Reactor Structure for the Decomposition of ADN-Based Monopropellant
Abstract
:1. Introduction
2. Materials and Methods
2.1. ADN-Based Monopropellant
2.2. Catalyst
2.3. Reactor Design
2.4. Experimental Setup
3. Results and Discussion
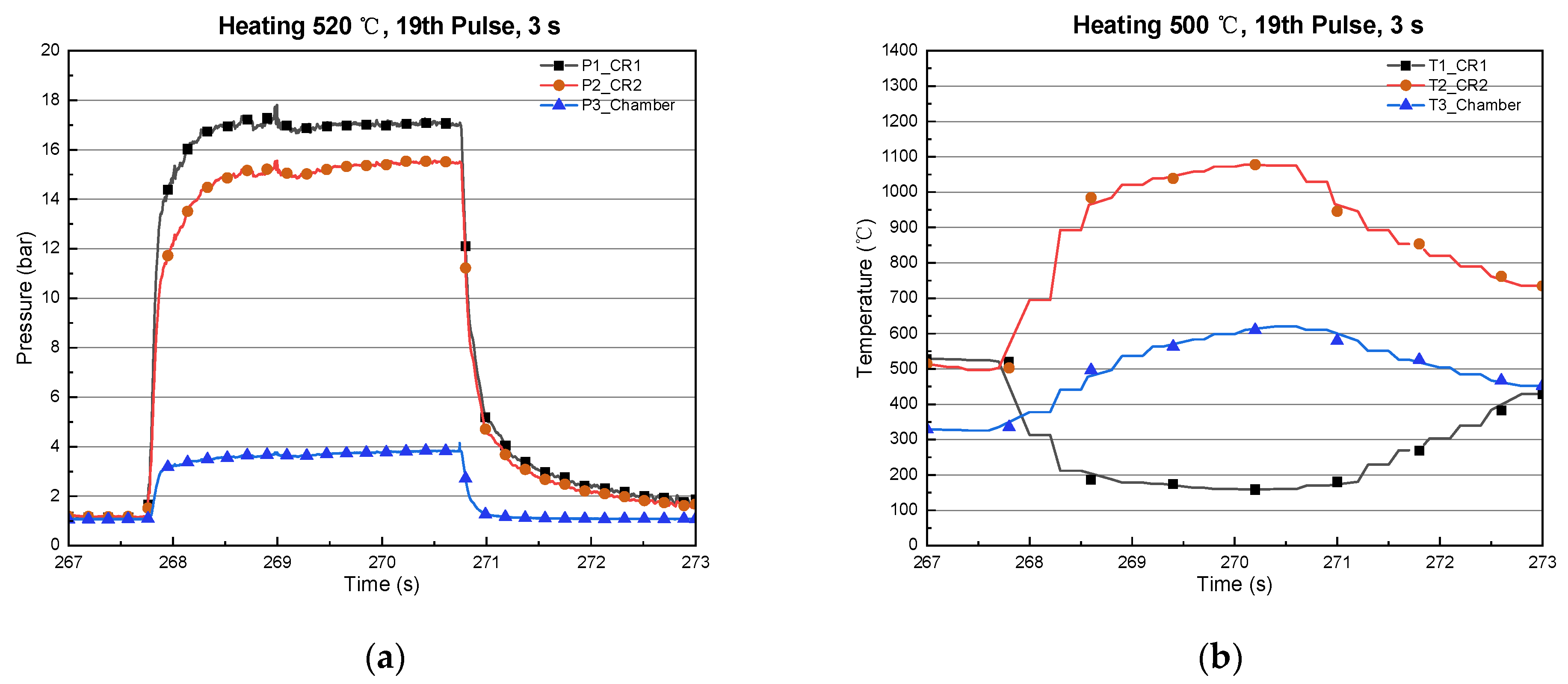
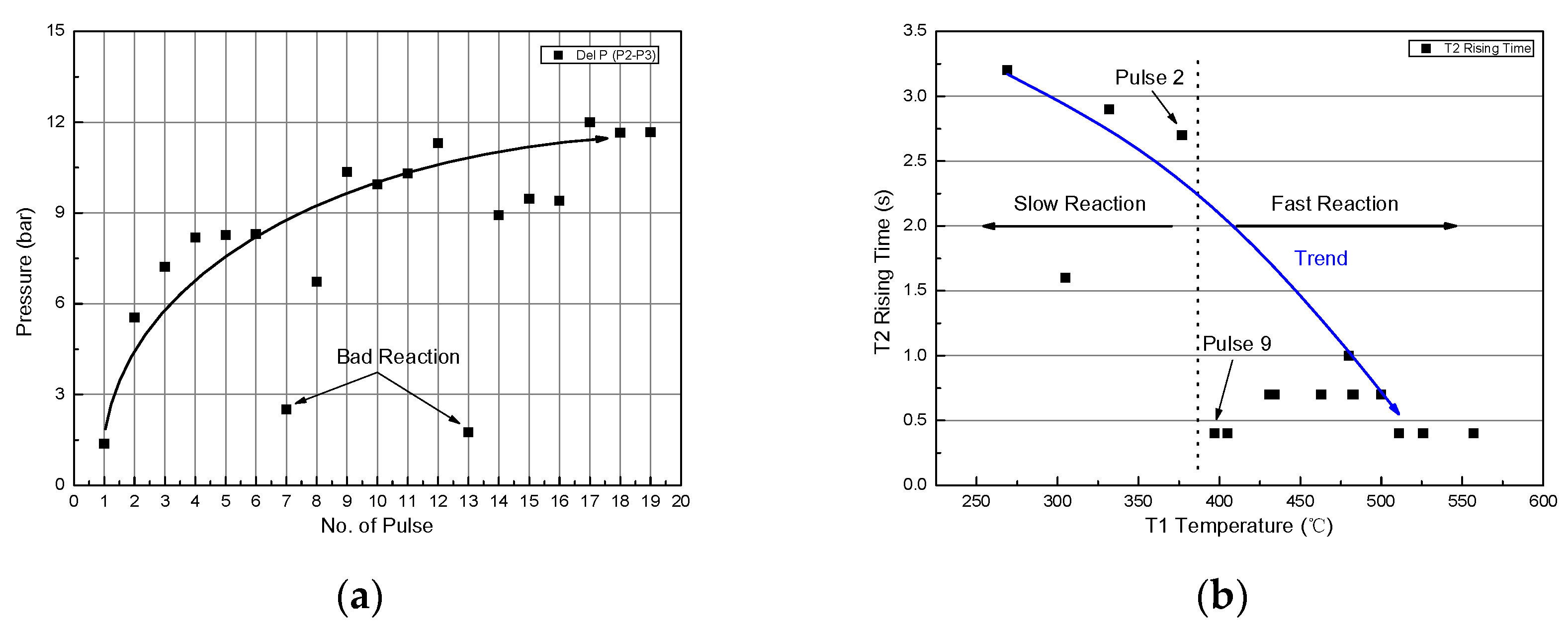
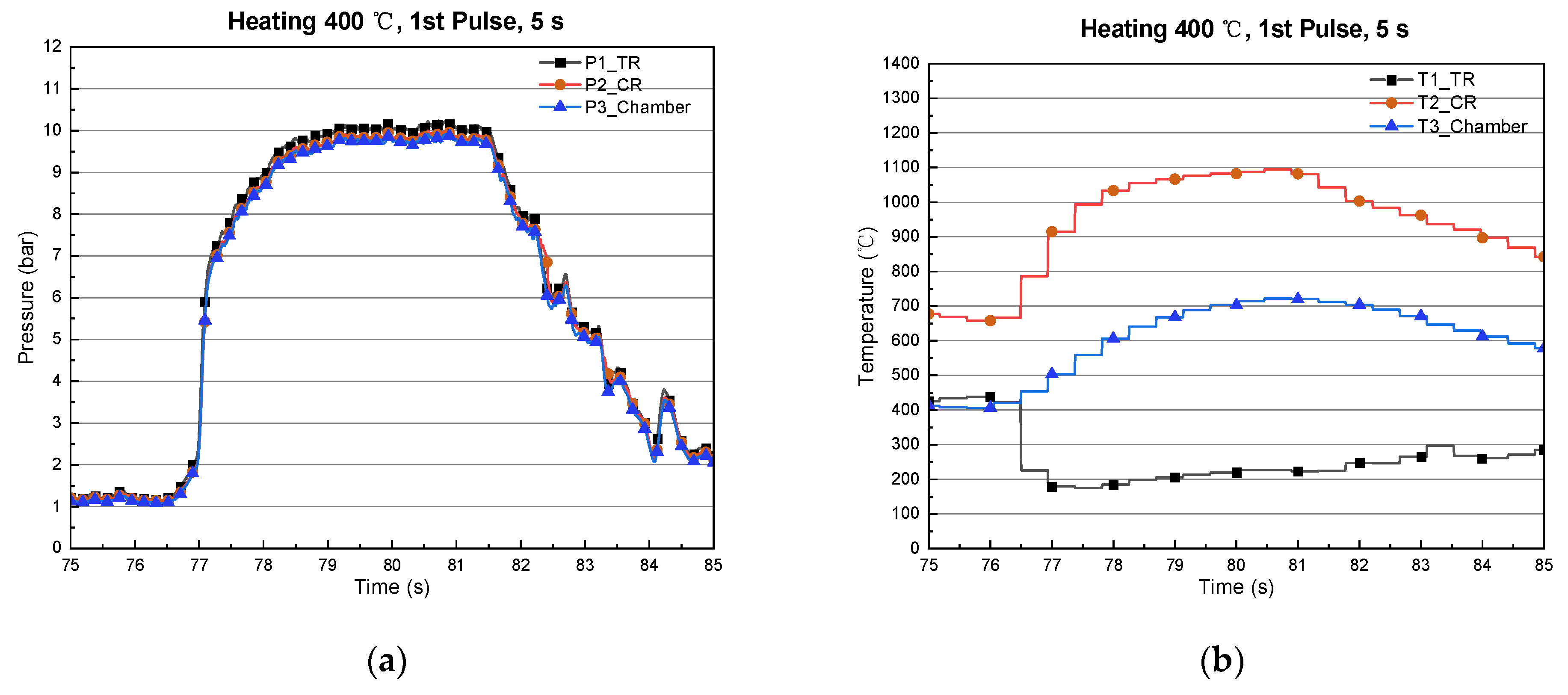
3.1. Type-1 (Catalyst Reactor)
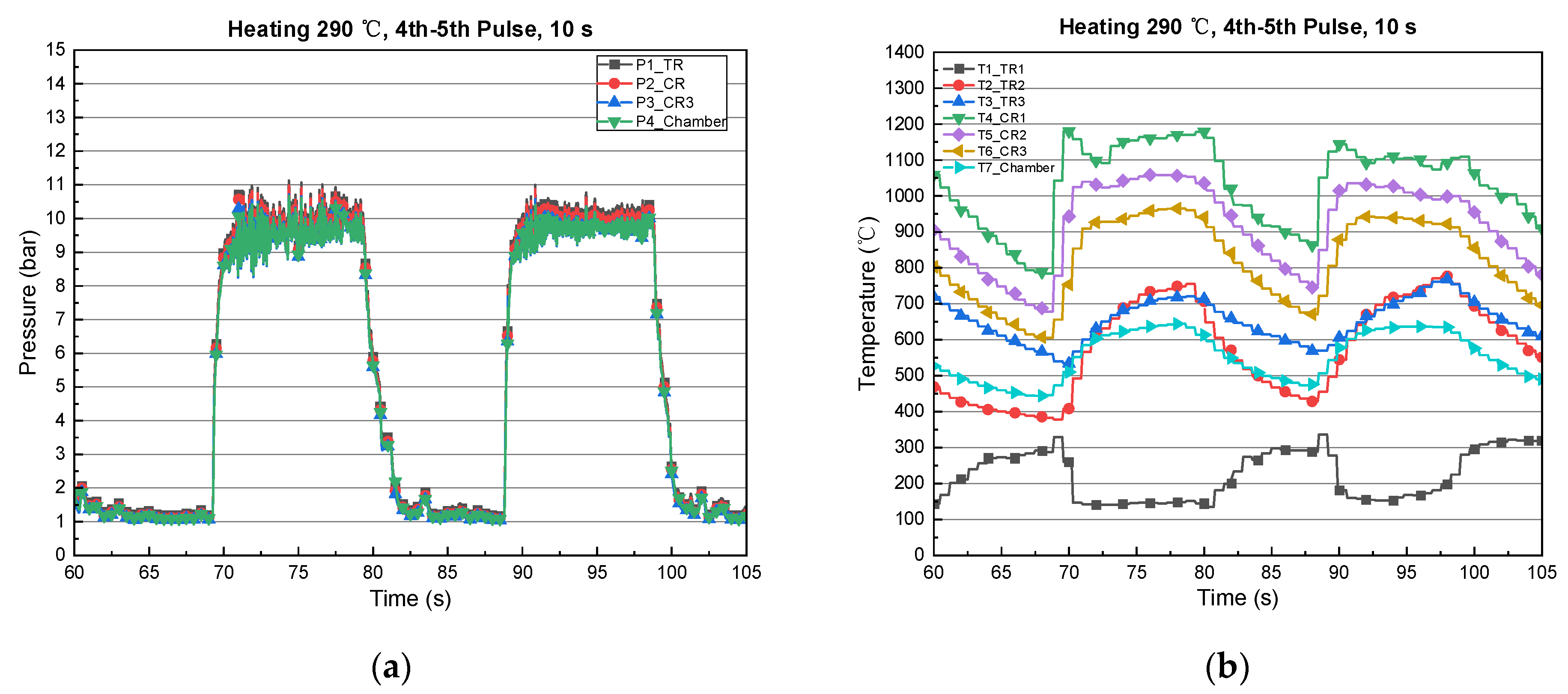
3.2. Type-2 (Thermal/Catalyst Reactor)
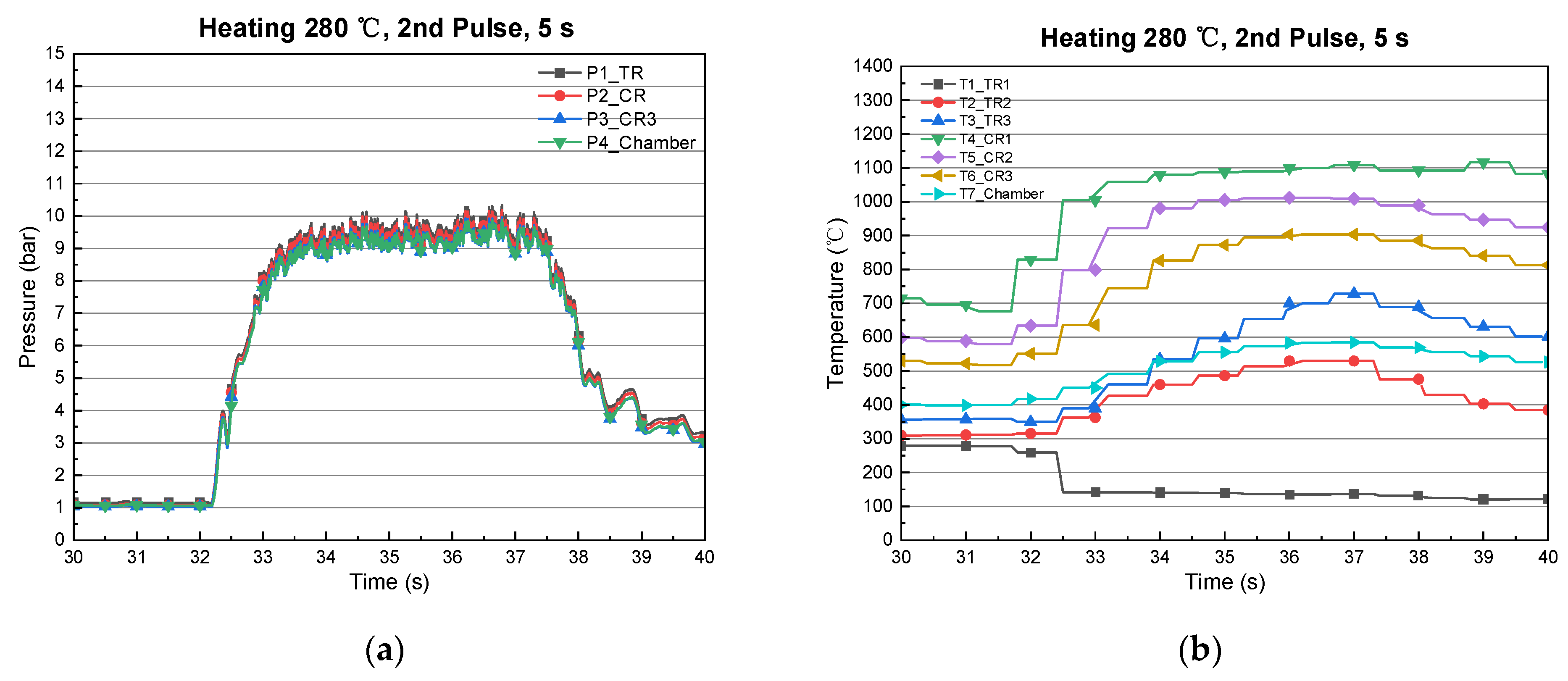
3.3. Type-3 (Long Thermal/Catalyst Reactor)
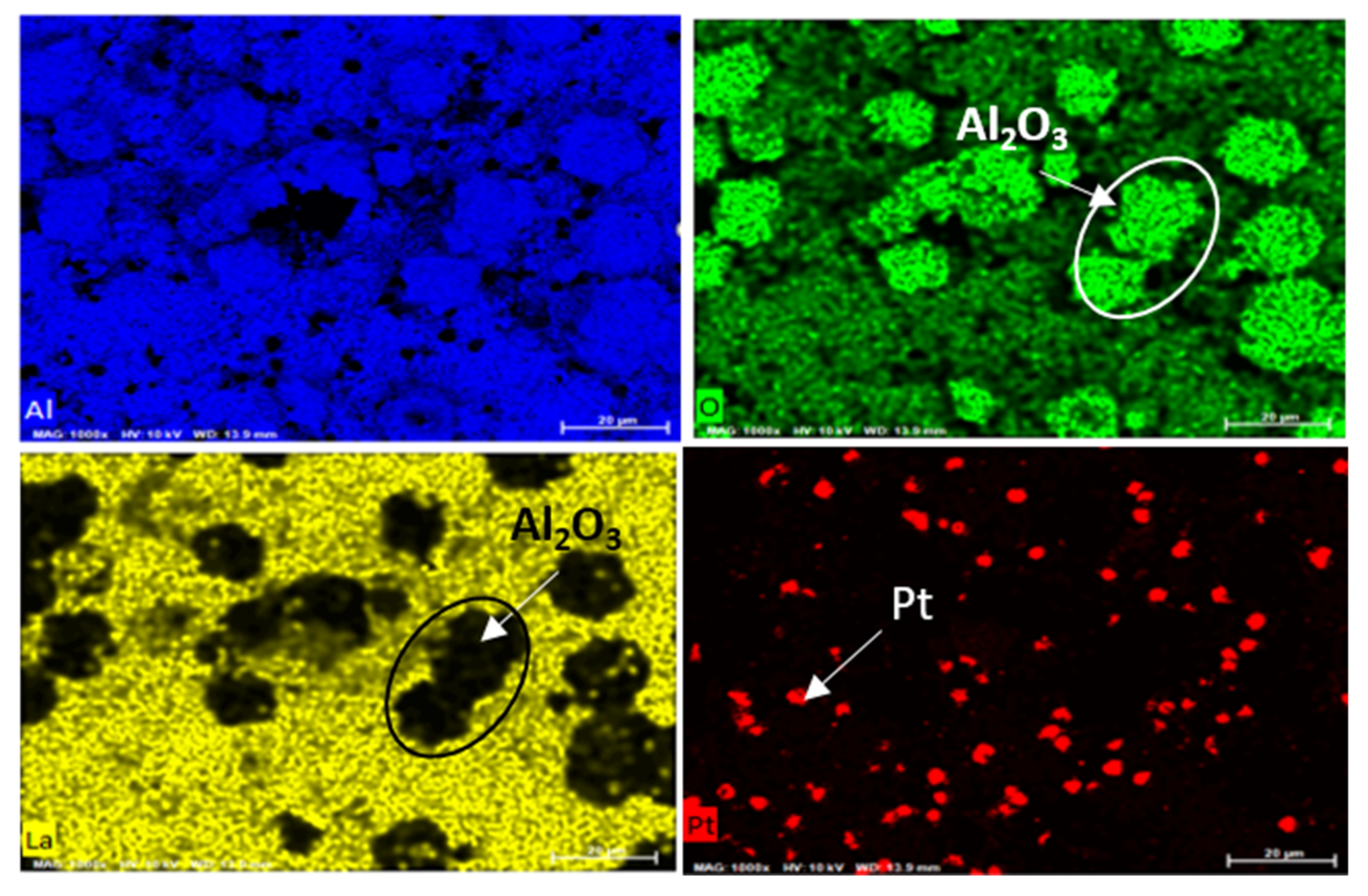
3.4. Catalyst Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anflo, K.; Möllerberg, R. Flight demonstration of new thruster and green propellant technology on the PRISMA satellite. Acta Astronaut. 2009, 65, 1238–1249. [Google Scholar] [CrossRef] [Green Version]
- Dinardi, A.; Anflo, K.; Friedhoff, P. On-Orbit Commissioning of High Performance Green Propulsion (HPGP) in the SkySat Constellation. In Proceedings of the 31st Annual AIAA/USU Conference on Small Satellites, Logan, UT, USA, 5 August–10 August 2017. [Google Scholar]
- Gohardani, A.S.; Stanojev, J.; Demairé, A.; Anflo, K.; Persson, M.; Wingborg, N.; Nilsson, C. Green space propulsion: Opportunities and prospects. Prog. Aerosp. Sci. 2014, 71, 128–149. [Google Scholar] [CrossRef]
- Persson, M.; Anflo, K.; Friedhoff, P. Flight Heritage of Ammonium Dinitramide (ADN) Based High Performance Green Propulsion (HPGP) Systems. Propellants Explos. Pyrotech. 2019, 44, 1073–1079. [Google Scholar] [CrossRef]
- Freudenmann, D.; Ciezki, H.K. ADN and HAN-Based Monopropellants—A Minireview on Compatibility and Chemical Stability in Aqueous Media. Propellants Explos. Pyrotech. 2019, 44, 1084–1089. [Google Scholar] [CrossRef]
- Rahm, M. Green Propellants. Doctoral Thesis, Physical Chemistry, KTH Chemical Science and Engineering, Royal Institute of Technology, Stockholm, Sweden, 2010. ISBN 9789174157581. [Google Scholar]
- ArianeGroup. Chemical Monopropellant Thruster Family; Arianegroup: Rue Camille Desmoulins, France, 2020; Available online: https://www.space-propulsion.com/brochures/hydrazine-thrusters/hydrazine-thrusters.pdf (accessed on 1 July 2023).
- Negri, M. Replacement of Hydrazine: Overview and First Results of the H2020 Project Rheform. In Proceedings of the 6th European Conference for Aeronautics and Space Sciences, Krakow, Poland, 29 June–3 July 2015; pp. 1–12. [Google Scholar]
- European Chemical Agency (ECHA). Agreement of the Member State Committee on the Identification of Hydrazine as a Substance of Very High Concern; European Chemical Agency (ECHA): 26 May 2011. Available online: https://echa.europa.eu/documents/10162/b3561467-aa0f-4551-9dff-dcdc5ba60eec (accessed on 1 July 2023).
- Anflo, K.; Persson, S.; Thormählen, P.; Bergman, G.; Hasanof, T.; Grönland, T.A.; Möllerberg, R. Flight demonstration of an ADN-based propulsion system. In Proceedings of the AIAA 57th International Astronautical Congress (IAC 2006), Valencia, Spain, 2–6 October 2006; pp. 6018–6027. [Google Scholar]
- Anflo, K.; Crowe, B. In-space demonstration of an ADN-based propulsion system. In Proceedings of the 47th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, San Diego, CA, USA, 31 July–3 August 2011; pp. 1–14. [Google Scholar] [CrossRef]
- Wingborg, N.; Johansson, M.; Bodin, L. Initial Development of a Laboratory Rocket Thruster for ADN-Based Liquid Monopropellants; FOI Technology Report; Swedish Defence Research Agency: Kista, Sweden, 2006. [Google Scholar]
- Persson, M.; Anflo, K.; Dinardi, A.; Bahu, J.M. A family of thrusters for ADN-Based monopropellant LMP-103S. In Proceedings of the 48th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Atlanta, GA, USA, 30 July–1 August 2012; pp. 1–15. [Google Scholar] [CrossRef]
- Wingborg, N.; Eldsäter, C.; Skifs, H. Formulations and Characterisation of ADN-Based Liquid Monopropellants. In Proceedings of the 2nd International Conference on Green Propellants for Space Propulsion (ESA SP-557), Cagliari, Sardinia, Italy, 7–8 June 2004. [Google Scholar]
- Karaliunaite, V. A Successful In-Orbit Test of the First Ever Chemical Propulsion System Running on-Board a CubeSat Was Performed. Available online: https://nanoavionics.com/news/successful-orbit-test-first-ever-chemical-propulsion-system-running-board-cubesat-performed/ (accessed on 1 July 2023).
- Zhaopu, Y.A.O.; Wei, Z.; Meng, W.; Jun, C.; Yan, S. Experimental investigation and on-orbit flying validation of an ADN-based liquid space engine. J. Rocket Propuls. 2018, 44, 8–14. [Google Scholar]
- Li, L.; Li, G.X.; Li, H.M.; Yao, Z.P. Experimental Study on Electrical Ignition Characteristics of Ammonium Dinitramide Based Propellant under Different Environmental Pressures. Propellants Explos. Pyrotech. 2020, 45, 1056–1065. [Google Scholar] [CrossRef]
- Hou, Y.; Yu, Y.; Liu, X.; Cao, J. Effect of Combustion Chamber Geometrical Parameters on the Decomposition and Combustion Characteristics of an ADN-Based Thruster. Micromachines 2022, 13, 605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, G.; Yu, Y.; Chen, J.; Wang, M. Effects of Catalytic Bed Thermal Characteristics on Liquid Monopropellant Decomposition and Combustion Characteristics within an Eco-Friendly Thruster Based on Ammonium Dinitramide. Combust. Sci. Technol. 2016, 188, 910–923. [Google Scholar] [CrossRef]
- Grönland, T.-A.; Anflo, K.; Bergman, G.; Nedar, R. ADN-Based Propulsion for Spacecraft, -Key Requirements and Experimental Verification. In Proceedings of the 40th Joint Propulsion Conference and Exhibit, Fort Lauderdale, FL, USA, 11–14 July 2004. [Google Scholar]
- Anflo, K.; Grönland, T.A.; Wingborg, N. Development and Testing of ADN-Based Monopropellants in Small Rocket Engines. In Proceedings of the 36th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Las Vegas, NV, USA, 24–28 July 2000. [Google Scholar] [CrossRef]
- Anflo, K.; Grönland, T.A.; Bergman, G.; Johansson, M.; Nedar, R. Towards Green Propulsion for Spacecraft with ADN-Based Monopropellants. In Proceedings of the 38th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Indianapolis, IN, USA, 7–10 July 2002; pp. 1–9. [Google Scholar] [CrossRef]
- Kim, J.W.; Baek, S.; Jung, Y.S.; Yoon, W.; Ban, H.S.; Kwon, S. An Alternative ADN Based Monopropellant Mixed with Tetraglyme. Acta Astronaut. 2021, 178, 241–249. [Google Scholar] [CrossRef]
- Anflo, K.; Crowe, B. In-Space Demonstration of High Performance Propulsion and Its Impact on Small Satellites. In Proceedings of the 25th Annual AIAA/USU Conference on Small Satellites, Logan, UT, USA; 2011; pp. 1–7. [Google Scholar]
- Zhang, T.; Li, G.; Yu, Y.; Sun, Z.; Wang, M.; Chen, J. Numerical Simulation of Ammonium Dinitramide (ADN)-Based Non-Toxic Aerospace Propellant Decomposition and Combustion in a Monopropellant Thruster. Energy Convers. Manag. 2014, 87, 965–974. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Ammonium Dinitramide: Kinetics and Mechanism of Thermal Decomposition. J. Phys. Chem. A 1997, 101, 5653–5658. [Google Scholar] [CrossRef]
- Amrousse, R.; Hori, K.; Fetimi, W.; Farhat, K. HAN and ADN as Liquid Ionic Monopropellants: Thermal and Catalytic Decomposition Processes. Appl. Catal. B Environ. 2012, 127, 121–128. [Google Scholar] [CrossRef]
- Shmakov, A.G.; Korobeinichev, O.P.; Bol’shova, T.A. Thermal Decomposition of Ammonium Dinitramide Vapor in a Two-Temperature Flow Reactor. Combust. Explos. Shock Waves 2002, 38, 284–294. [Google Scholar] [CrossRef]
- Wilhelm, M.; Negri, M.; Ciezki, H.; Schlechtriem, S. Preliminary Tests on Thermal Ignition of ADN-Based Liquid Monopropellants. Acta Astronaut. 2019, 158, 388–396. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Zhang, T.; Wang, M.; Yu, Y. Experimental Investigation of the Catalytic Decomposition and Combustion Characteristics of a Non-Toxic Ammonium Dinitramide (ADN)-Based Monopropellant Thruster. Acta Astronaut. 2016, 129, 367–373. [Google Scholar] [CrossRef]
- Anflo, K.; Thormahlen, P. Improved Reactor for Ammonium Dinitramide-Based Liquid Monopropellants, and Thruster Including the Reactor. EP2847452B1, 30 September 2016. [Google Scholar]
- Grönlandb, T.-A.; Westerberg, B.; BergmanKjell, G.; Anflo, K.; Brandt, J.; Ola, L.; Agrell, J.; Ersson, A.; Järås, S.; Buotonnet, M.; et al. Reactor for Decomposition of Ammonium Dinitramide-Based Liquid Monopropellants and Process for the Decomposition. WO2002095207A1, 28 November 2002. [Google Scholar]
- Kumar, P. An Overview on Properties, Thermal Decomposition, and Combustion Behavior of ADN and ADN Based Solid Propellants. Def. Technol. 2018, 14, 661–673. [Google Scholar] [CrossRef]

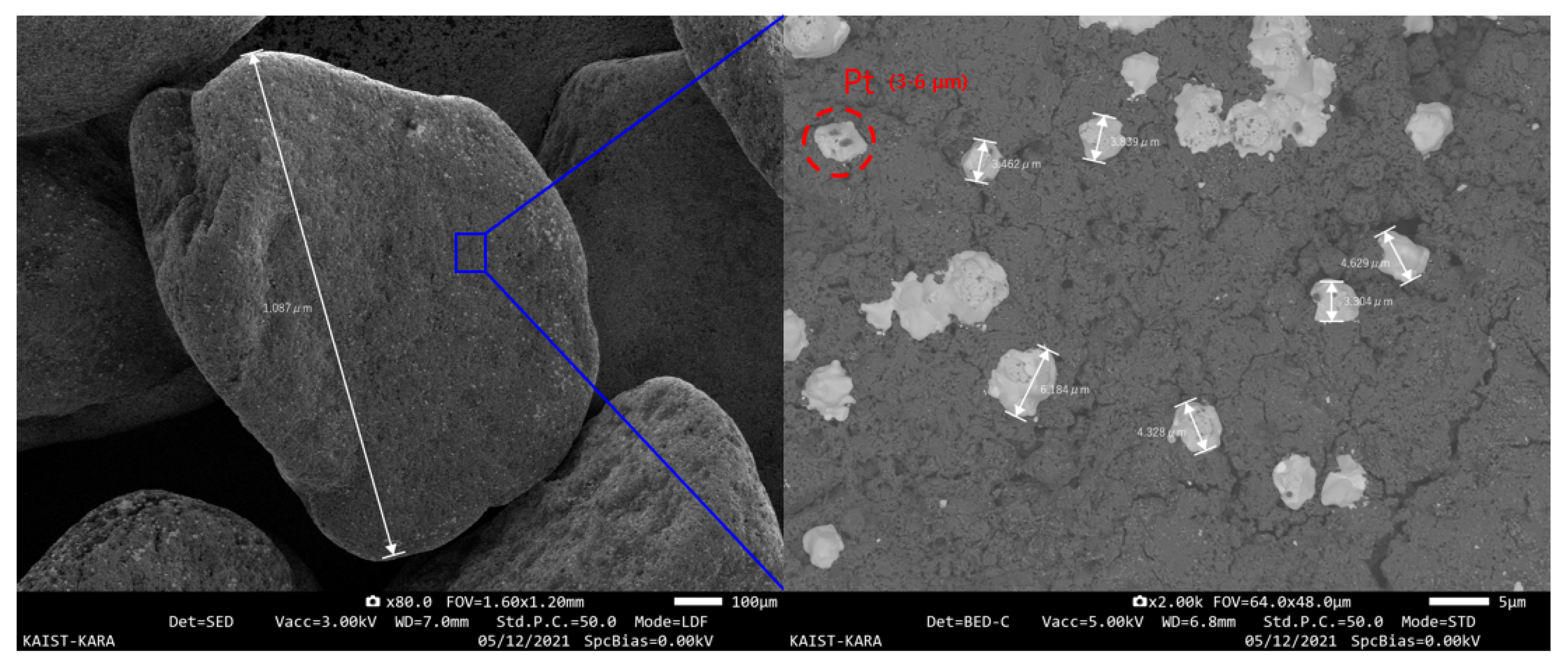
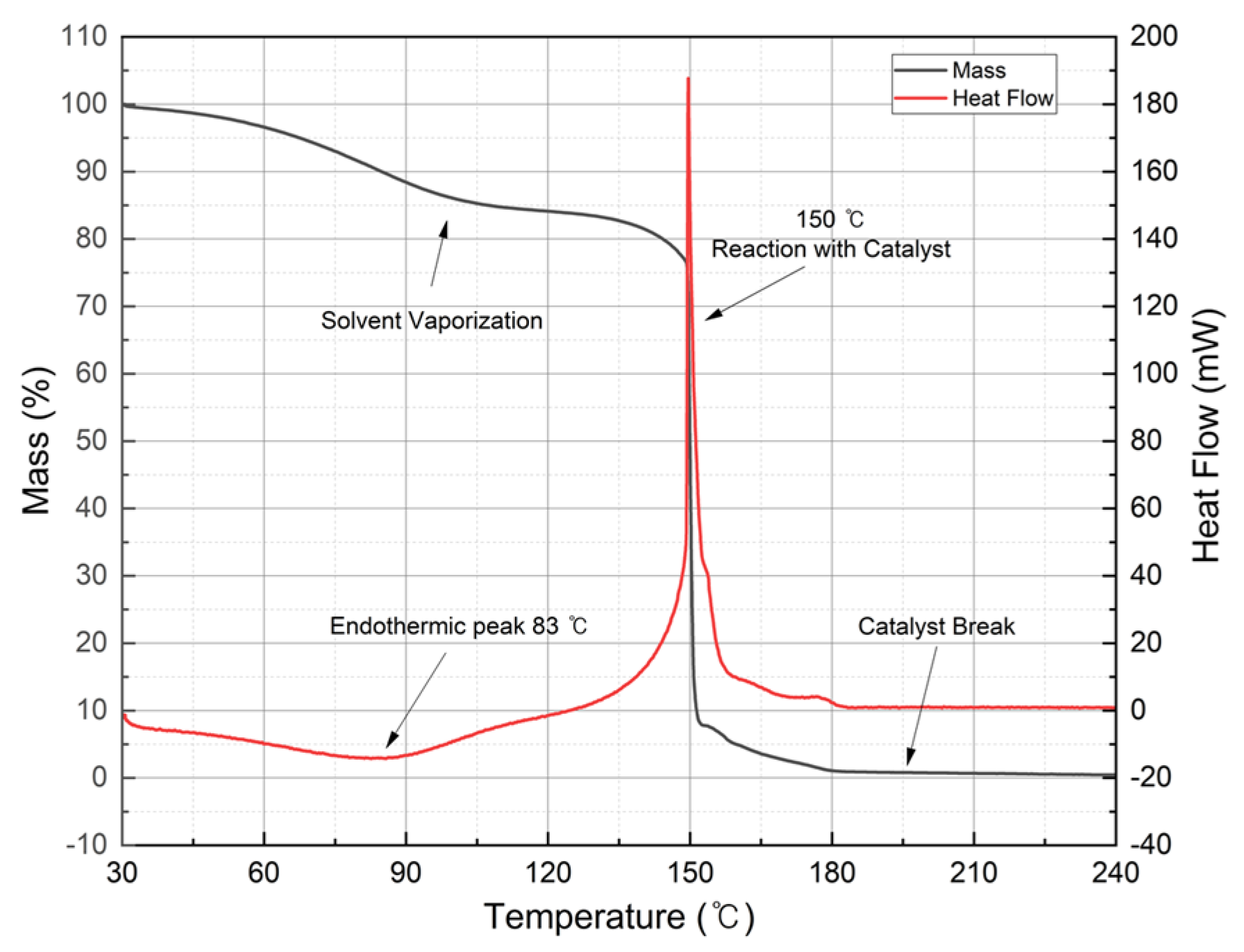
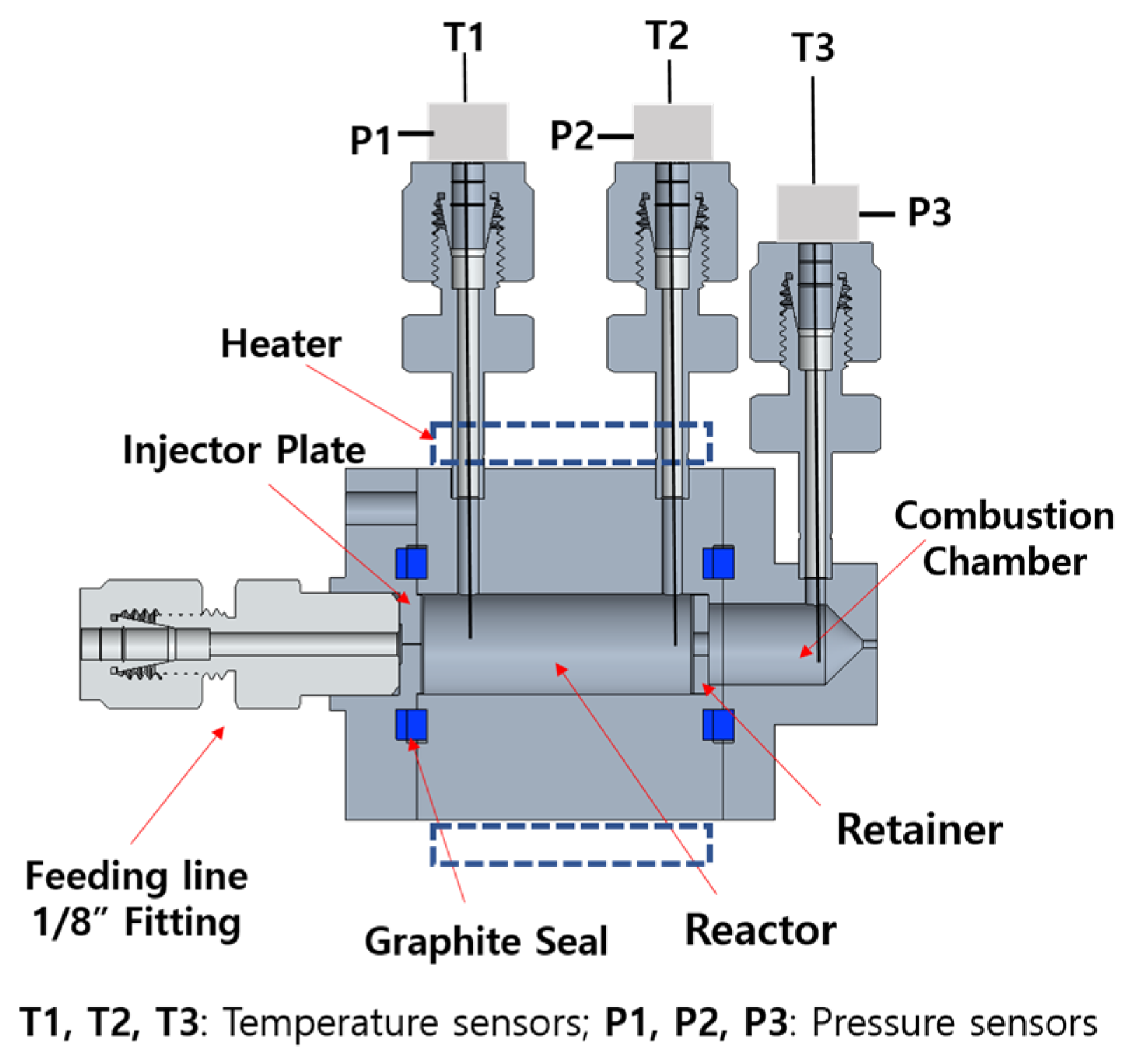


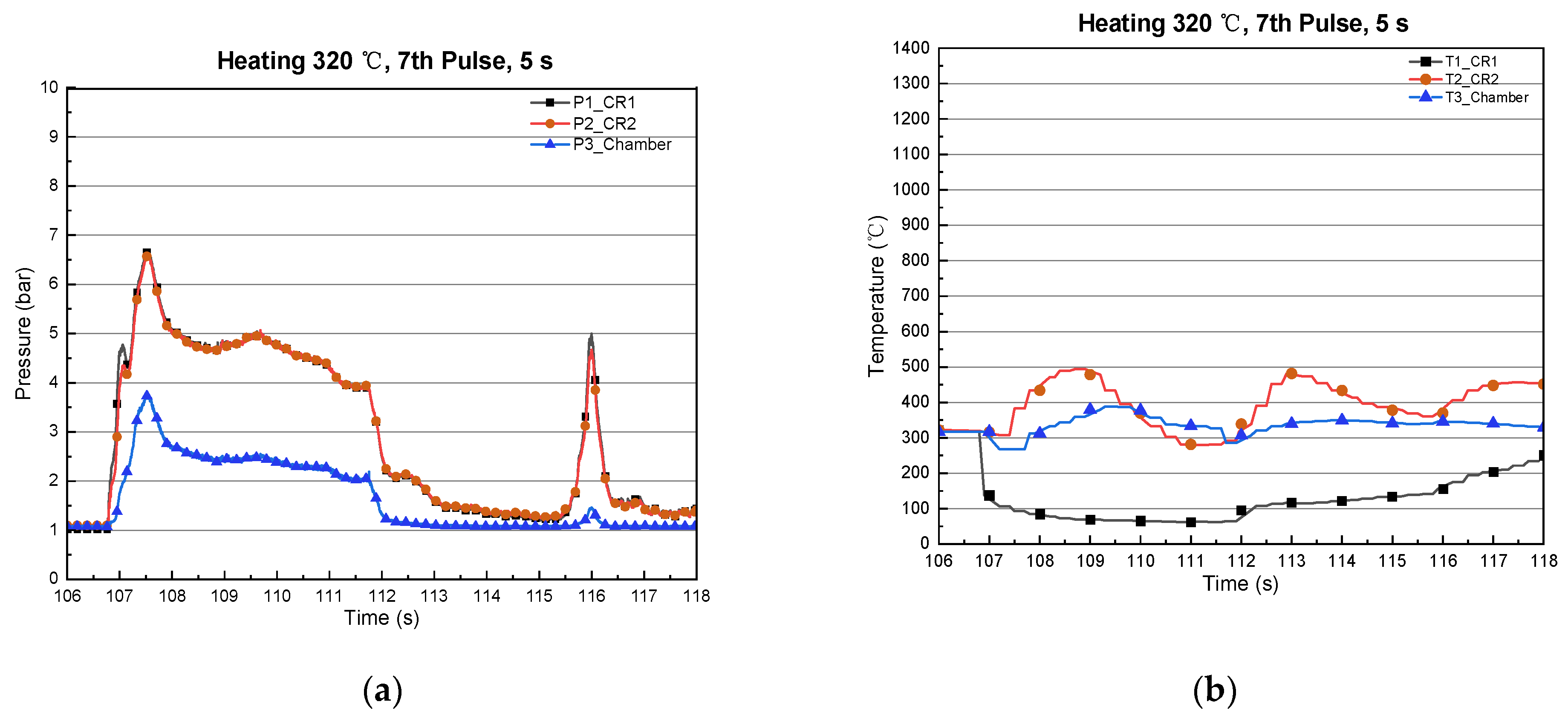




| Chemicals | Composition (wt%) |
|---|---|
| ADN | 63 |
| CH3OH | 18.4 |
| NH3 | 4.65 |
| H2O | 13.95 |
| Parameters | Type 1 | Type 2 | Type 3 | |
|---|---|---|---|---|
| Thrust | 1 N | |||
| Chamber pressure | 10 bar | |||
| Nozzle expansion ratio | 100 | |||
| Nozzle throat diameter | 0.82 mm | |||
| Characteristic velocity | 1389.7 m/s | |||
| Ideal specific impulse | 268.5 s | |||
| Diameter of reactor | 10 mm | |||
| Length of reactor | 30 mm | |||
| Injector orifice diameter | 0.16 mm | |||
| Number of injector orifice | 1 | |||
| Theoretical mass flow rate | 0.38 g/s | |||
| Heat of formation of ADN in monopropellant [8] | −110.22 kJ/mol | |||
| Diameter of reactor (Type 1~3) | 10 mm | |||
| Length of reactor | Thermal | X | 15 mm | 30 mm |
| Catalyst | 30 mm | 15 mm | 30 mm | |
| Expt./Pulses | Preheating Temperature (Actual) (°C) | Firing Time, (s) | Temperature during Combustion, (°C) | Catalyst Bed Pressure, P2 (bar) | Chamber Pressure, P3 (bar) | ||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | ||||
| 1 | 332 | 332 | 0.5 | 110 | 568 | 4.67 | 3.3 |
| 2 | 377 | 377 | 0.5 | 121 | 627 | 10.72 | 5.17 |
| 3 | 431 | 431 | 0.5 | 143 | 747 | 12.18 | 4.96 |
| 4 | 463 | 463 | 1 | 143 | 915 | 13.53 | 5.34 |
| 5 | 434 | 434 | 1 | 173 | 985 | 13.56 | 5.3 |
| 6 | 482 | 482 | 1 | 155 | 1019 | 13.4 | 5.1 |
| 7 | 316 | 316 | 5 | 62 | 493 | 5 | 2.5 |
| 8 | 305 | 287 | 1 | 139 | 604 | 10.7 | 3.97 |
| 9 | 397 | 311 | 1 | 384 | 658 | 14.15 | 3.8 |
| 10 | 405 | 326 | 1 | 395 | 673 | 13.65 | 3.7 |
| 11 | 557 | 486 | 1 | 557 | 786 | 14 | 3.7 |
| 12 | 500 | 432 | 3 | 173 | 1069 | 15.3 | 4 |
| 13 | 269 | 286 | 0.3 | 111 | 548 | 3.33 | 1.58 |
| 14 | 480 | 393 | 0.3 | 291 | 604 | 12.23 | 3.3 |
| 15 | 526 | 468 | 0.3 | 329 | 655 | 12.71 | 3.25 |
| 16 | 511 | 450 | 0.3 | 336 | 637 | 12.6 | 3.2 |
| 17 | 500 | 429 | 1 | 199 | 933 | 15.55 | 3.55 |
| 18 | 483 | 453 | 1 | 173 | 984 | 15.21 | 3.56 |
| 19 | 521 | 502 | 3 | 160 | 1077 | 15.5 | 3.83 |
| Expt./Pulses | Preheating Temperature (Actual), (°C) | Firing Time, (s) | Temperature during Combustion, (°C) | Catalyst Bed Pressure, P2 (bar) | Chamber Pressure, P3 (bar) | ||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | ||||
| 1 | 420 | 666 | 5 | 175 | 1094 | 9.8 | 9.7 |
| 2 | 327 | 739 | 5 | 135 | 1120 | 9.75 | 9.63 |
| 3 | 345 | 760 | 5 | 137 | 1151 | 10 | 9.9 |
| 4 | 408 | 778 | 5 | 151 | 1163 | 10 | 9.8 |
| 5 | 426 | 779 | 5 | 157 | 1151 | 10 | 9.8 |
| 6 | 432 | 831 | 5 | 164 | 1150 | 10 | 9.8 |
| 7 | 440 | 818 | 5 | 190 | 1168 | 10 | 9.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, W.; Bhosale, V.K.; Yoon, H. Reactor Structure for the Decomposition of ADN-Based Monopropellant. Aerospace 2023, 10, 686. https://doi.org/10.3390/aerospace10080686
Yoon W, Bhosale VK, Yoon H. Reactor Structure for the Decomposition of ADN-Based Monopropellant. Aerospace. 2023; 10(8):686. https://doi.org/10.3390/aerospace10080686
Chicago/Turabian StyleYoon, Wonjae, Vikas Khandu Bhosale, and Hosung Yoon. 2023. "Reactor Structure for the Decomposition of ADN-Based Monopropellant" Aerospace 10, no. 8: 686. https://doi.org/10.3390/aerospace10080686
APA StyleYoon, W., Bhosale, V. K., & Yoon, H. (2023). Reactor Structure for the Decomposition of ADN-Based Monopropellant. Aerospace, 10(8), 686. https://doi.org/10.3390/aerospace10080686





