Characterization of an Ignition System for Nitromethane-Based Monopropellants †
Abstract
:1. Introduction
- To determine the required mass ratio of oxygen to NMP-001 (ROF) for successful ignition with a glow plug/oxygen ignition system (test series S1).
- To evaluate if a similar low-pressure combustion limit can be achieved with the H2/O2 torch ignitor used in prior research presented in [29] (test series S2).
- To optimize the ignition procedure (test series S3).
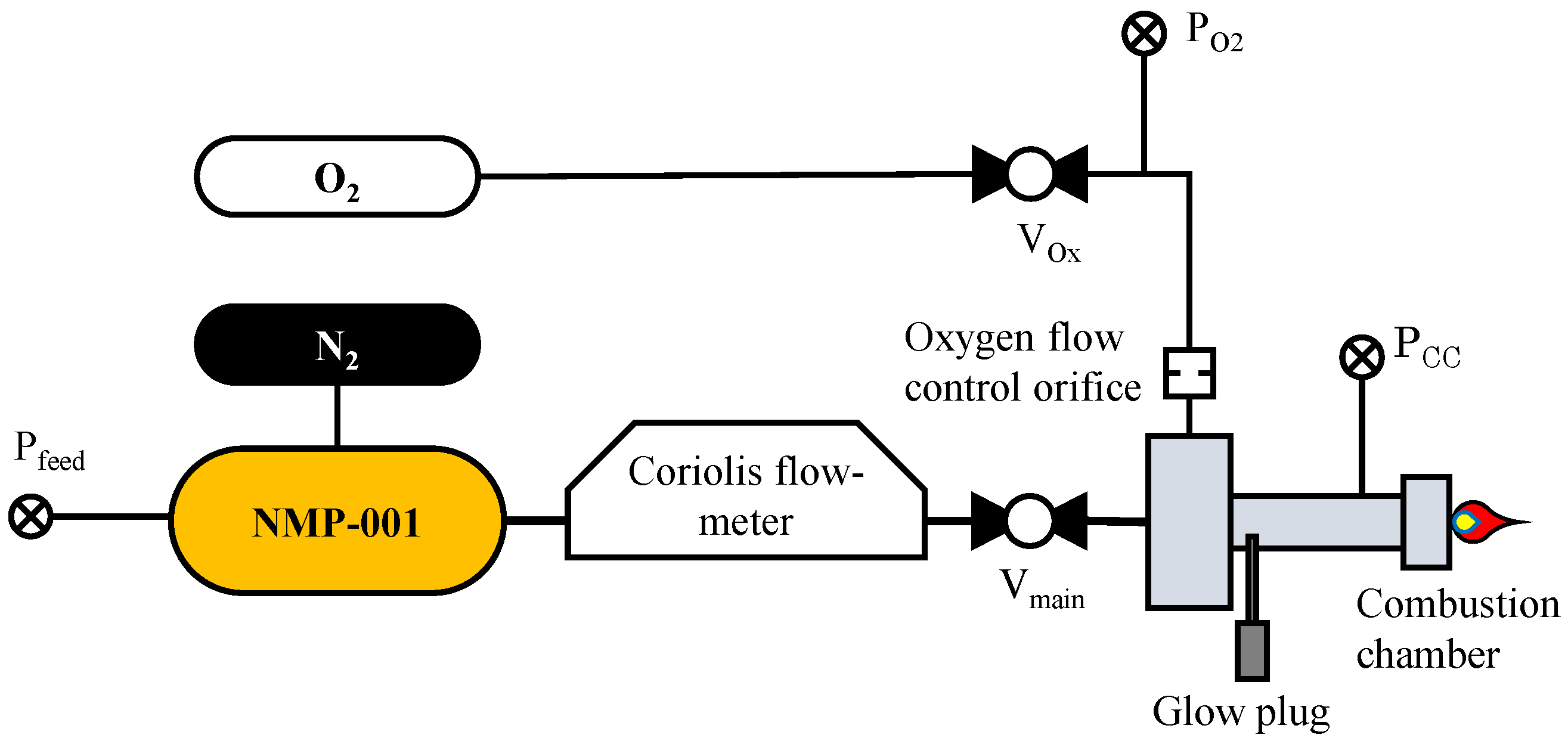
2. Materials and Methods
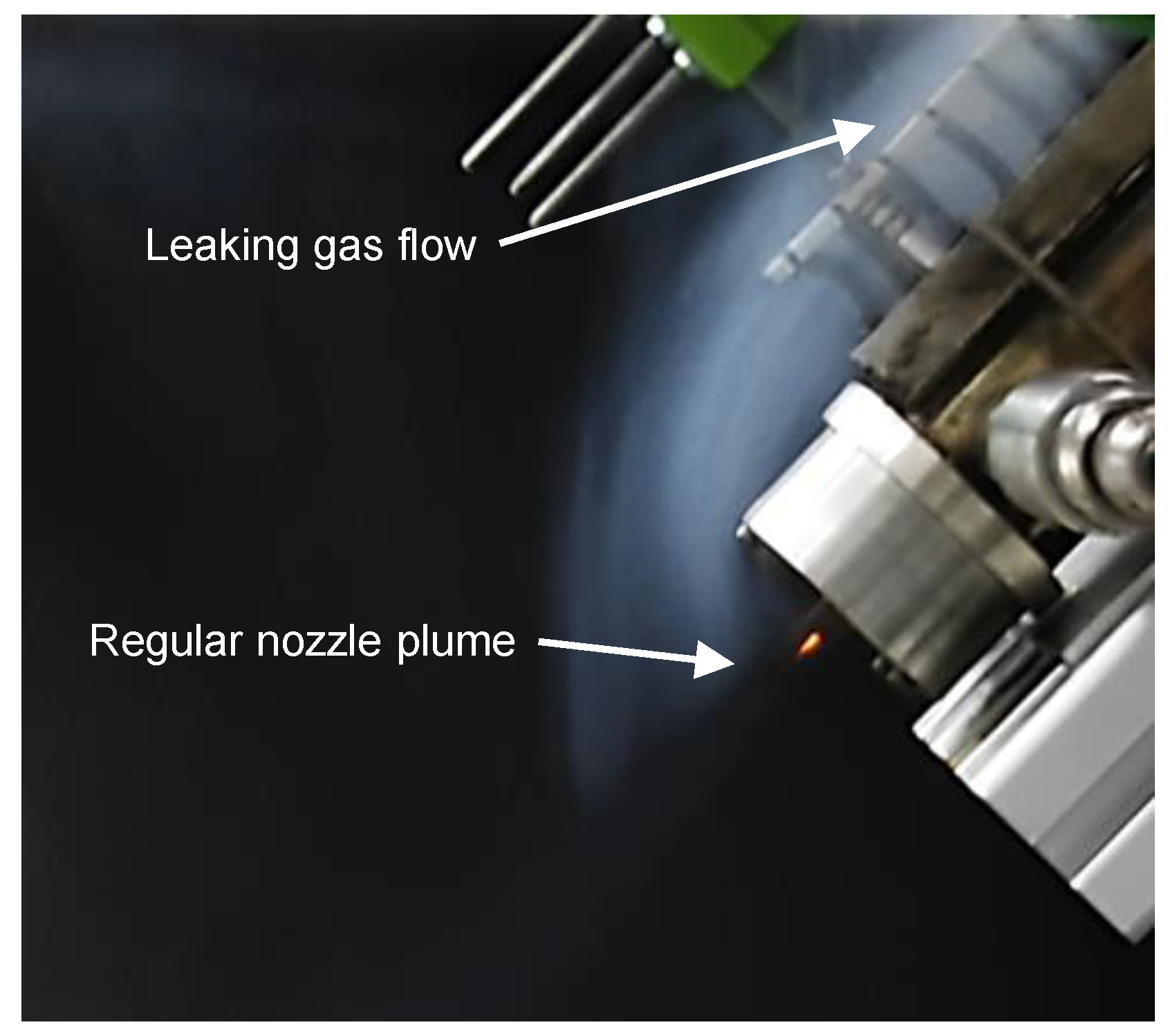
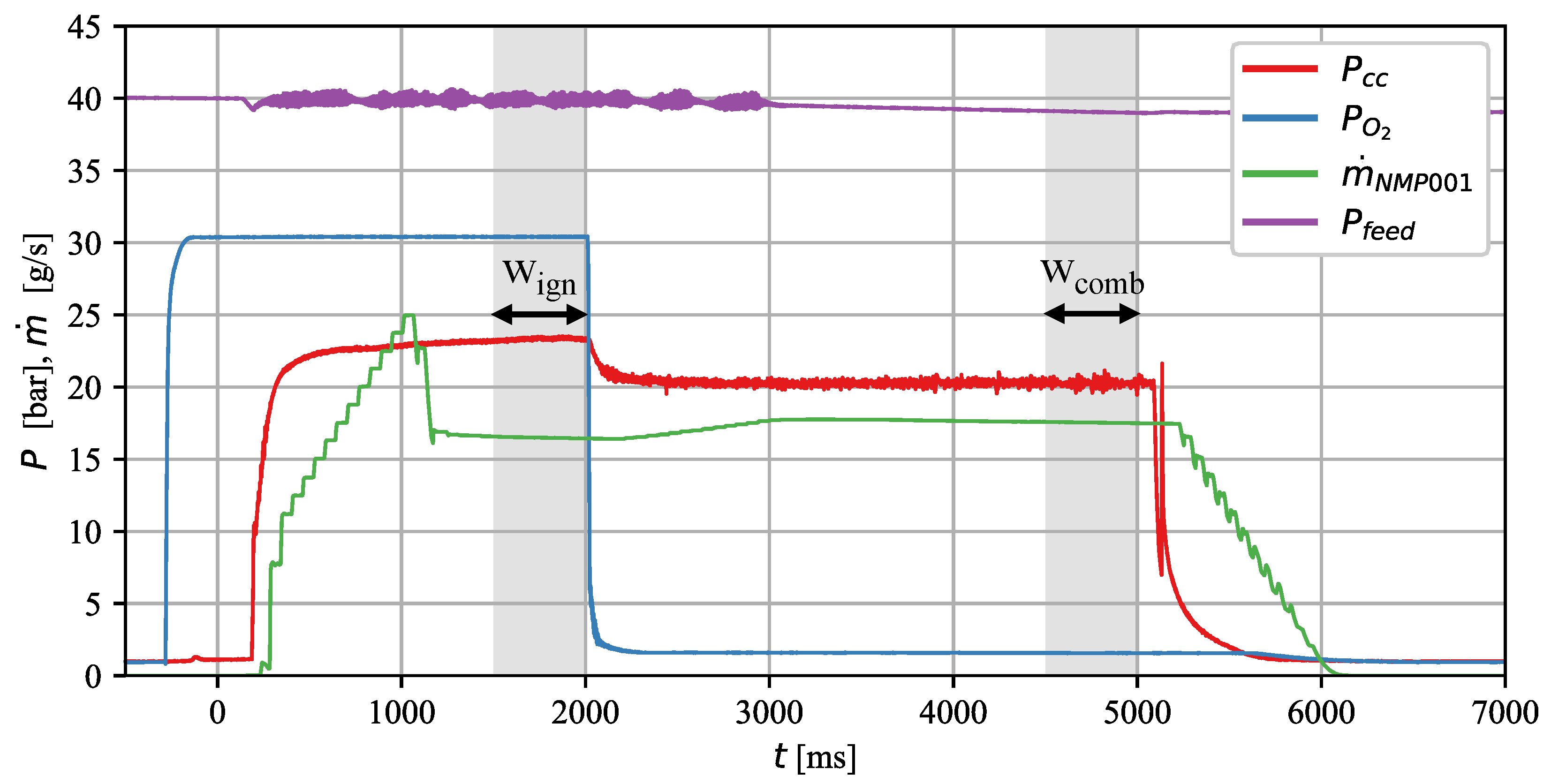
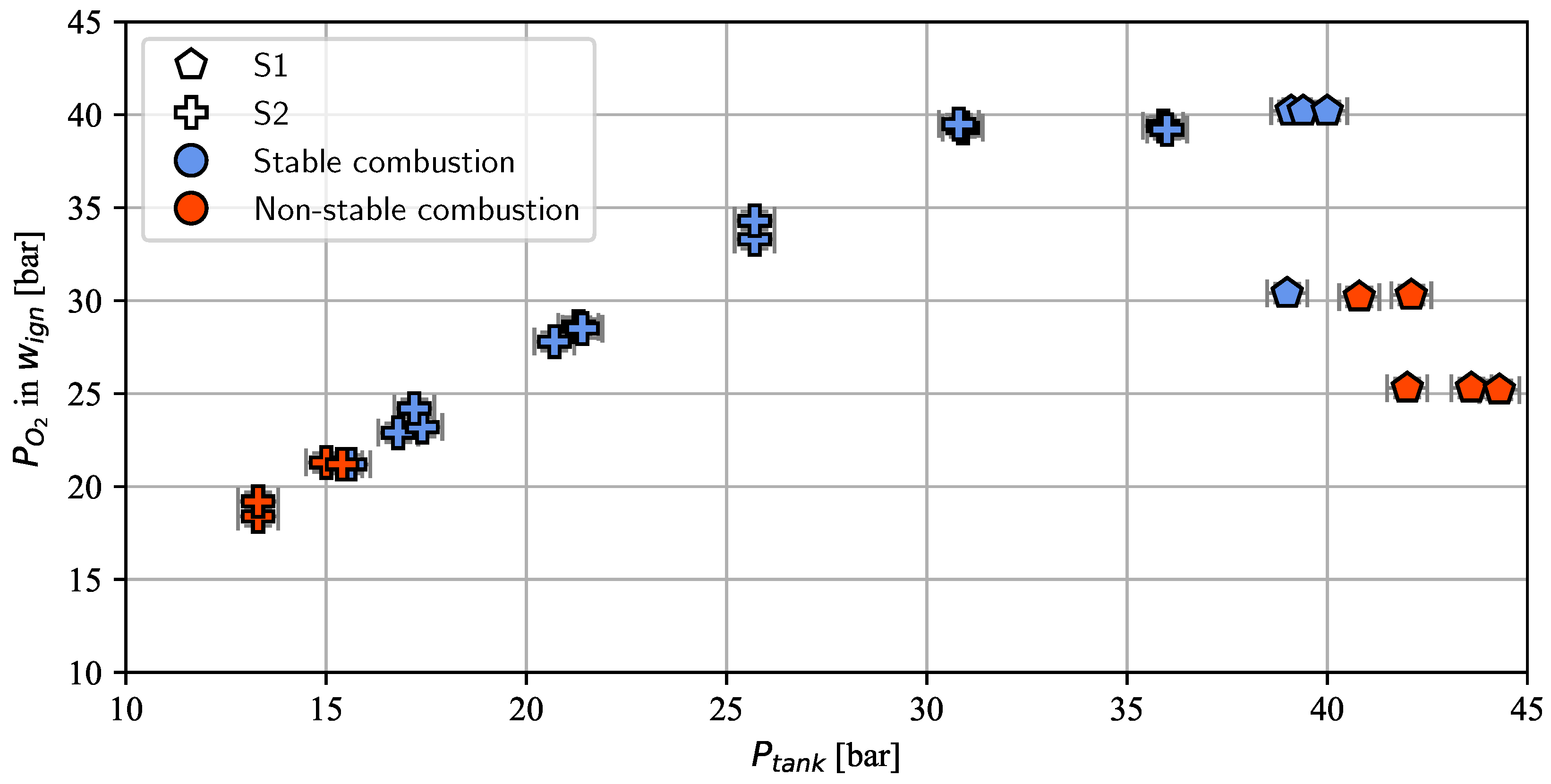
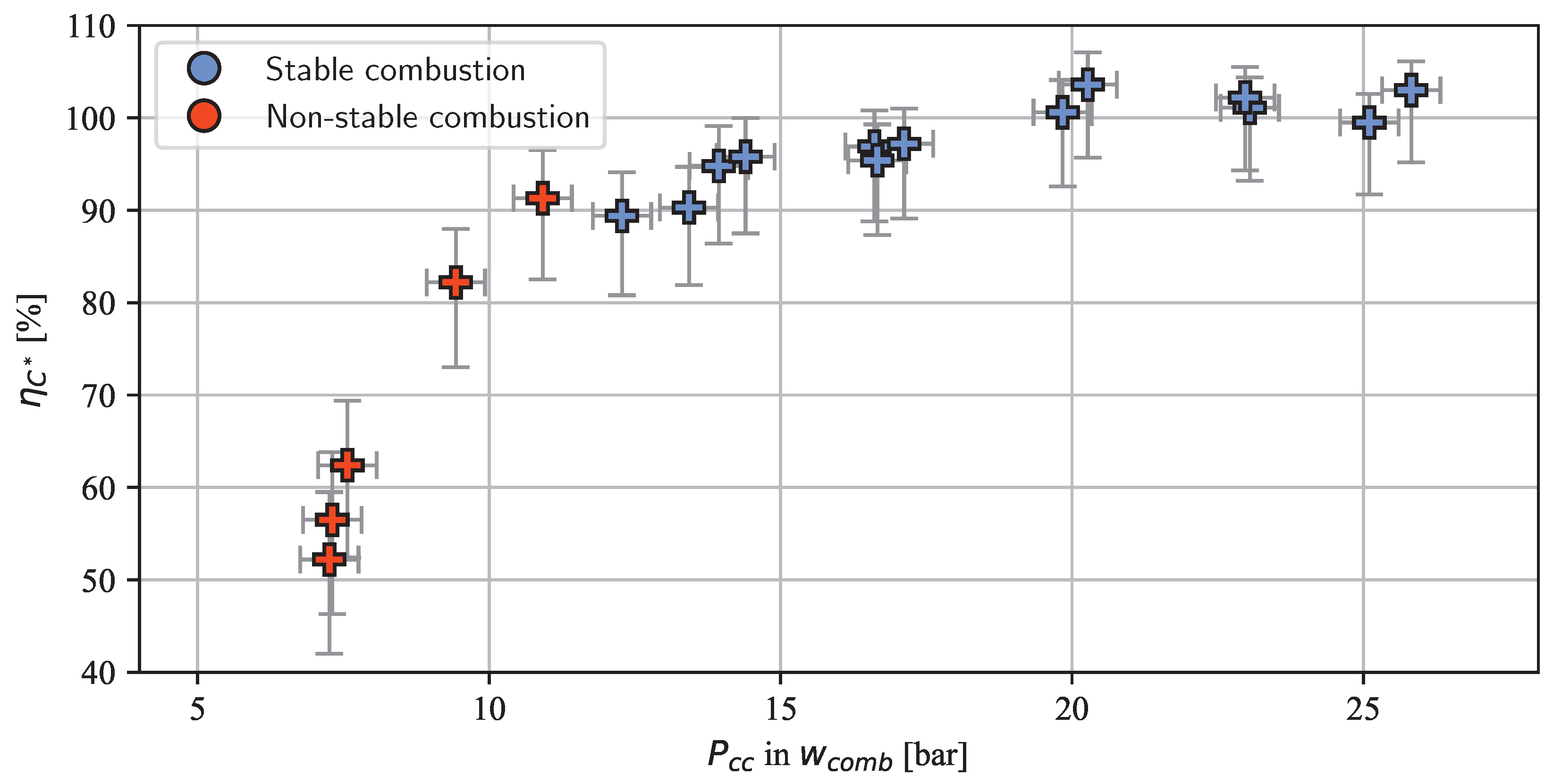
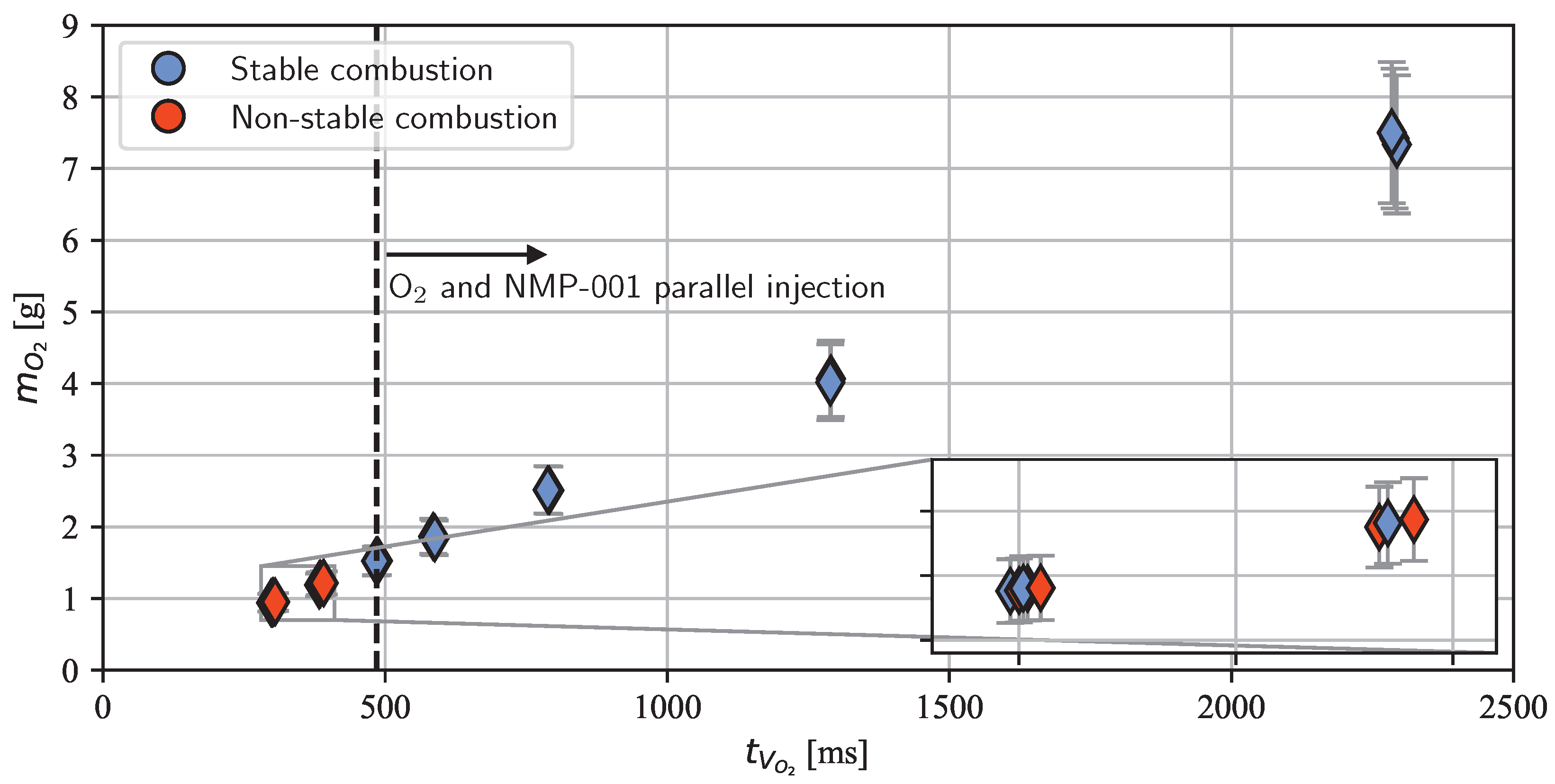
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADN | Ammonium dinitramide |
| HAN | Hydroxylammonium nitrate |
| NMP-001 | Nitromethane monopropellant 001 |
| PLC | Programmable Logic Controller |
| ROF | Oxygen-to-NMP-001 mass ratio |
| Calculated characteristic velocity, [m/s] | |
| Measured characteristic velocity, [m/s] | |
| Nozzle throat diameter, [mm] | |
| Characteristic combustion chamber length, [m] | |
| NMP mass flow rate inside , [g/s] | |
| NMP mass flow rate inside , [g/s] | |
| Oxygen mass rate flow inside , [g/s] | |
| Combustion chamber pressure, [bar] | |
| Average chamber pressure in , [bar] | |
| Propellant tank feed pressure, [bar] | |
| Ignition oxygen pressure, [bar] | |
| Combustion efficiency, [%] |
References
- Brown, C.D. Spacecraft Propulsion; AIAA: Las Vegas, NV, USA, 1996. [Google Scholar]
- Sutton, G.P.; Biblarz, O. Rocket Propulsion Elements, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Sarritzu, A.; Felix, L.; Lukas, W.; Pasini, A. Assessment of Propulsion System Architectures for Green Propellants-based Orbital Stages. In Proceedings of the International Astronautical Congress: IAC Proceedings, Paris, France, 8–22 September 2022; IAF (International Astronautical Federation): New Delhi, India, 2022. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 9321, Hydrazine. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hydrazine (accessed on 30 August 2024).
- European Chemicals Agency. Substance Infocard—Hydrazine. 2024. Available online: https://echa.europa.eu/de/substance-information/-/substanceinfo/100.005.560 (accessed on 29 August 2024).
- MacLean, M.; Rodriguez, H. A low-risk, reliable, operationally efficient auxiliary propulsion system for the Reusable Launch Vehicle. In Proceedings of the 32nd Joint Propulsion Conference and Exhibit, Lake Buena Vista, FL, USA, 1–3 July 1996; p. 3228. [Google Scholar]
- Marshall, W.M.; Deans, M.C. Recommended figures of merit for green monopropellants. In Proceedings of the 49th AIAA/ASME/SAE/ASEE Joint Propulsion Conference, San Jose, CA, USA, 15–17 July 2013; p. 3722. [Google Scholar]
- Bombelli, V.; Simon, D.; Moerel, J.L.; Marée, T. Economic benefits of the use of non-toxic mono-propellants for spacecraft applications. In Proceedings of the 39th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Huntsville, AL, USA, 20–23 July 2003; p. 4783. [Google Scholar]
- Masse, R.; Allen, M.; Spores, R.; Driscoll, E.A. AF-M315E propulsion system advances and improvements. In Proceedings of the 52nd AIAA/SAE/ASEE Joint Propulsion Conference, Salt Lake City, UT, USA, 25–27 July 2016; p. 4577. [Google Scholar]
- Gohardani, A.S.; Stanojev, J.; Demairé, A.; Anflo, K.; Persson, M.; Wingborg, N.; Nilsson, C. Green space propulsion: Opportunities and prospects. Prog. Aerosp. Sci. 2014, 71, 128–149. [Google Scholar] [CrossRef]
- Wingborg, N.; Larsson, A.; Elfsberg, M.; Appelgren, P. Characterization and ignition of ADN-based liquid monopropellants. In Proceedings of the 41st AIAA/ASME/SAE/ ASEE Joint Propulsion Conference & Exhibit, Tucson, AZ, USA, 10–13 July 2005; p. 4468. [Google Scholar]
- Wingborg, N.; Johansson, M.; Bodin, L. Initial Development of a Laboratory Rocket Thruster for ADN-Based Liquid Monopropellants; Swedish Defence Research Agency: Stockholm, Sweden, 2006.
- Larsson, A.; Wingborg, N. Green propellants based on ammonium dinitramide (ADN). Adv. Spacecr. Technol. 2011, 2, 139–156. [Google Scholar]
- Persson, M.; Anflo, K.; Friedhoff, P. Flight heritage of ammonium dinitramide (ADN) based high performance green propulsion (HPGP) systems. Propellants Explos. Pyrotech. 2019, 44, 1073–1079. [Google Scholar] [CrossRef]
- Amrousse, R.; Katsumi, T.; Azuma, N.; Hori, K. Hydroxylammonium nitrate (HAN)-based green propellant as alternative energy resource for potential hydrazine substitution: From lab scale to pilot plant scale-up. Combust. Flame 2017, 176, 334–348. [Google Scholar] [CrossRef]
- Hori, K.; Katsumi, T.; Sawai, S.; Azuma, N.; Hatai, K.; Nakatsuka, J. HAN-Based Green Propellant, SHP163–Its R&D and Test in Space. Propellants Explos. Pyrotech. 2019, 44, 1080–1083. [Google Scholar]
- Katsumi, T.; Hori, K. Successful development of HAN based green propellant. Energetic Mater. Front. 2021, 2, 228–237. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheah, K.H.; Wu, M.H.; Koh, K.S.; Sun, D.; Meng, H. A review on hydroxylammonium nitrate (HAN) decomposition techniques for propulsion application. Acta Astronaut. 2022, 196, 194–214. [Google Scholar] [CrossRef]
- Masse, R.K.; Spores, R.; Allen, M. AF-M315E advanced green propulsion–GPIM and beyond. In Proceedings of the AIAA Propulsion and Energy 2020 Forum, Virtual. 24–26 August 2020; p. 3517. [Google Scholar]
- Gonzalez Rueda Flores, P.; Cuevas, R.A.; Rahman, A.; Zubia, R.A.; Tobias, E.A.; Adams, S.C.; Ontiveros, N.; Quintana, J.; Greig, A.D.; Choudhuri, A.R. Implementing Test Methodology Improvements for Testing and Validation of a 1N AF-M315E Thruster. In Proceedings of the AIAA Propulsion and Energy 2021 Forum, Virtual. 9–11 August 2021; p. 3590. [Google Scholar]
- Kilcoin, M.; Cavender, D.; Hasanof, T.; Zaluki, M.; McKechnie, T.; Sedano, C.; Williams, H. Development of ASCENT propellant thrusters and propulsion systems. In Proceedings of the 36th Annual AIAA/USU Conference on Small Satellites, Propulsion, SSC22-X-08, Logan, UT, USA, 11–13 August 2022. [Google Scholar]
- Maleix, C.; Chabernaud, P.; Brahmi, R.; Beauchet, R.; Batonneau, Y.; Kappenstein, C.; Schwentenwein, M.; Koopmans, R.J.; Schuh, S.; Scharlemann, C. Development of catalytic materials for decomposition of ADN-based monopropellants. Acta Astronaut. 2019, 158, 407–415. [Google Scholar] [CrossRef]
- Negri, M.; Wilhelm, M.; Hendrich, C.; Wingborg, N.; Gediminas, L.; Adelöw, L.; Maleix, C.; Chabernaud, P.; Brahmi, R.; Beauchet, R.; et al. New technologies for ammonium dinitramide based monopropellant thrusters–The project RHEFORM. Acta Astronaut. 2018, 143, 105–117. [Google Scholar] [CrossRef]
- Gordon, S.; McBride, B.J. Computer Program for Calculation of Complex Chemical Equilibrium Compositions and Applications. Part 1: Analysis; Technical Report; NASA Lewis Research Cente: Cleveland, OH, USA, 1994.
- Price, T.; Evans, D. The status of Monopropellant Hydrazine Technology; Technical Report; Jet Propulsion Laboratory, NASA: Pasadena, CA, USA, 1968.
- Pasini, A.; Sales, L.; Puccinelli, E.; Lin, L.; Apollonio, A.; Simi, R.; Brotini, G.; d’Agostino, L. Design of an Affordable Hydrogen Peroxide Propulsion System for CubeSats. In Proceedings of the AIAA Propulsion and Energy 2021 Forum, Virtual. 9–11 August 2021; p. 3690. [Google Scholar]
- German, B.J.; Branscome, E.C.; Frits, A.P.; Yiakas, N.C.; Mavris, D.N. An evaluation of green propellants for an ICBM post-boost propulsion system. In Proceedings of the Missile Sciences Conference, Monterey, CA, USA, 5–7 November 2000. [Google Scholar]
- Davis, D.D.; Dee, L.; Greene, B.; Hornung, S.; McClure, M.; Rathgeber, K. Fire, Explosion, Compatibility and Safety Hazards of Hydrogen Peroxide; National Aeronautics and Space Administration NASA TM-2004-213151; NASA: Washington, DC, USA, 2005.
- Kurilov, M.; Werling, L.; Kirchberger, C.; Ciezki, H.; Schlechtriem, S. Combustion behavior of NMP-001, a nitromethane-based green rocket propellant. Acta Astronaut. 2024, 223, 1–14. [Google Scholar] [CrossRef]
- Markofsky, S.B. Nitro compounds, aliphatic. Ullmann’s Encycl. Ind. Chem. 2000, 24, 291–300. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6375, Nitromethane. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Nitromethane (accessed on 27 August 2024).
- U.S. Department of Defense. Performance specification - Propellant, Hydrazine, MIL-PRF-26536F.; 2011. Available online: http://everyspec.com/MIL-PRF/MIL-PRF-010000-29999/MIL-PRF-26536F_32241/ (accessed on 27 August 2024).
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004; Volume 85. [Google Scholar]
- U.S. Defence Logistics Agency. Standard Prices for Aerospace Products.; 2024. Available online: https://www.dla.mil/Energy/Business/Standard-Prices/ (accessed on 25 August 2024).
- Wingborg, N. Heat of Formation of ADN-Based Liquid Monopropellants. Propellants Explos. Pyrotech. 2019, 44, 1090–1095. [Google Scholar] [CrossRef]
- Anflo, K.; Möllerberg, R. Flight demonstration of new thruster and green propellant technology on the PRISMA satellite. Acta Astronaut. 2009, 65, 1238–1249. [Google Scholar] [CrossRef]
- Gotzig, U. Challenges and economic benefits of green propellants for satellite propulsion. In Proceedings of the 7th European Conference for Aeronautics and Space Sciences (EUCASS), Milan, Italy, 3–6 July 2017. [Google Scholar]
- Digital Solid State Propulsion Inc. AF-M315E Safety Data Sheet. 2024. Available online: https://static1.squarespace.com/static/59de9c9c18b27ddf3bac610a/t/5e3af2d0fb8df43f9b2ae377/1580921553250/AF-M315E+SDS.pdf (accessed on 24 August 2024).
- Digital Solid State Propulsion Inc. Chemicals and Propellants—AF-M315E Pricing. 2024. Available online: https://dssptech.com/propellant-products (accessed on 24 August 2024).
- Evonik Operations GmbH. Aeornautic Applications Brochure. 2024. Available online: https://active-oxygens.evonik.com/zh/attachment/140277?rev=9a24167032bd618d7f7ff672a62fa152 (accessed on 24 August 2024).
- Evonik Active Oxygens, LLC. Hydrogen Peroxide Physico-Chemical Properties. 2024. Available online: https://active-oxygens.evonik.com/en/products-and-services/hydrogen-peroxide/general-information/physico-chemical-properties (accessed on 24 August 2024).
- Jarosiewicz, M.; Szychlinski, J. Gas chromatographic determination of impurities in nitromethane. Chromatographia 1980, 13, 35–39. [Google Scholar] [CrossRef]
- House, W.C. Project SQUID. In Liquid Propellant Rockets, Volume II, Part 2; Filed Survey Report; Princeton University: Princeton, NJ, USA, 1947; pp. 48–50. [Google Scholar]
- Clark, J.D. Ignition!: An Informal History of Liquid Rocket Propellants; Rutgers University Press: New Brunswick, NJ, USA, 1972. [Google Scholar]
- Boyer, E.; Kuo, K. Characteristics of nitromethane for propulsion applications. In Proceedings of the 44th AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 9–12 January 2006; p. 361. [Google Scholar]
- Makovky, A.; Lenji, L. Nitromethane-physical properties, thermodynamics, kinetics of decomposition, and utilization as fuel. Chem. Rev. 1958, 58, 627–644. [Google Scholar] [CrossRef]
- Hermoni, A. Role of rate of decomposition of nitric oxide in the use of nitromethane as a monopropellant. J. Appl. Chem. 1959, 9, 420–422. [Google Scholar] [CrossRef]
- Benziger, J.B. Decomposition of Nitromethane over NiO and Cr2O3 Catalysts. Combust. Sci. Technol. 1982, 29, 191–205. [Google Scholar] [CrossRef]
- Benziger, J.B. A mechanistic study of nitromethane decomposition on nickel. Appl. Surf. Sci. 1984, 17, 309–323. [Google Scholar] [CrossRef]
- Kelzenberg, S.; Eisenreich, N.; Eckl, W.; Weiser, V. Modelling nitromethane combustion. Propellants Explos. Pyrotech. 1999, 24, 189–194. [Google Scholar] [CrossRef]
- Zoll, E.J.J.; Foster, J.W.; Hodges, E.S.; James, G.R.J.; Pefferle, F.R.; Egan, R.M.; Greenwald, C.W.; Heckenkamp, R.G.; Hart, T.B.; Thompson, C.; et al. Accident at Mt. Pulaski, Ill., on June 1 1958 Caused by the Explosion of a Tank Car Loaded with Nitromethane; Technical Report; Interstate Commerce Comission: Washington, DC, USA, 1958. [Google Scholar]
- Minato, R.; Tanaka, S. Fluid hammer phenomena for Nitromethane propellant feed system. Sci. Technol. Energetic Mater. 2022, 83, 111–116. [Google Scholar]
- Campbell, A.; Malin, M.; Holland, T. Temperature effects in the liquid explosive, nitromethane. J. Appl. Phys. 1956, 27, 963. [Google Scholar] [CrossRef]
- Bellinger, F.; Friedman, H.; Bauer, W.; Eastes, J.; Bull, W. Chemical Propellants. Stability of Mononitromethane. Ind. Eng. Chem. 1948, 40, 1320–1323. [Google Scholar] [CrossRef]
- Kusakabe, M.; Fujiwara, S. Effects of liquid diluents on detonation propagation in nitromethane. In Proceedings of the Sixth Symposium on Detonation, Coronado, CA, USA, 24–27 August 1976; pp. 133–142. [Google Scholar]
- Ananin, A.V.; Koldunov, S.A.; Garanin, V.V.; Sosikov, V.A.; Torunov, S.I. Shock wave sensitivity of nitromethane mixtures with nonexplosive liquids. Int. J. Energetic Mater. Chem. Propuls. 2013, 12, 87–94. [Google Scholar] [CrossRef]
- Kurilov, M.; Werling, L.; Negri, M.; Kirchberger, C.; Schlechtriem, S. Impact Sensitivity of Nitromethane-based Green-propellant Precursor Mixtures. Int. J. Energetic Mater. Chem. Propuls. 2023, 22, 35–43. [Google Scholar] [CrossRef]
- Klein, S.; Kurilov, M. Fluid hammer phenomena in a nitromethane-based green propellant in hot gas test runs. In Proceedings of the Space Propulsion Conference 2024, Glasgow, UK, 23 May 2024. [Google Scholar]
- Kindsvater, H.; Kendall, K.; Mueller, K.; Datner, P. Research on Nitromethane; Aeroject Engineering Cooperation, Report; Aeroject Engineering Cooperation: Rancho Cordova, CA, USA, 1951. [Google Scholar]
- Yetter, R.A.; Yang, V.; Wu, M.H.; Wang, Y.; Milius, D.; Aksay, I.A.; Dryer, F.L. Combustion issues and approaches for chemical microthrusters. Int. J. Energetic Mater. Chem. Propuls. 2007, 6, 393–424. [Google Scholar] [CrossRef]
- Shrestha, K.P.; Vin, N.; Herbinet, O.; Seidel, L.; Battin-Leclerc, F.; Zeuch, T.; Mauss, F. Insights into nitromethane combustion from detailed kinetic modeling–Pyrolysis experiments in jet-stirred and flow reactors. Fuel 2020, 261, 116349. [Google Scholar] [CrossRef]
- Kurilov, M.; Ziemer, L.; Weiser, V.; Ricker, S.; Kirchberger, C.; Schlechtriem, S. Ignition of Nitromethane-based Propellant Mixtures. Int. J. Energetic Mater. Chem. Propuls. 2024, 23, 53–67. [Google Scholar] [CrossRef]
- Kayser, J.C.; Shambaugh, R.L. Discharge coefficients for compressible flow through small-diameter orifices and convergent nozzles. Chem. Eng. Sci. 1991, 46, 1697–1711. [Google Scholar] [CrossRef]
- Suslov, D.; Woschnak, A.; Sender, J.; Oschwald, M.; Haidn, O. Test specimen design and measurement technique for investigation of heat transfer processes in cooling channels of rocket engines under real thermal conditions. In Proceedings of the 39th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Huntsville, AL, USA, 20–23 July 2003; p. 4613. [Google Scholar]
- Dieck, R.H. Measurement Uncertainty: Methods and Applications; ISA: Kowloon, Hongkong, 2007. [Google Scholar]
- Lindberg, V. Uncertainties and error propagation. Manual on Uncertainties, Graphing and the Vernier Caliper, Part I; Rochester Institute of Technology: New York, NY, USA, 2000; Available online: http://www.rit.edu/uphysics/uncertinities/Uncertinitiespart2.html#addsub (accessed on 30 August 2024).
- Boyer, E.; Kuo, K.K. Modeling of nitromethane flame structure and burning behavior. Proc. Combust. Inst. 2007, 31, 2045–2053. [Google Scholar] [CrossRef]



| Propellant | Composition | Liquid Range | Cost |
|---|---|---|---|
| Hydrazine | Impurities: 0.5 to2% H2O, NH4, aniline [32] | 1.4 to 114 °C [33] | 335 [34] |
| LMP-103S | 63.0% ADN, 18.4% CH3OH, 18.6% NH4 [35] | 10 to 50 °C [36] | >1000 [37] |
| AF-M315E | 44.5% HAN, 44.5% HEHN, 11% H2O [18] | −80 to 93 °C [38] | 726 [39] |
| Hydrogen peroxide (98%) | 2% H2O [40,41] | −2 to 147 °C [40,41] | ∼100 * |
| NMP-001 | 85% Nitromethane, 13% DMSO, 2% Ferrocene | 10 °C to N/A | N/A |
| Nitromethane (98.5%) | ≤0.3% H2O, nitroalkane impurities [42] | −28.3 to 101.2 °C [33] | <30 * |
| Pcc [bar] | PO2 [bar] | L* [m] | T0 [°C] | ΔTw, ign [°C] | Ignition Method | |
|---|---|---|---|---|---|---|
| S2 | 13.9 | 23.4 | 8.4 | 31 | 44 | Glow plug and O2 |
| C1 [29] | 15.1 | 28.2 | 11.6 | 29 | 87 | H2/O2 torch |
| C3 [29] | 16.6 | 36.2 | 7.3 | 33 | 116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurilov, M.; Kirchberger, C.U.; Schlechtriem, S. Characterization of an Ignition System for Nitromethane-Based Monopropellants. Aerospace 2024, 11, 1001. https://doi.org/10.3390/aerospace11121001
Kurilov M, Kirchberger CU, Schlechtriem S. Characterization of an Ignition System for Nitromethane-Based Monopropellants. Aerospace. 2024; 11(12):1001. https://doi.org/10.3390/aerospace11121001
Chicago/Turabian StyleKurilov, Maxim, Christoph U. Kirchberger, and Stefan Schlechtriem. 2024. "Characterization of an Ignition System for Nitromethane-Based Monopropellants" Aerospace 11, no. 12: 1001. https://doi.org/10.3390/aerospace11121001
APA StyleKurilov, M., Kirchberger, C. U., & Schlechtriem, S. (2024). Characterization of an Ignition System for Nitromethane-Based Monopropellants. Aerospace, 11(12), 1001. https://doi.org/10.3390/aerospace11121001







