Mapping Temperate Vegetation Climate Adaptation Variability Using Normalized Land Surface Phenology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Overview
2.2. Remotely Sensed Land Surface Phenology
2.3. Modeled Phenology Based on Cloned Plants
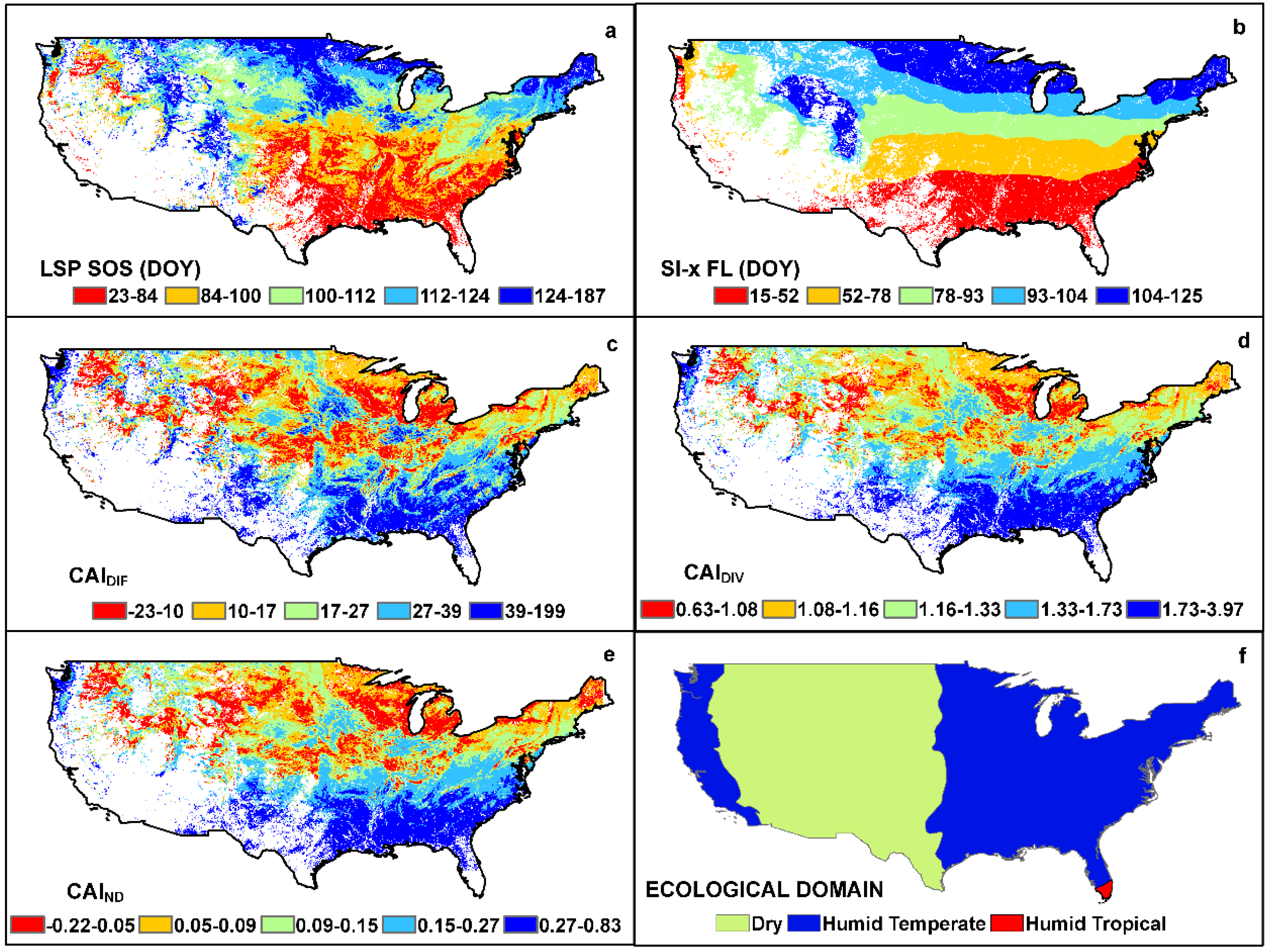
2.4. Rationale and Algorithms
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Badeck, F.W.; Bondeau, A.; Bottcher, K.; Doktor, D.; Lucht, W.; Schaber, J.; Sitch, S. Responses of spring phenology to climate change. New Phytol. 2004, 162, 295–309. [Google Scholar] [CrossRef]
- Liang, L.; Schwartz, M.D. Landscape phenology: An integrative approach to seasonal vegetation dynamics. Landsc. Ecol. 2009, 24, 465–472. [Google Scholar] [CrossRef]
- Pau, S.; Wolkovich, E.M.; Cook, B.I.; Davies, T.J.; Kraft, N.J.B.; Bolmgren, K.; Betancourt, J.L.; Cleland, E.E. Predicting phenology by integrating ecology, evolution and climate science. Glob. Chang. Biol. 2011, 17, 3633–3643. [Google Scholar] [CrossRef]
- Hopkins, A.D. Periodical Events and Natural Law as Guides to Agricultural Research and Practice; US Government Printing Office: Washington, DC, USA, 1918.
- Hopkins, A.D. The bioclimatic law. Mon. Weather Rev. 1920, 48, 34–40. [Google Scholar] [CrossRef]
- Celton, J.M.; Martinez, S.; Jammes, M.J.; Bechti, A.; Salvi, S.; Legave, J.M.; Costes, E. Deciphering the genetic determinism of bud phenology in apple progenies: A new insight into chilling and heat requirement effects on flowering dates and positional candidate genes. New Phytol. 2011, 192, 378–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gienapp, P.; Teplitsky, C.; Alho, J.; Mills, J.; Merila, J. Climate change and evolution: Disentangling environmental and genetic responses. Mol. Ecol. 2008, 17, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.B.; Lacourse, T.; Hawkins, B.J.; Yanchuk, A. Adaptive variation in growth, phenology, cold tolerance and nitrogen fixation of red alder (Alnus rubra Bong). For. Ecol. Manag. 2013, 291, 357–366. [Google Scholar] [CrossRef]
- Olson, M.S.; Levsen, N.; Soolanayakanahally, R.Y.; Guy, R.D.; Schroeder, W.R.; Keller, S.R.; Tiffin, P. The adaptive potential of Populus balsamifera L. To phenology requirements in a warmer global climate. Mol. Ecol. 2013, 22, 1214–1230. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.S.; Hansen, J.K. Geographical variation in phenology of Quercus petraea (Matt.) Liebl and Quercus robur L. Oak grown in a greenhouse. Scand. J. For. Res. 2008, 23, 179–188. [Google Scholar] [CrossRef]
- Kriebel, H.B.; Wang, C.W. The interaction between provenance and degree of chilling in bud-break of sugar maple. Silvae Genet. 1962, 11, 125–130. [Google Scholar]
- Li, H.L.H.; Wang, X.W.X.; Hamann, A.H.A. Genetic adaptation of aspen (Populus tremuloides) populations to spring risk environments: A novel remote sensing approach. Can. J. For. Res. 2010, 40, 2082–2090. [Google Scholar] [CrossRef]
- Cech, F.C.; Carter, K.K. Geographic variation in black cherry: Ten-year results of a West Virginia provenance test. In Proceeding of the First North Central Tree Improvement Conference, Madison, WI, USA, 21–23 August 1979; pp. 21–27.
- McGee, C. Elevation of seed sources and planting sites affects phenology and development of red oak seedlings. For. Sci. 1974, 20, 160–164. [Google Scholar]
- Cesaraccio, C.; Spano, D.; Snyder, R.L.; Duce, P. Chilling and forcing model to predict bud-burst of crop and forest species. Agric. For. Meteorol. 2004, 126, 1–13. [Google Scholar] [CrossRef]
- Schwartz, M.D.; Hanes, J.M. Continental scale phenology: Warming and chilling. Int. J. Climatol. 2010, 30, 1595–1598. [Google Scholar] [CrossRef]
- McMillan, C. The role of ecotypic variation in the distribution of the central grassland of North America. Ecol. Monogr. 1959, 29, 286–308. [Google Scholar] [CrossRef]
- Basler, D.; Körner, C. Photoperiod sensitivity of bud burst in 14 temperate forest tree species. Agric. For. Meteorol. 2012, 165, 73–81. [Google Scholar] [CrossRef]
- Caffarra, A.; Donnelly, A. The ecological significance of phenology in four different tree species: Effects of light and temperature on bud burst. Int. J. Biometeorol. 2011, 55, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Basler, D.; Körner, C. Photoperiod and temperature responses of bud swelling and bud burst in four temperate forest tree species. Tree Physiol. 2014, 34, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Chuine, I.; Morin, X.; Bugmann, H. Warming, photoperiods, and tree phenology. Science 2010, 329, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Zohner, C.M.; Renner, S.S. Common garden comparison of the leaf-out phenology of woody species from different native climates, combined with herbarium records, forecasts long-term change. Ecol. Lett. 2014, 17, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Schwartz, M.D. Testing a growth efficiency hypothesis with continental-scale phenological variations of common and cloned plants. Int. J. Biometeorol. 2014, 58, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Wielgolaski, F.E. Phenological modifications in plants by various edaphic factors. Int. J. Biometeorol. 2001, 45, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Justice, C.; Holben, B.; Gwynne, M. Monitoring East African vegetation using AVHRR data. Int. J. Remote Sens. 1986, 7, 1453–1474. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Pedelty, J.; Devadiga, S.; Masuoka, E.; Brown, M.; Pinzon, J.; Tucker, C.; Roy, D.; Ju, J.; Vermote, E.; Prince, S. Generating a long-term land data record from the AVHRR and MODIS instruments. In Proceeding of the IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Barcelona, Spain, 23–28 July 2007; pp. 1021–1025.
- Vegetation Index & Phenology Lab. Land Surface Phenology Data. Available online: http://vip.arizona.edu/viplab-data-explorer (accessed on 14 April 2016).
- Huete, A.R.; Didan, K.; Shimabukuro, Y.E.; Ratana, P.; Saleska, S.R.; Hutyra, L.R.; Yang, W.; Nemani, R.R.; Myneni, R. Amazon rainforests green-up with sunlight in dry season. Geophys. Res. Lett. 2006, 33, L06405. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.R.; Didan, K.; Miura, T. Development of a two-band enhanced vegetation index without a blue band. Remote Sens. Environ. 2008, 112, 3833–3845. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Tan, B.; Yu, Y. Interannual variations and trends in global land surface phenology derived from enhanced vegetation index during 1982–2010. Int. J. Biometeorol. 2014, 58, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Friedl, M.A.; Schaaf, C.B. Global vegetation phenology from moderate resolution imaging spectroradiometer (MODIS): Evaluation of global patterns and comparison with in situ measurements. J. Geophys. Res. Biogeosci. 2006, 111, G04017. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Hodges, J.; Schaaf, C.; Friedl, M.; Strahler, A.; Gao, F. Global vegetation phenology from AVHRR and MODIS data. In Proceeding of the IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Sydney, NSW, Australia, 9–13 July 2001; pp. 2262–2264.
- Zhang, X.Y. Reconstruction of a complete global time series of daily vegetation index trajectory from long-term AVHRR data. Remote Sens. Environ. 2015, 156, 457–475. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Friedl, M.A.; Schaaf, C.B.; Strahler, A.H.; Hodges, J.C.F.; Gao, F.; Reed, B.C.; Huete, A. Monitoring vegetation phenology using MODIS. Remote Sens. Environ. 2003, 84, 471–475. [Google Scholar] [CrossRef]
- Schwartz, M.D.; Ault, T.R.; Betancourt, J.L. Spring onset variations and trends in the continental United States: Past and regional assessment using temperature-based indices. Int. J. Climatol. 2013, 33, 2917–2922. [Google Scholar] [CrossRef]
- Schwartz, M.D.; Ahas, R.; Aasa, A. Onset of spring starting earlier across the Northern Hemisphere. Glob. Chang. Biol. 2006, 12, 343–351. [Google Scholar] [CrossRef]
- Schwartz, M.D.; Marotz, G.A. Synoptic events and spring phenology. Phys. Geogr. 1988, 9, 151–161. [Google Scholar]
- Ault, T.R.; Zurita-Milla, R.; Schwartz, M.D. A Matlab© toolbox for calculating spring indices from daily meteorological data. Comput. Geosci. 2015, 83, 46–53. [Google Scholar] [CrossRef]
- McCabe, G.J.; Ault, T.R.; Cook, B.I.; Betancourt, J.L.; Schwartz, M.D. Influences of the El Niño Southern Oscillation and the Pacific Decadal Oscillation on the timing of the North American spring. Int. J. Climatol. 2012, 32, 2301–2310. [Google Scholar] [CrossRef]
- EPA Climate Change Indicators in the United States: Leaf and Bloom Dates. Available online: http://www.epa.gov/climatechange/science/indicators/ecosystems/leaf-bloom-dates.html (accessed on 17 March 2016).
- Kenney, M.A.; Janetos, A.C. National Climate Indicators System Report, National Climate Assessment and Development Advisory Committee. Available online: http://www.globalchange.gov/sites/globalchange/files/Pilot-Indicator-System-Report_final.pdf (accessed on 14 April 2016).
- Bailey, R.; Avers, P.; King, T.; McNab, W. Ecoregions and Subregions of the United States (Map); US Geological Survey: Washington, DC, USA, 1994.
- Jolicoeur, P.; Pontier, J. Population growth and decline: A four-parameter generalization of the logistic curve. J. Theor. Biol. 1989, 141, 563–571. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Koppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Tarpley, D.; Sullivan, J.T. Diverse responses of vegetation phenology to a warming climate. Geophys. Res. Lett. 2007, 34, L19405. [Google Scholar] [CrossRef]
- Perry, T.O.; Wu, W.C. Genetic variation in the winter chilling requirement for date of dormancy break for Acer rubrum. J. Ecol. 1960, 41, 790–794. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, X. Coupled spatiotemporal variability of temperature and spring phenology in the eastern United States. Int. J. Climatol. 2015. [Google Scholar] [CrossRef]
- Richardson, A.D.; O’Keefe, J. Phenological differences between understory and overstory: A case study using the long-term Harvard forest records. In Phenology of Ecosystem Processes: Applications in Global Change Research; Noormets, A., Ed.; Springer: New York, NY, USA, 2009; pp. 87–117. [Google Scholar]
- Monserud, R.A.; Rehfeldt, G.E. Genetic and environmental components of variation of site index in inland douglas-fir. For. Sci. 1990, 36, 1–9. [Google Scholar]
- Howe, G.T.; Aitken, S.N.; Neale, D.B.; Jermstad, K.D.; Wheeler, N.C.; Chen, T.H.H. From genotype to phenotype: Unraveling the complexities of cold adaptation in forest trees. Can. J. Bot. 2003, 81, 1247–1266. [Google Scholar] [CrossRef]
- Schwartz, M.D.; Reed, B.C. Surface phenology and satellite sensor-derived onset of greenness: An initial comparison. Int. J. Remote Sens. 1999, 20, 3451–3457. [Google Scholar] [CrossRef]
- Fisher, J.I.; Richardson, A.D.; Mustard, J.F. Phenology model from surface meteorology does not capture satellite-based Greenup estimations. Glob. Chang. Biol. 2007, 13, 707–721. [Google Scholar] [CrossRef]
- Vitasse, Y.; Delzon, S.; Bresson, C.C.; Michalet, R.; Kremer, A. Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Can. J. For. Res. 2009, 39, 1259–1269. [Google Scholar] [CrossRef]
- Vitasse, Y.; Delzon, S.; Dufrêne, E.; Pontailler, J.Y.; Louvet, J.M.; Kremer, A.; Michalet, R. Leaf phenology sensitivity to temperature in European trees: Do within-species populations exhibit similar responses? Agric. For. Meteorol. 2009, 149, 735–744. [Google Scholar] [CrossRef]
- Conover, D.O.; Duffy, T.A.; Hice, L.A. The covariance between genetic and environmental influences across ecological gradients. Ann. N. Y. Acad. Sci. 2009, 1168, 100–129. [Google Scholar] [CrossRef] [PubMed]
- Soularue, J.P.; Kremer, A. Evolutionary responses of tree phenology to the combined effects of assortative mating, gene flow and divergent selection. Heredity 2014, 113, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Liang, L. Geographic variations in spring and autumn phenology of white ash in a common garden. Phys. Geogr. 2015, 36, 1–21. [Google Scholar] [CrossRef]
- Jeong, S.J.; Medvigy, D.; Shevliakova, E.; Malyshev, S. Predicting changes in temperate forest budburst using continental-scale observations and models. Geophys. Res. Lett. 2013, 40, 359–364. [Google Scholar] [CrossRef]
- Hargrove, W.W.; Spruce, J.P.; Gasser, G.E.; Hoffman, F.M. Toward a national early warning system for forest disturbances using remotely sensed canopy phenology. Photogramm. Eng. Remote Sens. 2009, 75, 1150–1156. [Google Scholar]
- Hoffman, F.M.; Mills, R.T.; Kumar, J.; Vulli, S.S.; Hargrove, W.W. Geospatiotemporal data mining in an early warning system for forest threats in the United States. In Proceeding of the IEEI International Geoscience and Remote Sensing Symposium (IGARSS), Honolulu, HI, USA, 25–30 July 2010; pp. 170–173.
- Norman, S.P.; Hargrove, W.W. Land Surface Phenology as a Coarse-Filter Indicator of Disturbance and Climatic Effects Across the Coast Redwood Range; Pacific Southwest Research Station, Forest Service; U.S. Department of Agriculture: Albany, CA, USA, 2012; pp. 657–665.
- Hargrove, W.W.; Hoffman, F.M.; Kumar, J.; Spruce, J.P.; Mills, R.T. National Phenological Ecoregions (2000–2011). Available online: https://www.geobabble.org/phenoregions (accessed on 17 March 2016).

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, L.; Schwartz, M.D.; Zhang, X. Mapping Temperate Vegetation Climate Adaptation Variability Using Normalized Land Surface Phenology. Climate 2016, 4, 24. https://doi.org/10.3390/cli4020024
Liang L, Schwartz MD, Zhang X. Mapping Temperate Vegetation Climate Adaptation Variability Using Normalized Land Surface Phenology. Climate. 2016; 4(2):24. https://doi.org/10.3390/cli4020024
Chicago/Turabian StyleLiang, Liang, Mark D. Schwartz, and Xiaoyang Zhang. 2016. "Mapping Temperate Vegetation Climate Adaptation Variability Using Normalized Land Surface Phenology" Climate 4, no. 2: 24. https://doi.org/10.3390/cli4020024
APA StyleLiang, L., Schwartz, M. D., & Zhang, X. (2016). Mapping Temperate Vegetation Climate Adaptation Variability Using Normalized Land Surface Phenology. Climate, 4(2), 24. https://doi.org/10.3390/cli4020024





