu) | 55.67 | Serine (Ser) | 49.42 |
| Glutamine (Gln) | 1.01 | Tau proteins (Tau) | 8.06 |
| Glycine (Gly) | 15.98 | Threonine (Thr) | 34.52 |
| Histidine (His) | 10.31 | Tryptophan (Try) | 1.51 |
| Isoleucine (Ile) | 30.78 | Tyrosine (Tyr) | 19.15 |
| Leucine (leu) | 70.46 | Valine (Val) | 40.74 |
| Characteristics | Number or Mean ± Standard Deviation |
|---|---|
| Substrate | 50% bark, 25% peat, 25% pumice |
| Fertility and lime amendments | 5 kg m−3 Osmocote, 1 kg m−3 Osmoform, 3 kg m−3 Dolomag, 1 kg m−3 gypsum, 2.5 kg m−3 lime |
| Moisture content (% volume) | 37.9 ± 4.13 |
| Bulk density (g mL−1) | 0.39 ± 90.02 |
| Porosity (% volume) | 45.9 ± 1.02 |
| Air space (% volume) | 16.8 ± 0.33 |
| Water holding capacity (%) | 29.1 ± 0.69 |
| pH | 4.46 ± 0.25 |
| Electrical conductivity (µg cm−1) | 1366 ± 6 |
| Soluble salt (%) | 0.47 ± 0.22 |
| Humic Acids (HA) | |||||
|---|---|---|---|---|---|
| Description | Extra low | Lower dose | Recommended dose from manufacturer | Higher dose | Extra high |
| Application rate | 0.5 g L−1 | 1.5 g L−1 | 3 g L−1 | 6 g L−1 | 12 g L−1 |
| Abbreviation | HA 0.5 | HA 1.5 | HA 3 | HA 6 | HA 12 |
| Protein Hydrolysates (PH) | |||||
| Description | Extra low | Lower dose | Recommended dose from manufacturer | Higher dose | Extra high |
| Application rate | 0.12 mL L−1 | 0.24 mL L−1 | 0.48 mL L−1 | 2.4 mL L−1 | 4.8 mL L−1 |
| Abbreviation | PH 0.12 | PH 0.24 | PH 0.48 | PH 2.4 | PH 4.8 |
| Seaweed Extract (SW) | |||||
| Description | Extra low | Lower dose | Recommended dose from manufacturer | Higher dose | Extra high |
| Application rate | 0.1 g L−1 | 0.25 g L−1 | 0.5 g L−1 | 1 g L−1 | 10 g L−1 |
| Abbreviation | SW 0.1 | SW 0.25 | SW 0.5 | SW 1 | SW 10 |
| Shoot growth and development scoring criteria | ||
| Score | Criteria | |
| Shoot length | 5 | Shoot length > 4 cm |
| 4 | 3 cm < Shoot length ≤ 4cm | |
| 3 | 2 cm < Shoot length ≤ 3cm | |
| 2 | 1.5 cm < Shoot length ≤ 2cm | |
| 1 | Shoot length ≤ 1.5 cm | |
| 0 | Shoot dead | |
| Leaf growth and development scoring criteria | ||
| Leaf number | 5 | Leaf number > 8 |
| 4 | 6 < Leaf number ≤ 8 | |
| 3 | 4 < Leaf number ≤ 6 | |
| 2 | 2 < Leaf number ≤ 4 | |
| 1 | Leaf number at 2 | |
| 0 | Leaves dead and dried | |
| Leaf size | 5 | Leaf diameter is mostly over 1.5 cm |
| 4 | Leaf diameter mostly in the range of 1 cm to 1.5 cm | |
| 3 | Leaf diameter mostly in the range of 0.5 cm to 1.0 cm | |
| 2 | Leaf diameter is mostly around 0.5 cm | |
| 1 | leaf diameter is less than 0.5 cm | |
| 0 | Leaves dead and dried | |
| Root growth and development scoring criteria | ||
| Development of root hairs | 5 | Secondary and tertiary roots developed with massive root hairs |
| 4 | Secondary roots developed with a medium number of root hairs | |
| 3 | Few secondary roots and sparse root hairs | |
| 2 | Little to no secondary roots and root hairs | |
| 1 | Only primary root without root hairs | |
| 0 | Root dead | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Dickinson, N. Revealing the Complex Interplay of Biostimulant Applications. Plants 2024, 13, 2188. https://doi.org/10.3390/plants13162188
Yuan Y, Dickinson N. Revealing the Complex Interplay of Biostimulant Applications. Plants. 2024; 13(16):2188. https://doi.org/10.3390/plants13162188
Chicago/Turabian StyleYuan, Ye, and Nicholas Dickinson. 2024. "Revealing the Complex Interplay of Biostimulant Applications" Plants 13, no. 16: 2188. https://doi.org/10.3390/plants13162188
APA StyleYuan, Y., & Dickinson, N. (2024). Revealing the Complex Interplay of Biostimulant Applications. Plants, 13(16), 2188. https://doi.org/10.3390/plants13162188






