Potential Effects of Climate and Human Influence Changes on Range and Diversity of Nine Fabaceae Species and Implications for Nature’s Contribution to People in Kenya
Abstract
:1. Introduction
2. Materials and Methods
2.1. Occurrence Data
2.2. Environmental Variables
2.3. Climate and Population Change Scenarios
2.4. Species Distribution and Diversity Indicators by MaxEnt
3. Results
3.1. Climate Change by RCP 4.5 Scenario
3.2. Urban Area and HII Change by SSP Scenarios
3.3. Species Distribution Modeled by MaxEnt
3.4. Species Range Change by Climate and Socioeconomic Scenarios
4. Discussion
4.1. Species Distribution Modeling Accuracy by MaxEnt
4.2. Environmental Variables Affecting Species Habitats
4.3. Interaction of Climate and Human Influence Changes and Effects on Species Ranges
4.4. Possible Implications for NCP in Future
4.5. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kulindwa, K.; Kameri-Mbote, P.; Mohamed-Katerere, J.; Chenje, M. Chapter 1: The human dimension. In Africa Environment Outlook; United Nations Environment Programme: Nairobi, Kenya, 2006; p. 34. [Google Scholar]
- Diaz, S.; Demissew, S.; Carabias, J.; Joly, C.; Lonsdale, M.; Ash, N.; Larigauderie, A.; Adhikari, J.A.; Arico, A.; Báldi, A.; et al. The IPBES conceptual framework—connecting nature and people. Curr. Opin. Environ. Sustain. 2015, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Diaz, S.; Pascual, U.; Stenseke, M.; Martín-López, B.; Watson, R.; Molnar, Z.; Hill, R.; Chan, K.; Baste, I.; Brauman, K.; et al. Assessing nature’s contributions to people. Science 2018, 359, 270–272. [Google Scholar] [CrossRef] [Green Version]
- Failler, P.; Kasisi, R.; Akachuku, C.; Assogbadjo, A.; Boyd, E.; Effiom, E.; Elias, P.; Halmy, M.W.A.; Heubach, K.; Mohamed, A.; et al. Chapter 2: Nature’s contributions to people and quality of life. In IPBES: The IPBES Regional Assessment Report on Biodiversity and Ecosystem Services for Africa; Archer, E., Dziba, L., Mulongoy, K.J., Maoela, M.A., Walters, M., Eds.; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2018; pp. 77–130. [Google Scholar]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [Green Version]
- Scholes, R.J. Climate change and ecosystem services. Wiley Interdiscipl. Rev. Clim. Chang. 2016, 7, 537–550. [Google Scholar] [CrossRef]
- Crimmins, S.M.; Dobrowski, S.Z.; Greenberg, J.A.; Abatzoglou, J.T.; Mynsberge, A.R. Changes in climatic water balance drive downhill shifts in plant species’ optimum elevations. Science 2011, 331, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Hill, J.K.; Ohlemueller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Dziba, L.E.; Mulongoy, K.J.; Maoela, M.A.; Walters, M.; Biggs, R.O.; Cormier-Salem, M.-C.; Declerk, F.; Chimere Diaw, M.; Dunham, A.E.; et al. Summary for Policymakers of the Regional Assessment Report on Biodiversity and Ecosystem Services for Africa of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2018. [Google Scholar]
- Hay, S.I.; Guerra, C.A.; Tatem, A.J.; Atkinson, P.M.; Show, R.W. Urbanization, malaria transmission and disease burden in Africa. Nat. Rev. Microbiol. 2005, 3, 81–90. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change. Impacts, Adaptation and Vulnerability; IPCC Secretariat: Geneva, Switzerland, 2007. [Google Scholar]
- Ouko, C.A.; Mulwa, R.; Kibugi, R.; Thorn Jessica, P.R.; Oguge, N. Prospects of scenario planning for Kenya’s protected ecosystems: An example of Mount Marsabit. Curr. Res. Environ. Sustain. 2020, 1, 7–15. [Google Scholar] [CrossRef]
- Bai, Z.G.; Dent, D.L.; Olsson, L.; Schaepman, M.E. Proxy global assessment of land degradation. Soil Use Manag. 2008, 24, 223–234. [Google Scholar] [CrossRef]
- KC, S.; Lutz, W. The human core of the shared socioeconomic pathways: Population scenarios by age, sex and level of education for all countries to 2100. Glob. Environ. Chang. 2017, 42, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Seto, K.C.; Güneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarus, G.K.; Muthama, N.J.; Githaiga, J.M.; Onwonga, R. Projecting the impacts of climate change on tree biomass in Arabuko-Sokoke Forest, Kenya. J. Clim. Chang. Sustain. 2018, 1, 95–107. [Google Scholar] [CrossRef]
- Franklin, J. Mapping Species Distributions; Spatial Inference and Prediction; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists: Statistical explanation of MaxEnt. Divers. Distrib. 2011, 17, 43–47. [Google Scholar] [CrossRef]
- Popp, A.; Calvin, K.; Fujimori, S.; Havlik, P.; Humpenöder, F.; Stehfest, E.; Bodirsky, B.L.; Dietrich, J.P. Land-use futures in the shared socio-economic pathways. Glob. Environ. Chang. 2017, 42, 331–345. [Google Scholar] [CrossRef] [Green Version]
- Van Vuuren, D.P.; van Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.-F.; et al. The representative concentration pathways: An overview. Clim. Chang. 2011, 109, 5–31. [Google Scholar] [CrossRef]
- Cheche, W.W.; Githae, E.W.; Omondi, S.F.; Magana, A.M. An inventory and assessment of exotic and native plant species diversity in the Kenyan rangelands: Case study of Narok North Sub-County. J. Ecol. Nat. Environ. 2015, 7, 238–246. [Google Scholar] [CrossRef]
- Gachathi, F.; Eriksen, S. Gums and resins: The potential for supporting sustainable adaptation in Kenya’s drylands. Clim. Dev. 2011, 3, 59–70. [Google Scholar] [CrossRef]
- Dharani, N. Field Guide to Acacias of East Africa; Struik Publishers: Cape Town, South Africa, 2006. [Google Scholar]
- Roothaert, R.L.; Franzel, S. Farmers’ preferences and use of local fodder trees and shrubs in Kenya. Agrofor. Syst. 2001, 52, 239–252. [Google Scholar] [CrossRef]
- Kiringe, J.W.; Okello, M.M. Use and availability of tree and shrub resources on Maasai communal rangelands near Amboseli, Kenya. Afr. J. Range Sci. 2005, 22, 37–45. [Google Scholar] [CrossRef]
- Kimondo, J.; Miaron, J.; Mutai, P.; Njogu, P. Ethnobotanical survey of food and medicinal plants of the Ilkisonko Maasai community in Kenya. J. Ethnopharmacol. 2015, 175, 463–469. [Google Scholar] [CrossRef]
- Ruiz Guajardo, J.C.; Schnabel, A.; Ennos, R.; Preuss, S.; Otero-Arnaiz, A.; Stone, G. Landscape genetics of the key African acacia species Senegalia mellifera (Vahl): The importance of the Kenyan Rift Valley. Mol. Ecol. 2010, 19, 5126–5139. [Google Scholar] [CrossRef]
- Omondi, S.F.; Kireger, E.; Dangasuk, O.G. Genetic Diversity and Population Structure of Acacia Senegal Willd. in Kenya. Tropic. Plant Biol. 2010, 3, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Franz, T.E.; Caylor, K.K.; Nordbotten, J.M.; Rodríguez-Iturbe, I.; Celia, M.A. An ecohydrological approach to predicting regional woody species distribution patterns in dryland ecosystems. Adv. Water Resour. 2010, 33, 215–230. [Google Scholar] [CrossRef]
- GBIF.org. GBIF Occurrence Download. Available online: https://www.gbif.org/occurrence/download/0050043-191105090559680 (accessed on 27 January 2020). [CrossRef]
- Kyalangalilwa, B.; Boatwright, J.S.; Daru, B.H.; Maurin, O.; Van der Bank, M. Phylogenetic position and revised classification of Acacia S.L. (Fabaceae: Mimosoideae) in Africa, including new combinations in Vachellia and Senegalia. Botan. J. Linn. Soc. 2013, 172, 500–523. [Google Scholar] [CrossRef] [Green Version]
- Ersts, P.J. Geographic Distance Matrix Generator (version 1.2.3). American Museum of Natural History. Center for Biodiversity and Conservation. Available online: http://biodiversityinformatics.amnh.org/open_source/gdmg (accessed on 6 April 2020).
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Greve, M.; Lykke, A.M.; Fagg, C.W. Continental-scale variability in browser diversity is a major driver of diversity patterns in acacias across Africa. J. Ecol. 2012, 100, 1093–1104. [Google Scholar] [CrossRef] [Green Version]
- Marshall, A.R.; Platts, P.J.; Gereau, R.E.; Kindeketa, W.; Kang’ethe, S.; Marchant, R. The genus Acacia (Fabaceae) in East Africa: Distribution, biodiversity and the protected area network. Plant Ecol. Evol. 2012, 145, 289–301. [Google Scholar] [CrossRef] [Green Version]
- Yates, C.J.; McNeill, A.; Elith, J.; Midgley, G.F. Assessing the impacts of climate change and land transformation on Banksia in the South West Australian floristic region. Div. Distr. 2010, 16, 187–201. [Google Scholar] [CrossRef]
- Ma, B.; Sun, J. Predicting the distribution of Stipa purpurea across the Tibetan Plateau via the MaxEnt model. BMC Ecol. 2018, 18, 10. [Google Scholar] [CrossRef] [Green Version]
- Burgess, N.D.; Balmford, A.; Cordeiro, N.J.; Fjeldså, J.; Küper, W.; Rahbek, C.; Sanderson, E.W.; Scharlemann, J.P.W.; Sommer, J.H.; Williams, P.H. Correlations among species distributions, human density and human infrastructure across the high biodiversity tropical mountains of Africa. Bio. Conserv. 2007, 134, 164–177. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, J.E.; DiTommaso, A. Predicting the potential distribution of Lantana camara L. under RCP scenarios using ISI-MIP models. Clim. Chang. 2016, 134, 193–208. [Google Scholar] [CrossRef]
- Valencia, J.; Lassaletta, L.; Velázquez, E.; Nicolau, J.M.; Gomóz-Sal, A. Factors controlling compositional changes in a northern Andean Páramo (La Rusia, Colombia). Biotropica 2013, 45, 18–26. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1km spatial resolution climate surfaces for global land areas. Int. J. Clim. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Choi, S.; Lee, W.K.; Kwak, D.A.; Lee, S.; Son, Y.; Lim, J.H.; Saborowski, J. Predicting forest cover changes in future climate using hydrological and thermal indices in South Korea. Clim. Res. 2011, 49, 229–245. [Google Scholar] [CrossRef] [Green Version]
- Lehner, B.; Verdin, K.; Jarvis, A. HydroSHEDS Technical Documentation; World Wildlife Fund: Washington, DC, USA, 2006. Available online: http://hydrosheds.cr.usgs.gov/ (accessed on 20 November 2019).
- Sanderson, E.W.; Jaiteh, M.; Levy, M.A.; Redford, K.H.; Wannebo, A.V.; Woolmer, G. The Human Footprint and The Last of the Wild. BioScience 2002, 52, 891–904. [Google Scholar] [CrossRef]
- Li, G.; Liu, C.; Liu, Y.; Yang, J.; Zhang, X. Effects of climate, disturbance and soil factors on the potential distribution of Liaotung oak (Quercus wutaishanica Mayr) in China. Ecol. Res. 2012, 27, 427–436. [Google Scholar] [CrossRef]
- De Thoisy, B.; Richard-Hansen, C.; Goguillon, B. Rapid evaluation of threats to biodiversity: Human footprint score and large vertebrate species responses in French Guiana. Biodiv. Conserv. 2010, 19, 1567–1584. [Google Scholar] [CrossRef]
- Tomohiro, H.; Manabu, A.; Osamu, A.; Tatsuo, S.; Yoshiki, K.; Tomoo, O.; Koji, O.; Michio, W.; Akitomo, Y.; Hiroaki, T.; et al. MIROC MIROC-ES2L model output prepared for CMIP6 CMIP historical. Version 20200407. Earth Syst. Grid Fed. 2019. [Google Scholar] [CrossRef]
- Thomson, A.M.; Calvin, K.V.; Smith, S.J. RCP4.5: A pathway for stabilization of radiative forcing by 2100. Clim. Chang. 2011, 109, 77. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; O’Neill, B.C. Global urbanization projections for the Shared Socioeconomic Pathways. Glob. Environ. Chang. 2017, 42, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Center for International Earth Science Information Network—CIESIN; Columbia University—CUNY; Institute for Demographic Research—CIDR; International Food Policy Research Institute—IFPRI; The World Bank; Centro Internacional de Agricultura Tropical—CIAT. Global Rural-Urban Mapping Project; Version 1 (GRUMPv1), Urban Extent Polygons, Revision 01; NASA Socioeconomic Data and Applications Center (SEDAC): Palisades, NY, USA, 2017. [CrossRef]
- Center for International Earth Science Information Network—CIESIN; Columbia University; Centro Internacional de Agricultura Tropical—CIAT. Gridded Population of the World; Version 3 (GPWv3) Population Density Grid; NASA Socioeconomic Data and Applications Center (SEDAC): Palisades, NY, USA, 2005. [CrossRef]
- Jueterbock, A.; Smolina, I.; Coyer, J.A.; Hoarau, G. The fate of the Arctic seaweed Fucus distichus under climate change: An ecological niche modeling approach. Ecol. Evol. 2016, 6, 1712–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to Maxent for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Package ‘Dismo’. 2017. Available online: https://cran.r-project.org/web/packages/dismo/dismo.pdf (accessed on 27 January 2020).
- Broennimann, O.; Di Cola, V.; Petitpierre, B.; Breiner, F.; Scherrer, D.; D’Amen, M.; Randin, C.; Engler, R.; Hordijk, W.; Mod, H.; et al. R Package ‘Ecospat’. Available online: https://cran.r-project.org/web/packages/ecospat/ecospat.pdf (accessed on 11 September 2020).
- Gallardo, B. Europe’s top 10 invasive species: Relative importance of climatic, habitat and socio-economic factors. Ethol. Ecol. Evol. 2014, 26, 130–151. [Google Scholar] [CrossRef] [Green Version]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1949. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity, 2nd ed.; Blackwell Science Ltd.: Oxford, UK, 2004. [Google Scholar]
- Morris, E.K.; Caruso, T.; Buscot, F.; Fischer, M.; Hancock, C.; Maier, T.S. Choosing and using diversity indices: Insights for ecological applications from the German biodiversity exploratories. Ecol. Evol. 2014, 4, 3514–3524. [Google Scholar] [CrossRef] [Green Version]
- Bradie, J.; Leung, B. A quantitative synthesis of the importance of variables used in MaxEnt species distribution models. J. Biogeogr. 2017, 44, 1344–1361. [Google Scholar] [CrossRef]
- Walthert, L.; Meier, E.S. Tree species distribution in temperate forests is more influenced by soil than by climate. Ecol. Evol. 2017, 7, 9473–9484. [Google Scholar] [CrossRef]
- Forester, B.R.; DeChaine, E.G.; Bunn, A.G. Integrating ensemble species distribution modelling and statistical phylogeography to inform projections of climate change impacts on species distributions. Div. Distr. 2013, 19, 1480–1495. [Google Scholar] [CrossRef]
- Beentje, H.J.; Adamson, J. Kenya Trees, Shrubs and Lianas; National Museums of Kenya: Nairobi, Kenya, 1994. [Google Scholar]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Kiage, L.M.; Liu, K. Palynological evidence of climate change and land degradation in the Lake Baringo area, Kenya, East Africa, since AD 1650. Palaeogeogr. Palaeoclim. Palaeoecol. 2009, 279, 60–72. [Google Scholar] [CrossRef]
- Shekede, M.D.; Murwira, A.; Masocha, M. Spatial distribution of Vachellia karroo in Zimbabwean savannas under a changing climate. Ecol. Res. 2018, 33, 1181–1191. [Google Scholar] [CrossRef]
- Bucini, G.; Hanan, N. A continental-scale analysis of tree cover in African savannas. Glob. Ecol. Biogeogr. 2007, 16, 593. [Google Scholar] [CrossRef]
- Castro-Diez, P.; Godoy, O.; Saldaña, A.; Richardson, D.M. Predicting invasiveness of Australian acacias on the basis of their native climatic affinities, life history traits and human use. Div. Distr. 2011, 17, 934–945. [Google Scholar] [CrossRef]
- Wabuyele, E.; Kang’ethe, S.; Newton, L. Digital Knowledge of Kenyan Succulent Flora and Priorities for Future Inventory and Documentation. Biodiv. Infor. 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Wania, A.; Kühn, I.; Klotz, S. Plant richness patterns in agricultural and urban landscapes in Central Germany—Spatial gradients of species richness. Landsc. Urban Plan. 2006, 75, 97–110. [Google Scholar] [CrossRef]
- Seto, K.C.; Fragkias, M.; Güneralp, B.; Reilly, M.K. A meta-analysis of global urban land expansion. PLoS ONE 2011, 6, e23777. [Google Scholar] [CrossRef]
- Yusuf, H.; Treydte, A.C.; Demissew, S.; Woldu, Z. Assessment of woody species encroachment in the grasslands of Nechisar National Park, Ethiopia. Afr. J. Ecol. 2011, 49, 397–409. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S., III; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Bauer, N.; Calvin, K.; Emmerling, J.; Fricko, O.; Fujimori, S.; Hilaire, J.; Eom, J.; Krey, V.; Kriegler, E.; Mouratiadou, I.; et al. Shared Socio-Economic Pathways of the Energy Sector—Quantifying the Narratives. Glob. Environ. Chang. 2017, 42, 316–330. [Google Scholar] [CrossRef] [Green Version]
- Lambin, E.F.; Geist, H.J.; Lepers, E. Dynamics of land-use and land-cover change in tropical regions. Annu. Rev. Environ. Resour. 2003, 28, 205–241. [Google Scholar] [CrossRef] [Green Version]
- Onaindia, M.; Domínguez, I.; Albizu, I.; Garbisu, C.; Amezaga, I. Vegetation diversity and vertical structure as indicators of forest disturbance. For. Ecol. Manag. 2004, 195, 341–354. [Google Scholar] [CrossRef]
- Estoque, R.C.; Ooba, M.; Togawa, T.; Hijioka, Y. Projected land-use changes in the Shared Socioeconomic Pathways: Insights and implications. Ambio 2020. [Google Scholar] [CrossRef]
- Bai, Z.G.; Dent, D.L. Global Assessment of Land Degradation and Improvement; Pilot study in Kenya; ISRIC—World Soil Information: Wageningen, The Netherlands, 2006. [Google Scholar]
- Gomes, V.H.; IJff, S.D.; Raes, N. Species Distribution Modelling: Contrasting presence-only models with plot abundance data. Science 2018, 8, 1003. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |


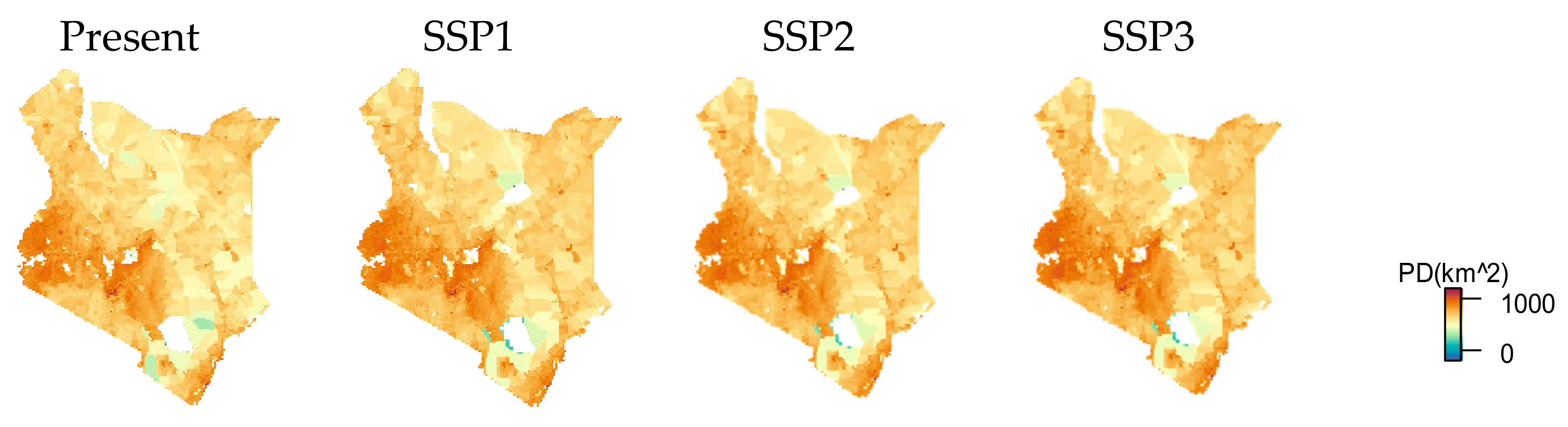


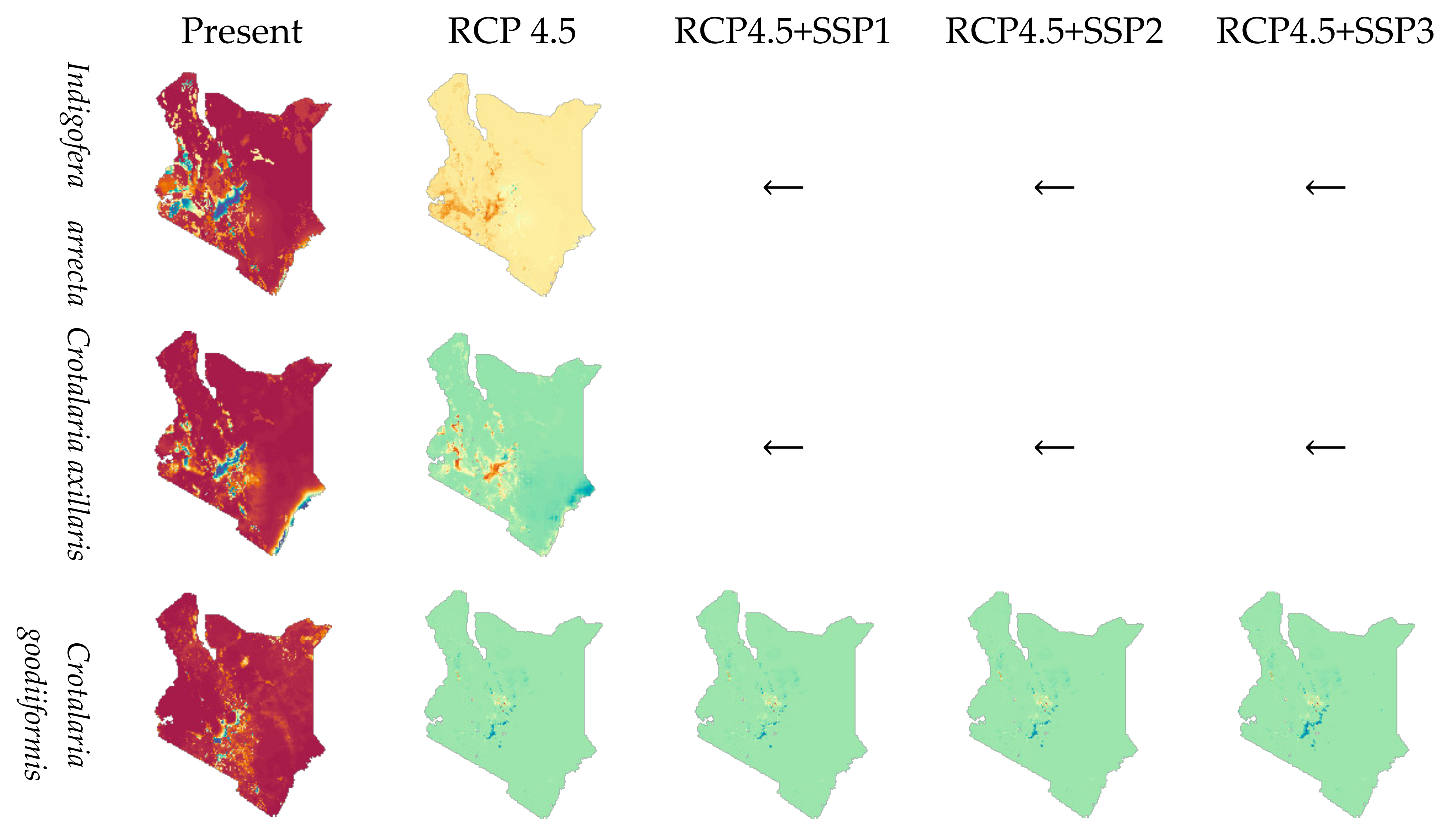


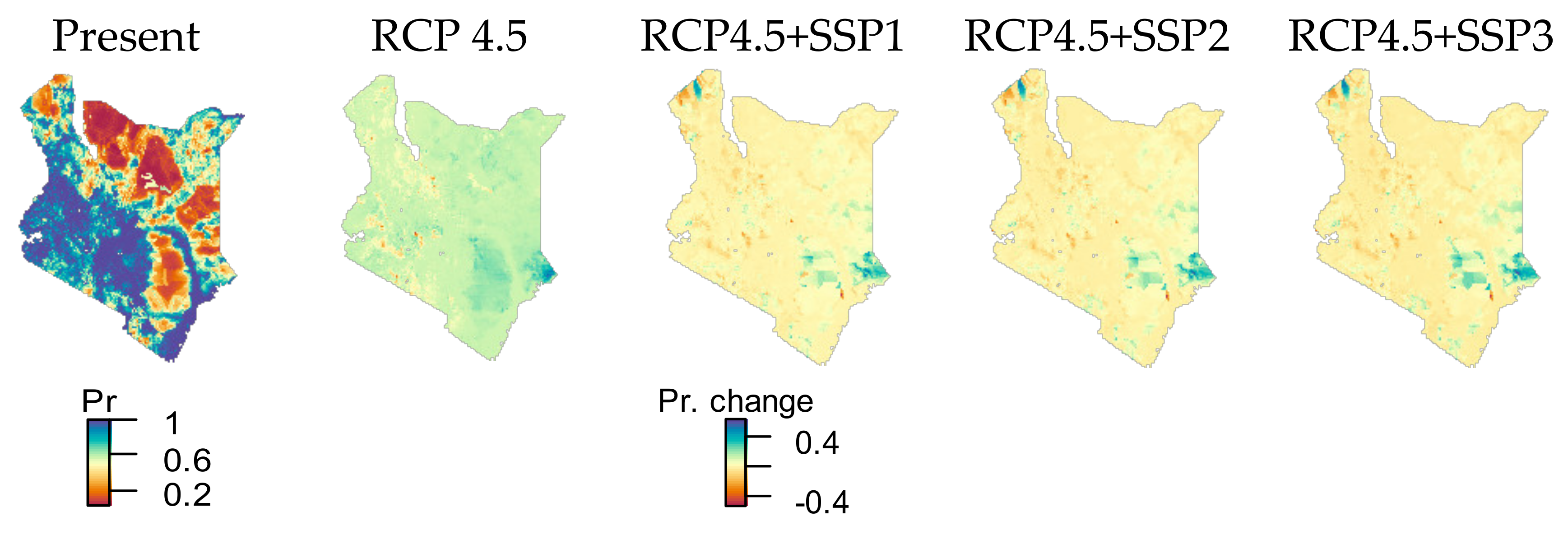
| Species Name | No. of 1 km Cells with Species Present | Species Values |
|---|---|---|
| Indigofera arrecta | 16 | Medicine, dye, food |
| Crotalaria axillaris | 17 | Medicine |
| Crotalaria goodiiformis | 16 | Fodder, fuel |
| Senegalia brevispica | 24 | Forage, fencing, fuelwood, medicine |
| Senegalia mellifera | 31 | medicine, fuelwood, fencing, beehive, soil conservation |
| Senegalia Senegal | 23 | Gum, resin, fuelwood, fencing, medicine, fodder, forage, soil nitrogen fixation |
| Vachellia etbaica | 16 | Fodder, beehive, medicine, poles, fuelwood |
| Vachellia hockii | 24 | Fuelwood, fodder, medicine, construction, soil nitrogen fixation |
| Vachellia nilotica | 41 | Fencing, furniture, fuelwood, gum, tannins, forage, medicine, dye, riverbank stabilization |
| Population Density (PD) Score | Urban Polygon Score | ||||
|---|---|---|---|---|---|
| PD (km−2) | Score | PD (km−2) | Score | Score | |
| 0–0.5 0.6–1.5 1.6–2.5 2.6–3.5 3.6–4.5 4.6–5.5 | 0 1 2 3 4 5 | 5.6–6.5 6.6–7.5 7.6–8.5 8.6–9.5 >9.5 | 6 7 8 9 10 | Inside urban polygon Outside urban polygon | 10 0 |
| Variable Name | Description (unit) | Source |
|---|---|---|
| Aspect | Slope direction (8 directions and flat) | Calculated from DEM (Worldclim) |
| Bio2 | Mean diurnal temperature range (mean of monthly mean daily values) (°C) | WorldClim |
| Bio3 | Isothermality (Bio2/Bio7 × 100%) | WorldClim |
| Bio12 | Annual precipitation (mm) | WorldClim |
| Bio13 | Precipitation of the wettest month (mm) | Worldclim |
| Bio14 | Precipitation of driest month (mm) | WorldClim |
| Bio16 | Precipitation of wettest quarter (mm) | WorldClim |
| Bio17 | Precipitation of driest quarter (mm) | WorldClim |
| Bio19 | Precipitation of coldest quarter (mm) | WorldClim |
| CEC | Soil cation exchange capacity (cmolc/kg) | Soil map of Kenya |
| Distrivers | Distance to rivers (km) | Calculated from HydroSHEDS layer |
| Elevation | Height above sea level (m) | WorldClim |
| ExchNa | Soil exchangeable sodium (cmolc/kg) | Soil map of Kenya |
| HII | Human influence index | NASA socioeconomic data and application center (SEDAC) |
| Landform | Features that make up the land surface (14 classes) | Soil map of Kenya |
| PD | Human population density (persons/km2) | NASA gridded population density |
| Rdensity | Road density (km/km2) | Calculated from Kenya road layer |
| Slope | The degree of inclination (decimal degrees) | Calculated from DEM (Worldclim) |
| Predictor | Species | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| I. arrec. | C. axilla | C. goodii. | S. brevis. | S. mellif. | S. seneg. | V. etbaic. | V. hockii | V. nilotic. | |
| Aspect | – | – | – | – | – | 0.0 | – | – | – |
| Bio2 | – | 38.5 | – | – | – | – | – | – | – |
| Bio3 | – | – | – | 13.4 | – | – | – | – | – |
| Bio12 | 0.0 | – | – | – | – | – | – | – | 37.2 |
| Bio13 | 3.6 | 32.4 | – | – | – | – | – | – | – |
| Bio14 | – | – | – | – | 16.3 | – | – | – | – |
| Bio16 | 1.7 | – | – | – | – | – | – | – | – |
| Bio17 | – | – | – | – | – | – | 0 | – | – |
| Bio19 | – | – | 52.2 | – | – | – | – | – | – |
| CEC | – | – | – | – | 17.9 | – | – | – | – |
| Distrivers | – | – | – | – | 41.6 | – | 58.9 | – | 5.5 |
| Elevation | – | – | – | – | – | – | 24.9 | 12.0 | 11.5 |
| ExchNa | – | – | – | – | 3.9 | – | – | – | – |
| HII | – | – | 9.4 | 82.1 | 18.9 | 94.9 | 13.2 | 59.1 | – |
| Landform | 94.7 | 23.2 | 38.4 | – | – | – | – | – | – |
| PD | 0.0 | 0.0 | 0.0 | 1.4 | – | – | 3.0 | 0.0 | 45.8 |
| Rdensity | – | 5.9 | – | – | – | 5.1 | – | 3.1 | – |
| Slope | 0.0 | – | – | 3.1 | 1.4 | – | – | 25.7 | – |
| AUC | 0.94 | 0.95 | 0.93 | 0.82 | 0.83 | 0.94 | 0.77 | 0.82 | 0.95 |
| CBI | 0.85 | 0.45 | 0.85 | 0.83 | 0.70 | 0.90 | 0.80 | 0.65 | 0.78 |
| Species | Threshold Pi at Max Sens. + Spec. | Habitat Range (km2) | ||||
|---|---|---|---|---|---|---|
| Present | In 2050 | |||||
| RCP4.5 | RCP4.5+ SSP1 | RCP4.5+ SSP2 | RCP4.5+ SSP3 | |||
| I. arrecta | 0.09 | 117,296 | 111,154 | ← | ← | ← |
| 75,117 | 69,620 | |||||
| C. axillaris | 0.31 | 37,511 | 21,715 | ← | ← | ← |
| 28,218 | 19,923 | |||||
| C. goodiiformis | 0.08 | 70,167 | 71,289 | 71,164 | 71,289 | 71,761 |
| 39,565 | 38,871 | 38,762 | 38,871 | 39,184 | ||
| S. brevispica | 0.41 | 64,018 | 69,515 | 75,709 | 77,500 | 81,640 |
| 63,261 | 69,195 | 75,270 | 77,049 | 81,140 | ||
| S. mellifera | 0.10 | 139,782 | 144,291 | 144,927 | 145,329 | 146,105 |
| 138,110 | 142,101 | 142,714 | 143,112 | 143,859 | ||
| S. Senegal | 0.73 | 76,897 | ← | 78,230 | 78,958 | 80,599 |
| 72,352 | 73,686 | 74,409 | 76,007 | |||
| V. etbaica | 0.13 | 239,066 | 239,530 | 244,055 | 245,058 | 246,817 |
| 168,143 | 168,248 | 172,276 | 173,105 | 174,780 | ||
| V. hockii | 0.03 | 171,484 | ← | 172,230 | 172,533 | 173,125 |
| 159,996 | 160,450 | 160,686 | 161,005 | |||
| V. nilotica | 0.44 | 115,545 | 119,552 | 145,650 | 149,882 | 161,890 |
| 114,172 | 118,234 | 143,817 | 147,847 | 158,471 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyairo, R.; Machimura, T. Potential Effects of Climate and Human Influence Changes on Range and Diversity of Nine Fabaceae Species and Implications for Nature’s Contribution to People in Kenya. Climate 2020, 8, 109. https://doi.org/10.3390/cli8100109
Nyairo R, Machimura T. Potential Effects of Climate and Human Influence Changes on Range and Diversity of Nine Fabaceae Species and Implications for Nature’s Contribution to People in Kenya. Climate. 2020; 8(10):109. https://doi.org/10.3390/cli8100109
Chicago/Turabian StyleNyairo, Risper, and Takashi Machimura. 2020. "Potential Effects of Climate and Human Influence Changes on Range and Diversity of Nine Fabaceae Species and Implications for Nature’s Contribution to People in Kenya" Climate 8, no. 10: 109. https://doi.org/10.3390/cli8100109
APA StyleNyairo, R., & Machimura, T. (2020). Potential Effects of Climate and Human Influence Changes on Range and Diversity of Nine Fabaceae Species and Implications for Nature’s Contribution to People in Kenya. Climate, 8(10), 109. https://doi.org/10.3390/cli8100109





