Gains or Losses in Forest Productivity under Climate Change? The Uncertainty of CO2 Fertilization and Climate Effects
Abstract
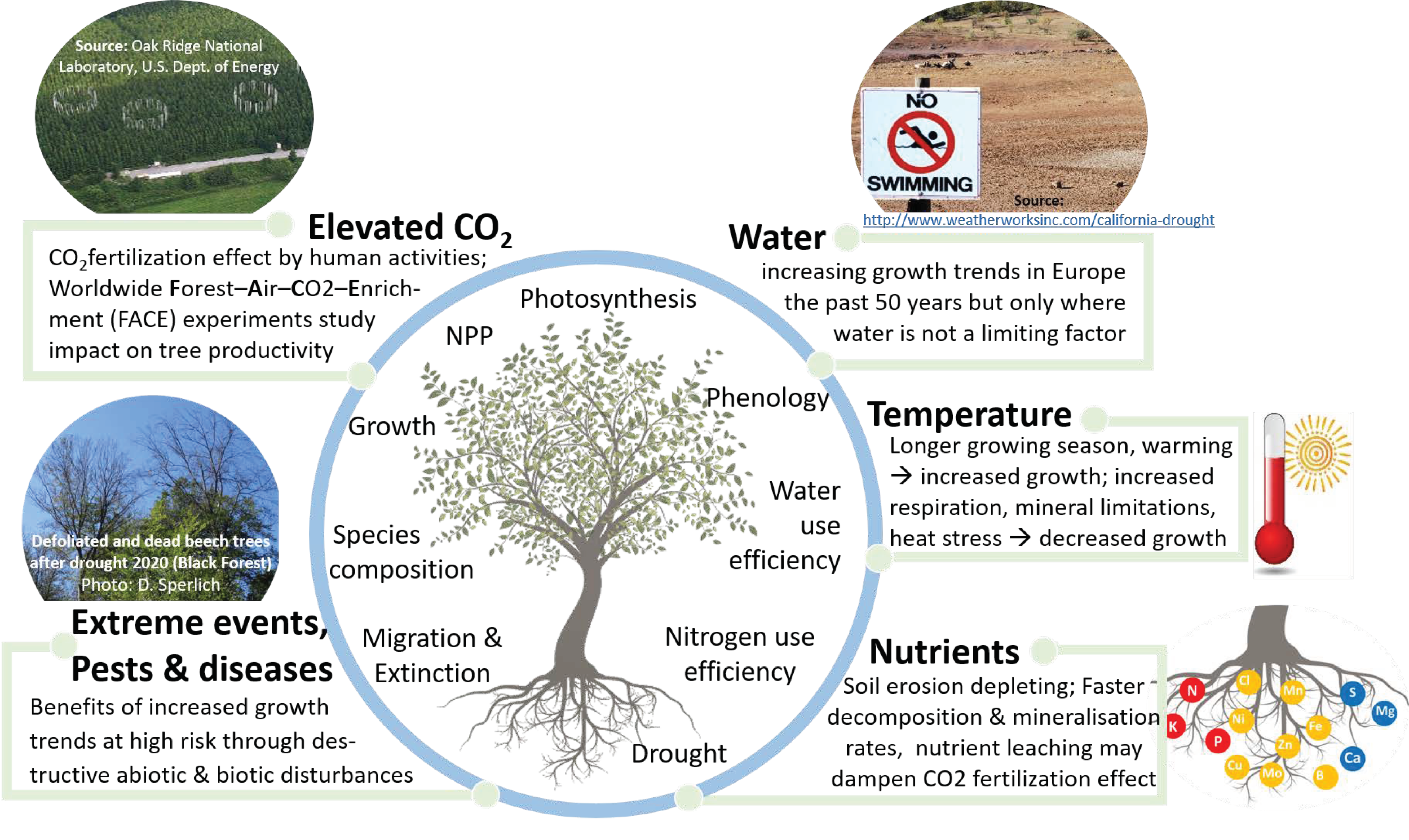
:1. Introduction
2. Material and Methods
2.1. The Biogeochemical Forest Growth Model GOTILWA+
2.2. Parametrization with Ecophysiological Field Data
2.3. Climate Data and Future Climate Scenarios
2.4. Management Regime
2.5. Validity of Simulation Results
3. Results
3.1. Effect of Species, Season and Leaf Position on Key Ecophysiological Model Parameters
3.2. Drought Trends in the Baseline Climate Data and Climate Scenarios
3.3. Productivity of Beech and Fir in the Reference Scenario and the Climate Change Scenarios
3.4. Climate Change’s Impacts on Net Primary Productivity
3.5. The Effect of CO2 Fertilization on Productivity
3.6. Accounting for Photosynthetic Downregulation as a Response to eCO2
3.7. Climate Change’s Impacts on Standing Volume, Basal Area and Mortality
3.8. The CO2 Fertilization and Climate Effects on Water-Use Efficiency
4. Discussion
4.1. Productivity Gains or Losses? The Uncertainty of the CO2 Fertilization Effect
4.2. Climate-Driven Mortality Increased, but at a Low Rate
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spiecker, H. Overview of Recent Growth Trends in European Forests. Water Air Soil Pollut. 1999, 116, 33–46. [Google Scholar] [CrossRef]
- Kahle, H.-P.; Karjalainen, T.; Schuck, A.; Ågren, G.I.; Kellomäki, S.; Mellert, K.H.; Prietzel, J.; Rehfuess, K.E.; Spiecker, H. Causes and Consequences of Forest Growth Trends in Europe–Results of the RECOGNITION Project; EFI Research Report 21; Brill: Leiden, The Netherlands, 2008; ISBN 9789004167056. [Google Scholar]
- Bravo-Oviedo, A.; Pretzsch, H.; Ammer, C.; Andenmatten, E.; Barbati, A.; Barreiro, S.; Brang, P.; Bravo, F.; Coll, L.; Corona, P.; et al. European Mixed Forests: Definition and research perspectives. For. Syst. 2014, 23, 518. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P.; Schütze, G.; Uhl, E.; Rötzer, T. Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat. Commun. 2014, 5, 4967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boisvenue, C.; Running, S.W. Impacts of climate change on natural forest productivity-Evidence since the middle of the 20th century. Glob. Chang. Biol. 2006, 12, 862–882. [Google Scholar] [CrossRef]
- Lindner, M.; Fitzgerald, J.B.; Zimmermann, N.E.; Reyer, C.; Delzon, S.; van der Maaten, E.; Schelhaas, M.J.; Lasch, P.; Eggers, J.; van der Maaten-Theunissen, M.; et al. Climate change and European forests: What do we know, what are the uncertainties, and what are the implications for forest management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef] [Green Version]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H. (Ted) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Jump, A.S.; Penuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Kane, J.M.; Anderegg, L.D.L. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Jump, A.S.; Hunt, J.M.; Peñuelas, J. Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Chang. Biol. 2006, 12, 2163–2174. [Google Scholar] [CrossRef] [Green Version]
- Liang, P.; Wang, X.; Sun, H.; Fan, Y.; Wu, Y.; Lin, X.; Chang, J. Forest type and height are important in shaping the altitudinal change of radial growth response to climate change. Sci. Rep. 2019, 9, 1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraus, C.; Zang, C.; Menzel, A. Elevational response in leaf and xylem phenology reveals different prolongation of growing period of common beech and Norway spruce under warming conditions in the Bavarian Alps. Eur. J. For. Res. 2016, 135, 1011–1023. [Google Scholar] [CrossRef]
- Bigler, C.; Bugmann, H. Climate-induced shifts in leaf unfolding and frost risk of European trees and shrubs. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Norby, R.J.; DeLucia, E.H.; Gielen, B.; Calfapietra, C.; Giardina, C.P.; Kings, J.S.; Ledford, J.; McCarthy, H.R.; Moore, D.J.P.; Ceulemans, R.; et al. Forest response to elevated CO2 is conserved across a broad range of productivity. Proc. Natl. Acad. Sci. USA 2005, 102, 18052–18056. [Google Scholar] [CrossRef] [Green Version]
- Flexas, J.; Loreto, F.; Medrano, H. Terrestrial Photosynthesis in a Changing Environment-A Molecular, Physiological and Ecological Approach; Flexas, J., Loretto, F., Medrano, H., Eds.; Cambridge University Press: Cambridge, UK, 2012; ISBN 978-0-521-89941-3. [Google Scholar]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. (Eds.) IPCC Summary for Policymakers. In Climate change 2013: The Physical Science Basis; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Kallarackal, J.; Roby, T.J. Responses of trees to elevated carbon dioxide and climate change. Biodivers. Conserv. 2012, 21, 1327–1342. [Google Scholar] [CrossRef]
- Reyer, C.; Gutsch, M.; Lasch, P. Simulated forest productivity and biomass changes under global change in Europe. In Proceedings of the COST FP0603 Spring School, Proceedings of the Modelling Forest Ecosystems-Concepts, Data and Application, Kaprun, Austria, 9–13 May 2011; Pötzelsberger, E., Mäkelä, A., Mohren, G., Palahí, M., Tomé, M., Hasenauer, H., Eds.; BOKU: Vienna, Austria, 2012; pp. 151–158. [Google Scholar]
- Reyer, C. Forest Productivity Under Environmental Change—A Review of Stand-Scale Modeling Studies. Curr. For. Rep. 2015, 1, 53–68. [Google Scholar] [CrossRef] [Green Version]
- Piao, S.; Sitch, S.; Ciais, P.; Friedlingstein, P.; Peylin, P.; Wang, X.; Ahlström, A.; Anav, A.; Canadell, J.G.; Cong, N.; et al. Evaluation of terrestrial carbon cycle models for their response to climate variability and to CO2 trends. Glob. Chang. Biol. 2013, 19, 2117–2132. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Piao, S.; Gasser, T.; Ciais, P.; Yang, H.; Wang, H.; Keenan, T.F.; Huang, M.; Wan, S.; Song, J.; et al. Field-experiment constraints on the enhancement of the terrestrial carbon sink by CO2 fertilization. Nat. Geosci. 2019, 12, 809–814. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Medlyn, B.E.; Drake, J.E.; Duursma, R.A.; Anderson, I.C.; Barton, C.V.M.; Boer, M.M.; Carrillo, Y.; Castañeda-Gómez, L.; Collins, L.; et al. The fate of carbon in a mature forest under carbon dioxide enrichment. Nature 2020, 580, 227–231. [Google Scholar] [CrossRef]
- Körner, C.; Asshoff, R.; Bignucolo, O.; Hättenschwiler, S.; Keel, S.G.; Peláez-Riedl, S.; Pepin, S.; Siegwolf, R.T.W.; Zotz, G. Carbon Flux and Growth in Mature Deciduous Forest Trees Exposed to Elevated CO2. Science 2005, 309, 1360–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaehle, S.; Friedlingstein, P.; Friend, A.D. Terrestrial nitrogen feedbacks may accelerate future climate change. Geophys. Res. Lett. 2010, 37, L01401. [Google Scholar] [CrossRef]
- Norby, R.J.; Zak, D.R. Ecological Lessons from Free-Air CO2 Enrichment (FACE) Experiments. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 181–203. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.P.; De Kauwe, M.G.; Medlyn, B.E.; Zaehle, S.; Iversen, C.M.; Asao, S.; Guenet, B.; Harper, A.; Hickler, T.; Hungate, B.A.; et al. Decadal biomass increment in early secondary succession woody ecosystems is increased by CO2 enrichment. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Trugman, A.T.; Anderegg, L.D.L.; Wolfe, B.T.; Birami, B.; Ruehr, N.K.; Detto, M.; Bartlett, M.K.; Anderegg, W.R.L. Climate and plant trait strategies determine tree carbon allocation to leaves and mediate future forest productivity. Glob. Chang. Biol. 2019, 25, 3395–3405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellsworth, D.S.; Anderson, I.C.; Crous, K.Y.; Cooke, J.; Drake, J.E.; Gherlenda, A.N.; Gimeno, T.E.; Macdonald, C.A.; Medlyn, B.E.; Powell, J.R.; et al. Elevated CO2 does not increase eucalypt forest productivity on a low-phosphorus soil. Nat. Clim. Chang. 2017, 7, 279–282. [Google Scholar] [CrossRef] [Green Version]
- Leuzinger, S.; Körner, C. Water savings in mature deciduous forest trees under elevated CO2. Glob. Chang. Biol. 2007, 13, 2498–2508. [Google Scholar] [CrossRef]
- Barton, C.V.M.; Duursma, R.A.; Medlyn, B.E.; Ellsworth, D.S.; Eamus, D.; Tissue, D.T.; Adams, M.A.; Conroy, J.; Crous, K.Y.; Liberloo, M.; et al. Effects of elevated atmospheric [CO2] on instantaneous transpiration efficiency at leaf and canopy scales in Eucalyptus saligna. Glob. Chang. Biol. 2012, 18, 585–595. [Google Scholar] [CrossRef]
- Keenan, T.F.; Hollinger, D.Y.; Bohrer, G.; Dragoni, D.; Munger, J.W.; Schmid, H.P.; Richardson, A.D. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 2013, 499, 324–327. [Google Scholar] [CrossRef]
- Field, C.B.; Jackson, R.B.; Mooney, H.A. Stomatal responses to increased CO2: Implications from the plant to the global scale. Plant Cell Environ. 1995, 18, 1214–1225. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Barton, C.V.M.; Broadmeadow, M.S.J.; Ceulemans, R.; De Angelis, P.; Forstreuter, M.; Freeman, M.; Jackson, S.B.; Kellomaki, S.; Laitat, E.; et al. Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: A synthesis. New Phytol. 2001, 149, 247–264. [Google Scholar] [CrossRef]
- Fatichi, S.; Leuzinger, S.; Paschalis, A.; Adam Langley, J.; Barraclough, A.D.; Hovenden, M.J. Partitioning direct and indirect effects reveals the response of water-limited ecosystems to elevated CO2. Proc. Natl. Acad. Sci. USA 2016, 113, 12757–12762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birami, B.; Nägele, T.; Gattmann, M.; Preisler, Y.; Gast, A.; Arneth, A.; Ruehr, N.K. Hot drought reduces the effects of elevated CO2 on tree water-use efficiency and carbon metabolism. New Phytol. 2020, 226, 1607–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, H.; Duursma, R.A.; Huang, G.; Smith, R.A.; Choat, B.; O’Grady, A.P.; Tissue, D.T. Elevated [CO2] does not ameliorate the negative effects of elevated temperature on drought-induced mortality in Eucalyptus radiata seedlings. Plant Cell Environ. 2014, 37, 1598–1613. [Google Scholar] [CrossRef] [PubMed]
- Metz, J.; Annighöfer, P.; Schall, P.; Zimmermann, J.; Kahl, T.; Schulze, E.-D.; Ammer, C. Site-adapted admixed tree species reduce drought susceptibility of mature European beech. Glob. Chang. Biol. 2016, 22, 903–920. [Google Scholar] [CrossRef] [PubMed]
- Bolte, A.; Ammer, C.; Löf, M.; Madsen, P.; Nabuurs, G.-J.; Schall, P.; Spathelf, P.; Rock, J. Adaptive forest management in central Europe: Climate change impacts, strategies and integrative concept. Scand. J. For. Res. 2009, 24, 473–482. [Google Scholar] [CrossRef]
- Geßler, A.; Keitel, C.; Kreuzwieser, U.; Matyssek, R.; Seiler, W.; Rennenberg, H.; Cochard, H.; Geßler, A.; Keitel, C.; Kreuzwieser, J.; et al. Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees 2007, 21, 1–11. [Google Scholar] [CrossRef]
- Tegel, W.; Seim, A.; Hakelberg, D.; Hoffmann, S.; Panev, M.; Westphal, T.; Büntgen, U. A recent growth increase of European beech (Fagus sylvatica L.) at its Mediterranean distribution limit contradicts drought stress. Eur. J. For. Res. 2014, 133, 61–71. [Google Scholar] [CrossRef]
- Valladares, F. A Mechanistic View of the Capacity of Forests to Cope with Climate Change. In Managing Forest Ecosystems: The Challenge of Climate Change; Bravo, F., Jandl, R., LeMay, V., Gadow, K., Eds.; Managing Forest Ecosystems; Springer: Dordrecht, The Netherlands, 2008; Volume 17, pp. 15–40. ISBN 978-1-4020-8342-6. [Google Scholar]
- Büntgen, U.; Tegel, W.; Kaplan, J.O.; Schaub, M.; Hagedorn, F.; Bürgi, M.; Brázdil, R.; Helle, G.; Carrer, M.; Heussner, K.-U.; et al. Placing unprecedented recent fir growth in a European-wide and Holocene-long context. Front. Ecol. Environ. 2014, 12, 100–106. [Google Scholar] [CrossRef]
- FVA-BW. Waldzustandsbericht 2019; FVA: Freiburg, Germany, 2019. [Google Scholar]
- Kramer, K.; Leinonen, I.; Bartelink, H.H.; Berbigier, P.; Borghetti, M.; Bernhofer, C.; Cienciala, E.; Dolman, A.J.; Froer, O.; Gracia, C.A.; et al. Evaluation of six process-based forest growth models using eddy-covariance measurements of CO2 and H2O fluxes at six forest sites in Europe. Glob. Chang. Biol. 2002, 8, 213–230. [Google Scholar] [CrossRef]
- Morales, P.; Sykes, M.T.; Prentice, I.C.; Smith, P.; Smith, B.; Bugmann, H.; Zierl, B.; Friedlingstein, P.; Viovy, N.; Sabate, S.; et al. Comparing and evaluating process-based ecosystem model predictions of carbon and water fluxes in major European forest biomes. Glob. Chang. Biol. 2005, 11, 2211–2233. [Google Scholar] [CrossRef]
- Keenan, T.; García, R.; Friend, A.D.; Zaehle, S.; Gracia, C.; Sabate, S. Improved understanding of drought controls on seasonal variation in Mediterranean forest canopy CO2 and water fluxes through combined in situ measurements and ecosystem modelling. Biogeosciences 2009, 6, 2285–2329. [Google Scholar] [CrossRef] [Green Version]
- Nadal-Sala, D.; Hartig, F.; Gracia, C.A.; Sabaté, S. Global warming likely to enhance black locust (Robinia pseudoacacia L.) growth in a Mediterranean riparian forest. For. Ecol. Manag. 2019, 449, 117448. [Google Scholar] [CrossRef]
- Sabaté, S.; Gracia, A.C.; Sánchez, A. Likely effects of climate change on growth of Quercus ilex, Pinus halepensis, Pinus pinaster, Pinus sylvestris and Fagus sylvatica forests in the Mediterranean region. For. Ecol. Manag. 2002, 162, 23–37. [Google Scholar] [CrossRef]
- Drake, J.E.; Power, S.A.; Duursma, R.A.; Medlyn, B.E.; Aspinwall, M.J.; Choat, B.; Creek, D.; Eamus, D.; Maier, C.; Pfautsch, S.; et al. Stomatal and non-stomatal limitations of photosynthesis for four tree species under drought: A comparison of model formulations. Agric. For. Meteorol. 2017, 247, 454–466. [Google Scholar] [CrossRef]
- Nadal-Sala, D.; Keenan, T.F.; Sabaté, S.; Gracia, C. Forest eco-physiological models: Water use and carbon sequestration. In Managing Forest Ecosystems: The Challenge of Climate Change; Bravo, F., LeMay, V., Jandl, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 81–102. ISBN 9783319282503. [Google Scholar]
- Bugmann, H.; Seidl, R.; Hartig, F.; Bohn, F.; Brůna, J.; Cailleret, M.; François, L.; Heinke, J.; Henrot, A.-J.; Hickler, T.; et al. Tree mortality submodels drive simulated long-term forest dynamics: Assessing 15 models from the stand to global scale. Ecosphere 2019, 10, e02616. [Google Scholar] [CrossRef] [Green Version]
- Eller, C.B.; Rowland, L.; Mencuccini, M.; Rosas, T.; Williams, K.; Harper, A.; Medlyn, B.E.; Wagner, Y.; Klein, T.; Teodoro, G.S.; et al. Stomatal optimization based on xylem hydraulics (SOX) improves land surface model simulation of vegetation responses to climate. New Phytol. 2020, 226, 1622–1637. [Google Scholar] [CrossRef] [PubMed]
- Magh, R.-K.; Grün, M.; Knothe, V.E.; Stubenazy, T.; Tejedor, J.; Dannenmann, M.; Rennenberg, H. Silver-fir (Abies alba MILL.) neighbors improve water relations of European beech (Fagus sylvatica L.), but do not affect N nutrition. Trees 2017, 32, 337–348. [Google Scholar] [CrossRef]
- Sperlich, D.; Barbeta, A.; Ogaya, R.; Sabate, S.; Penuelas, J. Balance between carbon gain and loss under long-term drought: Impacts on foliar respiration and photosynthesis in Quercus ilex L. J. Exp. Bot. 2016, 67, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Sperlich, D.; Chang, C.T.; Peñuelas, J.; Gracia, C.; Sabaté, S. Seasonal variability of foliar photosynthetic and morphological traits and drought impacts in a Mediterranean mixed forest. Tree Physiol. 2015, 35, 501–520. [Google Scholar] [CrossRef]
- Sperlich, D.; Chang, C.T.; Peñuelas, J.; Sabaté, S. Responses of photosynthesis and component processes to drought and temperature stress: Are Mediterranean trees fit for climate change? Tree Physiol. 2019, 39, 1783–1805. [Google Scholar] [CrossRef] [PubMed]
- Breda, N.J.J. Ground-based measurements of leaf area index: A review of methods, instruments and current controversies. J. Exp. Bot. 2003, 54, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Pokorný, R.; Stojnič, S. Leaf area index of Norway spruce stands in relation to age and defoliation. Beskydy 2012, 5, 173–180. [Google Scholar] [CrossRef]
- Pokorný, R.; Tomášková, I.; Havránková, K. Temporal variation and efficiency of leaf area index in young mountain Norway spruce stand. Eur. J. For. Res. 2008, 127, 359–367. [Google Scholar] [CrossRef]
- Nadal-Sala, D.; Gracia, C.; Sabaté, S. The RheaG Weather Generator Algorithm: Evaluation in Four Contrasting Climates from the Iberian Peninsula. J. Appl. Meteorol. Climatol. 2019, 58, 55–69. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multiscalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef] [Green Version]
- ESRL Earth System Research Laboratory-Global Greenhouse Gas Reference Network. Available online: https://www.esrl.noaa.gov/gmd/ccgg/trends/ (accessed on 28 May 2018).
- LFBW Richtlinie Landesweiter Waldentwicklungstypen; LFBW: 2014. Available online: www.forstbw.de/fileadmin/forstbw_infothek/forstbw_praxis/wet/ForstBW_Waldentwicklung_web.pdf (accessed on 30 November 2020).
- Klädtke, J.; Abetz, P. Die Durchforstungshilfe 2010–Eine Entscheidungshilfe für die Praxis; FVA: Freiburg, Germany, 2010. [Google Scholar]
- Bösch, B. Neue Bonitierungs- und Zuwachshilfen. In Schriftenreihe Freiburger Forstliche Forschung, Wissenstransfer in Praxis und Gesellschaft, FVA-Forschungstage, Band 18; FVA Baden-Württemberg: Freiburg, Germany, 2001. [Google Scholar]
- Niinemets, U.; Flexas, J.; Peñuelas, J. Evergreens favored by higher responsiveness to increased CO2. Trends Ecol. Evol. 2011, 26, 136–142. [Google Scholar] [CrossRef]
- Schwalm, C.R.; Glendon, S.; Duffy, P.B. RCP8.5 tracks cumulative CO2 emissions. Proc. Natl. Acad. Sci. USA 2020, 117, 19656–19657. [Google Scholar] [CrossRef]
- Fu, Y.H.; Zhao, H.; Piao, S.; Peaucelle, M.; Peng, S.; Zhou, G.; Ciais, P.; Huang, M.; Menzel, A.; Peñuelas, J.; et al. Declining global warming effects on the phenology of spring leaf unfolding. Nature 2015, 526, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant phenology and global climate change: Current progresses and challenges. Glob. Chang. Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef] [PubMed]
- Hickler, T.; Rammig, A.; Werner, C. Modelling CO2 impacts on forest productivity. Curr. For. Reports 2015, 1, 69–80. [Google Scholar] [CrossRef]
- Reyer, C.; Lasch-Born, P.; Suckow, F.; Gutsch, M.; Murawski, A.; Pilz, T. Projections of regional changes in forest net primary productivity for different tree species in Europe driven by climate change and carbon dioxide. Ann. For. Sci. 2014, 71, 211–225. [Google Scholar] [CrossRef]
- Keenan, T.; Maria Serra, J.; Lloret, F.; Ninyerola, M.; Sabate, S. Predicting the future of forests in the Mediterranean under climate change, with niche- and process-based models: CO2 matters! Glob. Chang. Biol. 2011, 17, 565–579. [Google Scholar] [CrossRef]
- Keenan, T.; Sabate, S.; Gracia, C. Soil water stress and coupled photosynthesis–conductance models: Bridging the gap between conflicting reports on the relative roles of stomatal, mesophyll conductance and biochemical limitations to photosynthesis. Agric. For. Meteorol. 2010, 150, 443–453. [Google Scholar] [CrossRef]
- Cramer, W.; Bondeau, A.; Woodward, F.I.; Prentice, I.C.; Betts, R.A.; Brovkin, V.; Cox, P.M.; Fisher, V.; Foley, J.A.; Friend, A.D.; et al. Global response of terrestrial ecosystem structure and function to CO2 and climate change: Results from six dynamic global vegetation models. Glob. Chang. Biol. 2001, 7, 357–373. [Google Scholar] [CrossRef] [Green Version]
- Sitch, S.; Huntingford, C.; Gedney, N.; Levy, P.E.; Lomas, M.; Piao, S.L.; Betts, R.; Ciais, P.; Cox, P.; Friedlingstein, P.; et al. Evaluation of the terrestrial carbon cycle, future plant geography and climate-carbon cycle feedbacks using five Dynamic Global Vegetation Models (DGVMs). Glob. Chang. Biol. 2008, 14, 2015–2039. [Google Scholar] [CrossRef]
- Hickler, T.; Smith, B.; Prentice, I.C.; Mjöfors, K.; Miller, P.; Arneth, A.; Sykes, M.T. CO2 fertilization in temperate FACE experiments not representative of boreal and tropical forests. Glob. Chang. Biol. 2008, 14, 1531–1542. [Google Scholar] [CrossRef]
- Zaehle, S.; Medlyn, B.E.; De Kauwe, M.G.; Walker, A.P.; Dietze, M.C.; Hickler, T.; Luo, Y.; Wang, Y.P.; El-Masri, B.; Thornton, P.; et al. Evaluation of 11 terrestrial carbon-nitrogen cycle models against observations from two temperate Free-Air CO2 Enrichment studies. New Phytol. 2014, 202, 803–822. [Google Scholar] [CrossRef] [Green Version]
- Norby, R.J.; Warren, J.M.; Iversen, C.M.; Medlyn, B.E.; McMurtrie, R.E. CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc. Natl. Acad. Sci. USA 2010, 107, 19368–19373. [Google Scholar] [CrossRef] [Green Version]
- Dannenmann, M.; Bimüller, C.; Gschwendtner, S.; Leberecht, M.; Tejedor, J.; Bilela, S.; Gasche, R.; Hanewinkel, M.; Baltensweiler, A.; Kögel-Knabner, I.; et al. Climate Change Impairs Nitrogen Cycling in European Beech Forests. PLoS ONE 2016, 11, e0158823. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, T.E.; McVicar, T.R.; O’Grady, A.P.; Tissue, D.T.; Ellsworth, D.S. Elevated CO2 did not affect the hydrological balance of a mature native Eucalyptus woodland. Glob. Chang. Biol. 2018, 24, 3010–3024. [Google Scholar] [CrossRef] [PubMed]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.G.; Scullion, J.; Ostle, N.; Levy, P.E.; Gwynn-Jones, D. Completing the FACE of elevated CO2 research. Environ. Int. 2014, 73, 252–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breshears, D.D.; Myers, O.B.; Meyer, C.W.; Barnes, F.J.; Zou, C.B.; Allen, C.D.; McDowell, N.G.; Pockman, W.T. Tree die-off in response to global change-type drought: Mortality insights from a decade of plant water potential measurements. Front. Ecol. Environ. 2009, 7, 185–189. [Google Scholar] [CrossRef] [Green Version]
- McDowell, N.G.; Beerling, D.J.; Breshears, D.D.; Fisher, R.A.; Raffa, K.F.; Stitt, M. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol. Evol. 2011, 26, 523–532. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Pretzsch, H.; Biber, P.; Ďurský, J. The single tree-based stand simulator SILVA: Construction, application and evaluation. For. Ecol. Manag. 2002, 162, 3–21. [Google Scholar] [CrossRef]
- Yoda, K.; Kira, T.; Ogawa, H.; Hozumi, K. Self-Thinning in Over-Crowded Pure Stands under Cultivated and Natural Conditions (Intraspecific Competition among Higher Plants XI). J. Inst. Polytech. Osaka City Univ. 1963, 14, 107–129. [Google Scholar]
- Hickler, T.; Vohland, K.; Feehan, J.; Miller, P.A.; Smith, B.; Costa, L.; Giesecke, T.; Fronzek, S.; Carter, T.R.; Cramer, W.; et al. Projecting the future distribution of European potential natural vegetation zones with a generalized, tree species-based dynamic vegetation model. Glob. Ecol. Biogeogr. 2012, 21, 50–63. [Google Scholar] [CrossRef]
- Merganičová, K.; Merganič, J.; Lehtonen, A.; Vacchiano, G.; Sever, M.Z.O.; Augustynczik, A.L.D.; Grote, R.; Kyselová, I.; Mäkelä, A.; Yousefpour, R.; et al. Forest carbon allocation modelling under climate change. Tree Physiol. 2019, 39, 1937–1960. [Google Scholar] [CrossRef] [PubMed]
- FVA-BW. Waldzustandsbericht 2018; FVA: Freiburg, Germany, 2018. [Google Scholar]
- Reyer, C.P.O.; Bathgate, S.; Blennow, K.; Borges, J.G.; Bugmann, H.; Delzon, S.; Faias, S.P.; Garcia-Gonzalo, J.; Gardiner, B.; Gonzalez-Olabarria, J.R.; et al. Are forest disturbances amplifying or canceling out climate change-induced productivity changes in European forests? Environ. Res. Lett. 2017, 12, 034027. [Google Scholar] [CrossRef] [PubMed]






| European Beech | Silver Fir | |||
|---|---|---|---|---|
| Freiamt Site | Simulated Stand | Freiamt Site | Simulated Stand | |
| N/ha | 404 (65%) | 214 (35%) | ||
| N/ha | 618 1 (100%) | 623 (100%) | 618 1 (100%) | 623 (100%) |
| DBH | 22.0 | 23.0 | 29.6 | 25.0 |
| H100 | 23.6 | 25.0 | 24.9 | 27.0 |
| MAI100 | 10.0 | 10.2 | 18.0 | 17.3 |
| age | 55 | 50 | 45 | 50 |
| (a) | ||||||
| Scenario | CO2 Base | CO2 Increase | PD | T Increase | P Decrease | P Factor |
| ppm | % year−1 | % | °C/10 years | %/100 years | - | |
| noCC | 370 | 0 | 0 | 0 | 0 | 0 |
| RCP2.6 | 370 | 0.21 | 0 | 0.18 | −24.8 | 5 |
| RCP4.5 | 370 | 1.38 | 0 | 0.25 | −23.5 | 5 |
| RCP8.5 | 370 | 5.36 | 0 | 0.44 | −26.4 | 10 |
| RCP2.6-CO2 | 370 | 0 | 0 | 0.18 | −24.8 | 5 |
| RCP4.5-CO2 | 370 | 0 | 0 | 0.25 | −23.5 | 5 |
| RCP8.5-CO2 | 370 | 0 | 0 | 0.44 | −26.4 | 10 |
| RCP8.5_PD100 | 370 | 5.36 | 100 | 0.44 | −26.4 | 10 |
| RCP8.5_PD75 | 370 | 5.36 | 75 | 0.44 | −26.4 | 10 |
| RCP8.5_PD50 | 370 | 5.36 | 50 | 0.44 | −26.4 | 10 |
| RCP8.5_PD25 | 370 | 5.36 | 25 | 0.44 | −26.4 | 10 |
| (b) | ||||||
| RCP2.6 | RCP4.5 | RCP8.5 | ||||
| Month | T in °C/100 year | P %/100 year | T in °C/100 year | P %/100 year | T in °C/100 year | P %/100 year |
| 1 | 1.61 | 14 | 2.47 | 14 | 4.04 | 16 |
| 2 | 1.01 | −22 | 1.29 | −24 | 3.29 | −13 |
| 3 | 0.35 | −13 | 0.56 | −4 | 1.64 | −4 |
| 4 | 2 | −20 | 2.07 | −4 | 3.21 | −5 |
| 5 | 1.01 | −23 | 1.87 | −32 | 3.37 | −29 |
| 6 | 2.46 | −20 | 2.96 | −15 | 5.31 | −35 |
| 7 | 2.79 | −53 | 3.86 | −63 | 6.14 | −64 |
| 8 | 1.96 | −7 | 3.11 | −19 | 6.18 | −32 |
| 9 | 3.15 | −44 | 4.01 | −47 | 6.86 | −62 |
| 10 | 1.74 | −50 | 1.96 | −38 | 4.74 | −43 |
| 11 | 2.41 | −51 | 3.26 | −39 | 5.34 | −37 |
| 12 | 1.36 | −9 | 1.94 | −11 | 3.15 | −9 |
| Tree Species | Leaf Position | n | Anet | gs | Ci | gm | Cc | LMA | Rn | Rd | Vc,max | Jmax | TPU | LAI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F. sylcatica | sun | 38 | 7.86 | 0.112 | 267 | 0.054 | 151 | 5.8 | 0.49 | 0.22 | 37.9 | 60.7 | 10.1 | 8.4 |
| SE | 0.53 | 0.010 | 7 | 0.006 | 28 | 0.3 | 0.04 | 0.03 | 2.9 | 5.2 | 2.5 | 0.2 | ||
| A. alba | Sun | 12 | 9.80 | 0.154 | 269 | 0.034 | 85 | 19.2 | 1.71 | 0.88 | 47.1 | 115.2 | 7.5 | - |
| SE | 1.67 | 0.027 | 9 | 0.009 | 20 | 1.6 | 0.26 | 0.14 | 6.6 | 13.4 | 0.7 | - | ||
| A. alba | shade | 12 | 5.80 | 0.066 | 214 | 0.063 | 76 | 11.3 | 2.63 | 0.29 | 31.4 | 73.9 | 4.4 | - |
| SE | 0.52 | 0.009 | 10 | 0.024 | 19 | 0.5 | 2.09 | 0.10 | 2.8 | 11.0 | 0.5 | - | ||
| A. alba | all | 24 | 7.80 | 0.110 | 241 | 0.047 | 81 | 15.2 | 2.17 | 0.57 | 39.3 | 94.6 | 6.0 | 14.9 |
| SE | 0.95 | 0.016 | 9 | 0.015 | 8 | 1.2 | 1.03 | 0.11 | 3.9 | 9.5 | 0.5 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sperlich, D.; Nadal-Sala, D.; Gracia, C.; Kreuzwieser, J.; Hanewinkel, M.; Yousefpour, R. Gains or Losses in Forest Productivity under Climate Change? The Uncertainty of CO2 Fertilization and Climate Effects. Climate 2020, 8, 141. https://doi.org/10.3390/cli8120141
Sperlich D, Nadal-Sala D, Gracia C, Kreuzwieser J, Hanewinkel M, Yousefpour R. Gains or Losses in Forest Productivity under Climate Change? The Uncertainty of CO2 Fertilization and Climate Effects. Climate. 2020; 8(12):141. https://doi.org/10.3390/cli8120141
Chicago/Turabian StyleSperlich, Dominik, Daniel Nadal-Sala, Carlos Gracia, Jürgen Kreuzwieser, Marc Hanewinkel, and Rasoul Yousefpour. 2020. "Gains or Losses in Forest Productivity under Climate Change? The Uncertainty of CO2 Fertilization and Climate Effects" Climate 8, no. 12: 141. https://doi.org/10.3390/cli8120141
APA StyleSperlich, D., Nadal-Sala, D., Gracia, C., Kreuzwieser, J., Hanewinkel, M., & Yousefpour, R. (2020). Gains or Losses in Forest Productivity under Climate Change? The Uncertainty of CO2 Fertilization and Climate Effects. Climate, 8(12), 141. https://doi.org/10.3390/cli8120141









