Effects of Wire-Wrapping Patterns and Low Temperature on Combustion of Propellant Embedded with Metal Wire
Abstract
:1. Introduction
2. Experimental Methodology
3. Results and Discussions
3.1. Thermal Reactivity
3.2. Ignition Delay and Burning Rate
3.3. Condensed Combustion Products
3.4. Effect of Low Temperature
3.5. Numerical Model and Model Calculation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rumbel, K.; Cohen, M.; Henderson, C.; Scurlock, A. A physical means of attaining high burning rate in solid propellants. In Proceedings of the Eleventh Army-Navy-Air Force Solid Propellant Meeting, Washington, DC, USA, 12–14 October 1955. [Google Scholar]
- King, M.K. Analytical modeling of effects of wires on solid motor ballistics. J. Propuls. Power 1991, 7, 312–321. [Google Scholar] [CrossRef]
- Gossant, B.; Godfroy, F.; Robert, P.H. Theoretical calculus of burning rate ratio in grains with embedded metal wires. In Proceedings of the 24th Joint Propulsion Conference, Boston, MA, USA, 11–13 July 1988; p. 3255. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X. Numerical Research for Adjusting Thrust of Solid Rocket Motor by Using the Embedded-wire Grain. Acta Armamentarii 2007, 28, 1218. [Google Scholar]
- Rumbel, K.E. Poly(vinyl chloride) Plastisol Propellants. In Propellants Manufacture, Hazards, and Testing; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1969; Volume 88, pp. 36–66. [Google Scholar]
- Shuling, C.; Fengsheng, L. Influence of long metal wires on combustion of double-base propellants. Combust. Flame 1982, 45, 213–218. [Google Scholar] [CrossRef]
- Caveny, L.H.; Glick, R.L. Influence of embedded metal fibers on solid- propellant burning rate. J. Spacecr. Rocket. 1967, 4, 79–85. [Google Scholar] [CrossRef]
- Bakhman, N.N.; Lobanov, I.N. Influence of the diameter of the heat-conducting elements on their efficiency during the combustion of condensed systems. Combust. Explos. Shock Waves 1983, 19, 42–46. [Google Scholar] [CrossRef]
- Kubota, N.; Ichida, M.; Fujisawa, T. Combustion Processes of Propellants with Embedded Metal Wires. AIAA J. 1982, 20, 116–121. [Google Scholar] [CrossRef]
- Wilson, P.B. Tactical Missile Performance for Single and Multi-Wire Embedded Propellant Configurations with Discontinuities. 2019. Available online: https://scholar.afit.edu/etd/2238 (accessed on 1 August 2024).
- Rajak, R.; Lim, D.; Gnanaprakash, K.; Oh, J.; Yoh, J.J. Peculiar Burning Characteristics of Electrically Controlled Solid Propellants. In Proceedings of the 29th International Colloquium on the Dynamics of Explosions and Reactive Systems, Siheung-si, Republic of Korea, 23–28 July 2023. [Google Scholar]
- Xiao, Z.; Wang, J.; Wang, J. Method of Ablation Heat Shield Design of End-Burning Grain with Embedded Wire. Aerosp. Hanghai 2010, 2, 1006–1630. [Google Scholar] [CrossRef]
- Yu, N.; Yang, T.; Liu, W.; Li, L. Theoretical Performance Analysis of End-burning Grain Gas Generator Embedded with Silver-wire. J. Proj. Rocket. Missiles Guid. 2009, 29. [Google Scholar] [CrossRef]
- Wu, Q.; Ren, Q. Tuning the Ballistic Performance of a Single-Burning-Rate Grain Solid Rocket Motor via New Discontinuous Embedded Metal Wires. Aerospace 2024, 11, 308. [Google Scholar] [CrossRef]
- Cohen, N.S.; Flanigan, D.A. Mechanisms and models of solid-propellant burn rate temperature sensitivity—A review. AIAA J. 1985, 23, 1538–1547. [Google Scholar] [CrossRef]
- Atwood, A.I.; Boggs, T.L.; Curran, P.O.; Parr, T.P.; Hanson-Parr, D.M.; Price, C.F.; Wiknich, J. Burning Rate of Solid Propellant Ingredients, Part 2: Determination of Burning Rate Temperature Sensitivity. J. Propuls. Power 1999, 15, 748–752. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Yuan, J.; Chen, X.; Zhao, Y.; Xie, W. Initial Temperature Effects on the Combustion Characteristics of Al. Propellants Explos. Pyrotech. 2022, 47, e202100365. [Google Scholar] [CrossRef]
- Atwood, A.I.; Boggs, T.L.; Curran, P.O.; Parr, T.P.; Hanson-Parr, D.M.; Price, C.F.; Wiknich, J. Burning Rate of Solid Propellant Ingredients, Part 1: Pressure and Initial Temperature Effects. J. Propuls. Power 1999, 15, 740–747. [Google Scholar] [CrossRef]
- Liu, L.; Ao, W.; Wen, Z.; Zhang, Y.; Lv, X.; Qin, Z.; Liu, P. Combustion promotion and agglomeration reduction of the composite propellant using graphene. Aerosp. Sci. Technol. 2021, 118, 106988. [Google Scholar] [CrossRef]
- Ji, S.; Wang, B.; Zhao, D. Numerical analysis on combustion instabilities in end-burning-grain solid rocket motors utilizing pressure-coupled response functions. Aerosp. Sci. Technol. 2020, 98, 105701. [Google Scholar] [CrossRef]
- Zou, X.; Wang, N.; Wang, J.; Feng, Y.; Shi, B. A numerical investigation on heterogeneous combustion of aluminum nanoparticle clouds. Aerosp. Sci. Technol. 2021, 112, 106604. [Google Scholar] [CrossRef]
- Li, C.; Wu, G.; Li, M.; Hu, C.; Wei, J. A heat transfer model for aluminum droplet/wall impact. Aerosp. Sci. Technol. 2020, 97, 105639. [Google Scholar] [CrossRef]
- Liu, T.-K.; Perng, H.-C.; Luh, S.-P.; Liu, F. Aluminum agglomeration in ammonium perchlorate/cyclotrimethylene trinitramine/aluminum/hydroxy-terminated polybutadiene propellant combustion. J. Propuls. Power 1992, 8, 1177–1184. [Google Scholar] [CrossRef]
- Jackson, T.L.; Najjar, F.; Buckmaster, J. New Aluminum Agglomeration Models and Their Use in Solid-Propellant-Rocket Simularions. J. Propuls. Power 2005, 21, 925–936. [Google Scholar] [CrossRef]
- Orlandi, O.; Plaud, M.; Godfroy, F.; Larrieu, S.; Cesco, N. Aluminium droplets combustion and SRM instabilities. Acta Astronaut. 2019, 158, 470–479. [Google Scholar] [CrossRef]
- Liang, D.; Xiao, R.; Liu, J.; Wang, Y. Ignition and heterogeneous combustion of aluminum boride and boron–aluminum blend. Aerosp. Sci. Technol. 2019, 84, 1081–1091. [Google Scholar] [CrossRef]
- Li, L.-B.; Chen, X.; Musa, O.; Zhou, C.-S.; Zhu, M. The effect of pressure and oxygen concentration on the ignition and combustion of aluminum–magnesium fuel-rich propellant. Aerosp. Sci. Technol. 2018, 76, 394–401. [Google Scholar] [CrossRef]
- Babuk, V.A.; Vasilyev, V.A.; Malakhov, M.S. Condensed Combustion Products at the Burning Surface of Aluminized Solid Propellant. J. Propuls. Power 1999, 15, 783–793. [Google Scholar] [CrossRef]
- Xiong, W.Q.; Liu, Y.J.; Zhang, T.F.; Wu, S.X.; Zeng, D.W.; Guo, X.; Pang, A.M. Effect of Al-Li Alloy on the Combustion Performance of AP/RDX/Al/HTPB Propellant. Aerospace 2023, 10, 222. [Google Scholar] [CrossRef]
- Yue, S.C.; Liu, L.; Liu, H.; Jiang, Y.F.; Liu, P.J.; Pang, A.M.; Zhang, G.X.; Ao, W. Agglomerate Size Evolution in Solid Propellant Combustion under High Pressure. Aerospace 2023, 10, 515. [Google Scholar] [CrossRef]
- Liu, Z.; Li, S.; Liu, M.; Guan, D.; Sui, X.; Wang, N. Experimental investigation of the combustion products in an aluminised solid propellant. Acta Astronaut. 2017, 133, 136–144. [Google Scholar] [CrossRef]
- Babuk, V.A.; Vassiliev, V.A.; Sviridov, V.V. Propellant Formulation Factors and Metal Agglomeration in Combustion of Aluminized Solid Rocket Propellant. Combust. Sci. Technol. 2001, 163, 261–289. [Google Scholar] [CrossRef]
- Liu, T.-K. Experimental and Model Study of Agglomeration of Burning Aluminized Propellants. J. Propuls. Power 2005, 21, 797–806. [Google Scholar] [CrossRef]
- Naya, T.; Kohga, M. Influences of particle size and content of RDX on burning characteristics of RDX-based propellant. Aerosp. Sci. Technol. 2014, 32, 26–34. [Google Scholar] [CrossRef]
- Takahashi, K.; Oide, S.; Kuwahara, T. Agglomeration Characteristics of Aluminum Particles in AP/AN Composite Propellants. Propellants Explos. Pyrotech. 2013, 38, 555–562. [Google Scholar] [CrossRef]
- Cohen, N.S. A pocket model for aluminum agglomeration in composite propellants. AIAA J. 1983, 21, 720–725. [Google Scholar] [CrossRef]
- Golub, G. Need for a variable burning-rate solid propellant. J. Spacecr. Rocket. 1965, 2, 593–594. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, Z.; Gou, D.; Shu, Y.; Liu, P.; Pang, A.; Ao, W. Elaborative collection of condensed combustion products of solid propellants: Towards a real Solid Rocket Motor (SRM) operational environment. Chin. J. Aeronaut. 2024, 37, 77–88. [Google Scholar] [CrossRef]
- Ao, W.; Liu, P.; Liu, H.; Wu, S.; Tao, B.; Huang, X.; Li, L.K.B. Tuning the agglomeration and combustion characteristics of aluminized propellants via a new functionalized fluoropolymer. Chem. Eng. J. 2020, 382, 122987. [Google Scholar] [CrossRef]
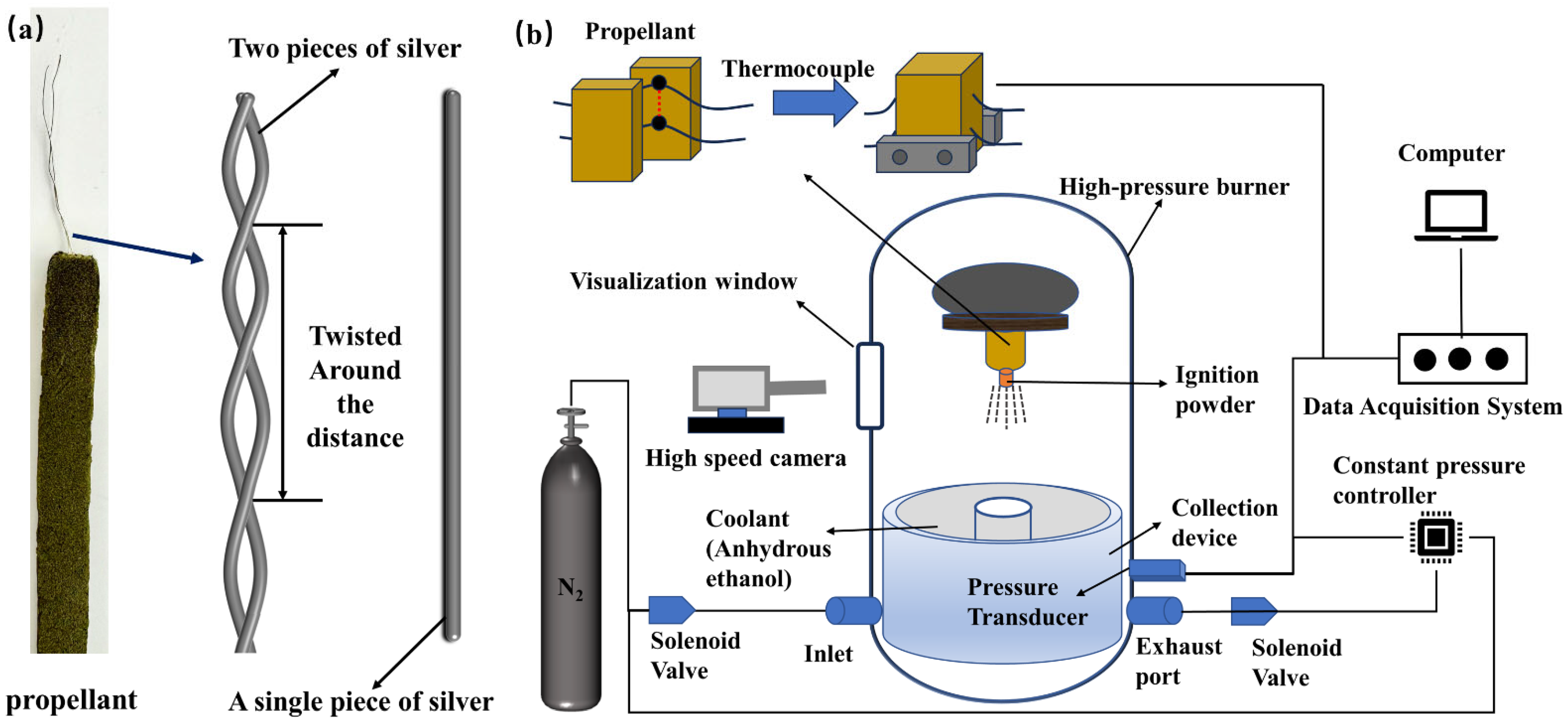

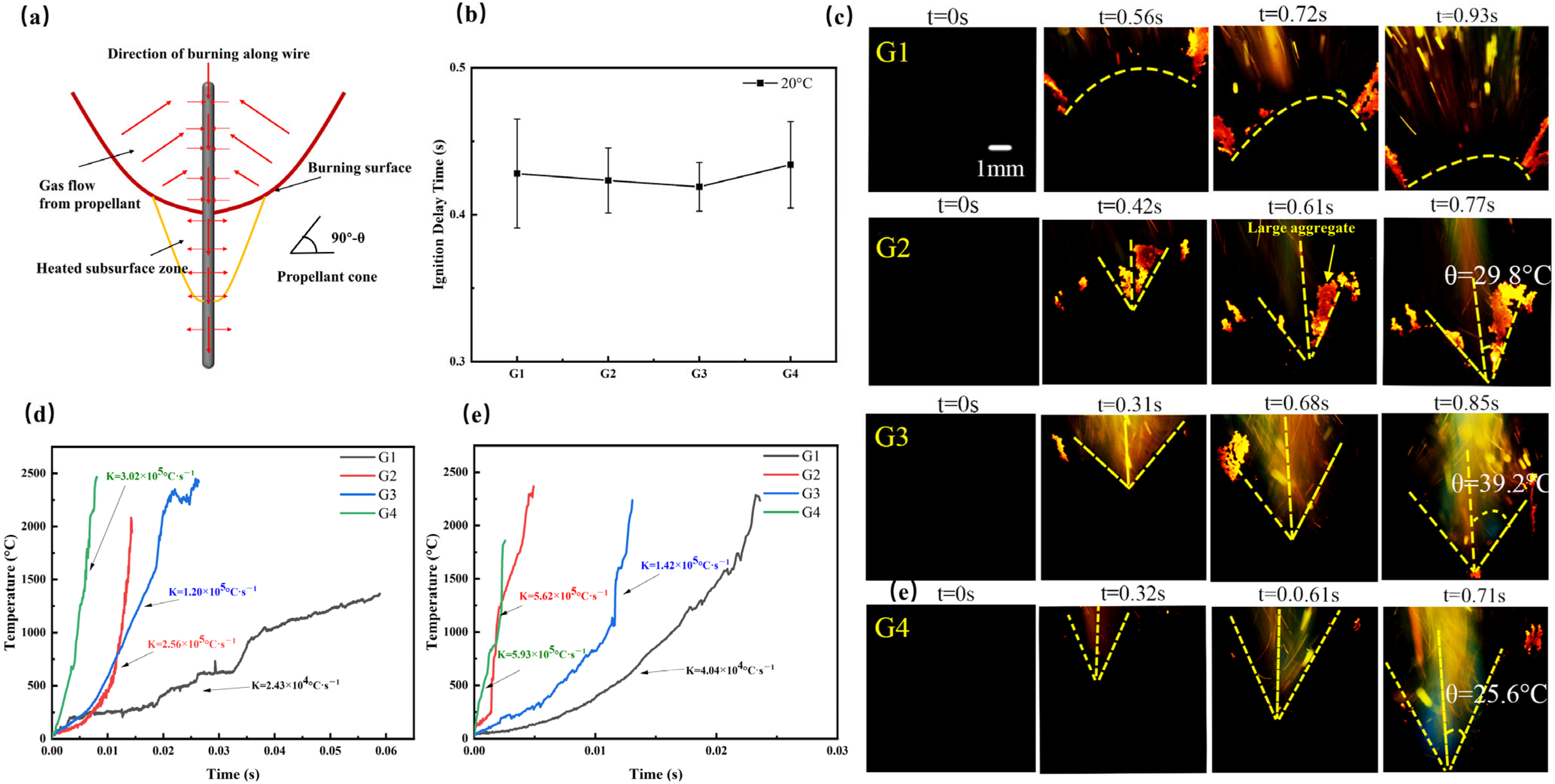
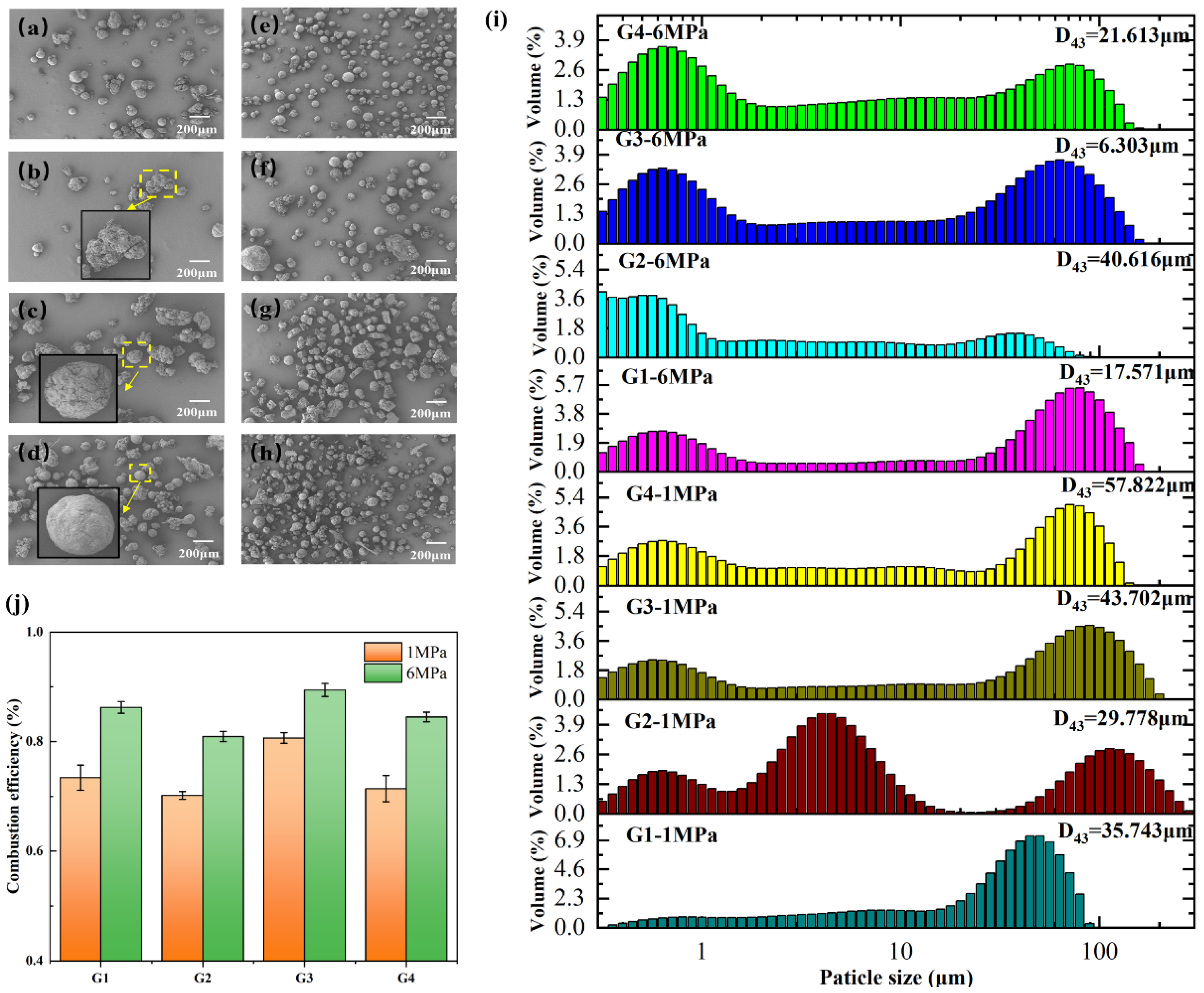
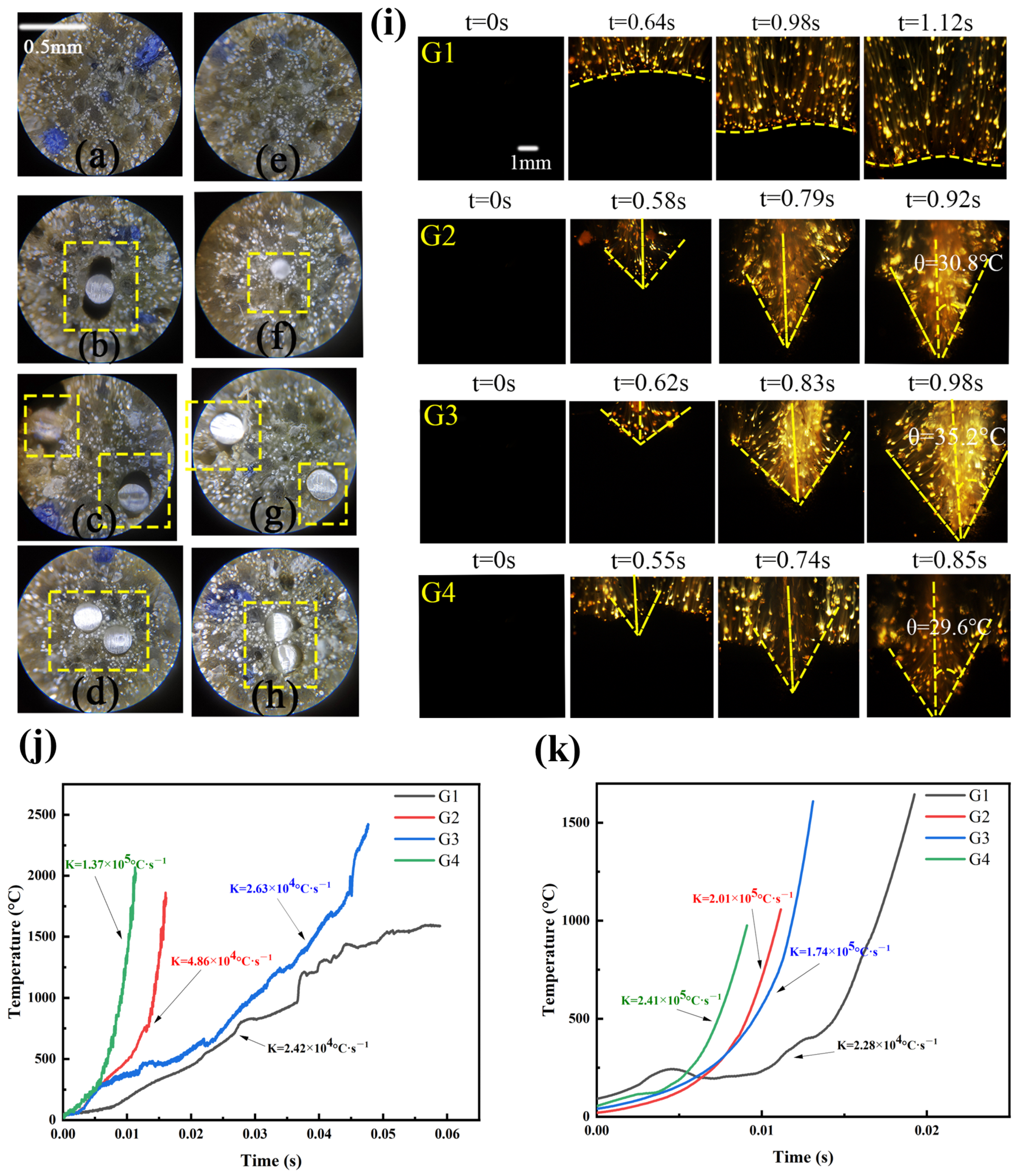

| Sample | Quantity | Wire Diameter (mm) | Twisted around the Distance (mm) | Surface Area of Silvered Wire per Unit Length of Propellant (mm2·mm−1) | Surface-Volume Ratio (mm2·mm−3) |
|---|---|---|---|---|---|
| No silver wire(G1) | 0 | - | - | - | - |
| A piece of silver(G2) | 1 | 0.25 | - | 0.78 | 16 |
| twisted sparsely(G3) | 2 | 0.25 | 100 | 1.56 | 16 |
| twisted tightly(G4) | 2 | 0.25 | 50 | 1.56 | 16 |
| Sample | TG | DTG | |||
|---|---|---|---|---|---|
| Ti | Te | MC | Tp | Lmax | |
| G1 | 132.53 | 306.18 | −102.79 | 302.61 304.35 305.53 | 2.624 −65.21 6.58 |
| G2 | 132.53 | 312.72 | −89.66 | 309.11 311.75 | −18.63 4.08 |
| G3 | 132.93 | 316.32 | −75.26 | 314.65 315.26 | −11.53 1.66 |
| G4 | 133.15 | 317.43 | −75.82 | 314.97 316.07 | −11.52 1.66 |
| Sample | Exotherm Peak | |||
|---|---|---|---|---|
| Tp | Ti | Te | Q | |
| G1 | 234.69 274.88 305.29 1127.01 | 197.97 241.38 288.93 1069.2 | 241.38 288.93 320.47 1150.13 | 9.17 60.66 54.83 5.95 |
| G2 | 228.92 274.31 323.58 660.32 1131.62 | 193.93 243.28 313.06 650.43 1072.54 | 243.28 289.58 338.73 667.61 1180.14 | 10.73 79.23 156.21 −3.12 23.18 |
| G3 | 226.37 277.13 317.78 1107.14 | 197.98 241.21 292.77 1073.21 | 241.21 292.77 337.36 1188.12 | 7.38 69.11 222.80 16.16 |
| G4 | 225.62 279.13 314.72 1109.28 | 198.43 239.31 291.76 1071.43 | 242.26 295.77 335.36 1186.22 | 8.18 64.51 224.88 18.42 |
| Sample | Stable Burning Length (mm) | Burning Time (ms) | Burning Rate (mm·s−1) |
|---|---|---|---|
| G1-1Mpa (20 °C) | 19.8 | 1.01404 (±0.021) | 30.52585 (±1.37) |
| G1-6Mpa (20 °C) | 19.9 | 0.425347 (±0.021) | 46.78532 (±2.1) |
| G1-1Mpa (−40 °C) | 20.1 | 1.487254 (±0.02) | 28.51484 (±1.17) |
| G1-6Mpa (−40 °C) | 20.1 | 0.47197 (±0.019) | 42.58747 (±1.73) |
| G2-1Mpa (20 °C) | 19.6 | 0.360963 (±0.019) | 54.29926 (±2.85) |
| G2-6Mpa (20 °C) | 20 | 0.199135 (±0.021) | 100.4343 (±3.16) |
| G2-1Mpa (−40 °C) | 20 | 0.437582 (±0.02) | 45.70569 (±1.99) |
| G2-6Mpa (−40 °C) | 19.5 | 0.205006 (±0.021) | 95.11936 (±3.45) |
| G3-1Mpa (20 °C) | 19.7 | 0.459847 (±0.019) | 42.84031 (±1.78) |
| G3-6Mpa (20 °C) | 19.8 | 0.234779 (±0.021) | 84.33481 (±2.62) |
| G3-1Mpa (−40 °C) | 19.8 | 0.486627 (±0.022) | 40.68823 (±1.60) |
| G3-6Mpa (−40 °C) | 20.1 | 0.221692 (±0.021) | 90.66646 (±2.50) |
| G4-1Mpa (20 °C) | 20.1 | 0.232092 (±0.02) | 86.60375 (±2.87) |
| G4-6Mpa (20 °C) | 20.2 | 0.175069 (±0.019) | 115.383 (±3.82) |
| G4-1Mpa (−40 °C) | 19.5 | 0.256719 (±0.02) | 75.95843 (±1.48) |
| G4-6Mpa (−40 °C) | 19.9 | 0.192009 (±0.019) | 103.6408 (±3.77) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Zhao, J.; Ren, Q. Effects of Wire-Wrapping Patterns and Low Temperature on Combustion of Propellant Embedded with Metal Wire. Aerospace 2024, 11, 639. https://doi.org/10.3390/aerospace11080639
Wu Q, Zhao J, Ren Q. Effects of Wire-Wrapping Patterns and Low Temperature on Combustion of Propellant Embedded with Metal Wire. Aerospace. 2024; 11(8):639. https://doi.org/10.3390/aerospace11080639
Chicago/Turabian StyleWu, Qiu, Jiangong Zhao, and Quanbin Ren. 2024. "Effects of Wire-Wrapping Patterns and Low Temperature on Combustion of Propellant Embedded with Metal Wire" Aerospace 11, no. 8: 639. https://doi.org/10.3390/aerospace11080639
APA StyleWu, Q., Zhao, J., & Ren, Q. (2024). Effects of Wire-Wrapping Patterns and Low Temperature on Combustion of Propellant Embedded with Metal Wire. Aerospace, 11(8), 639. https://doi.org/10.3390/aerospace11080639





