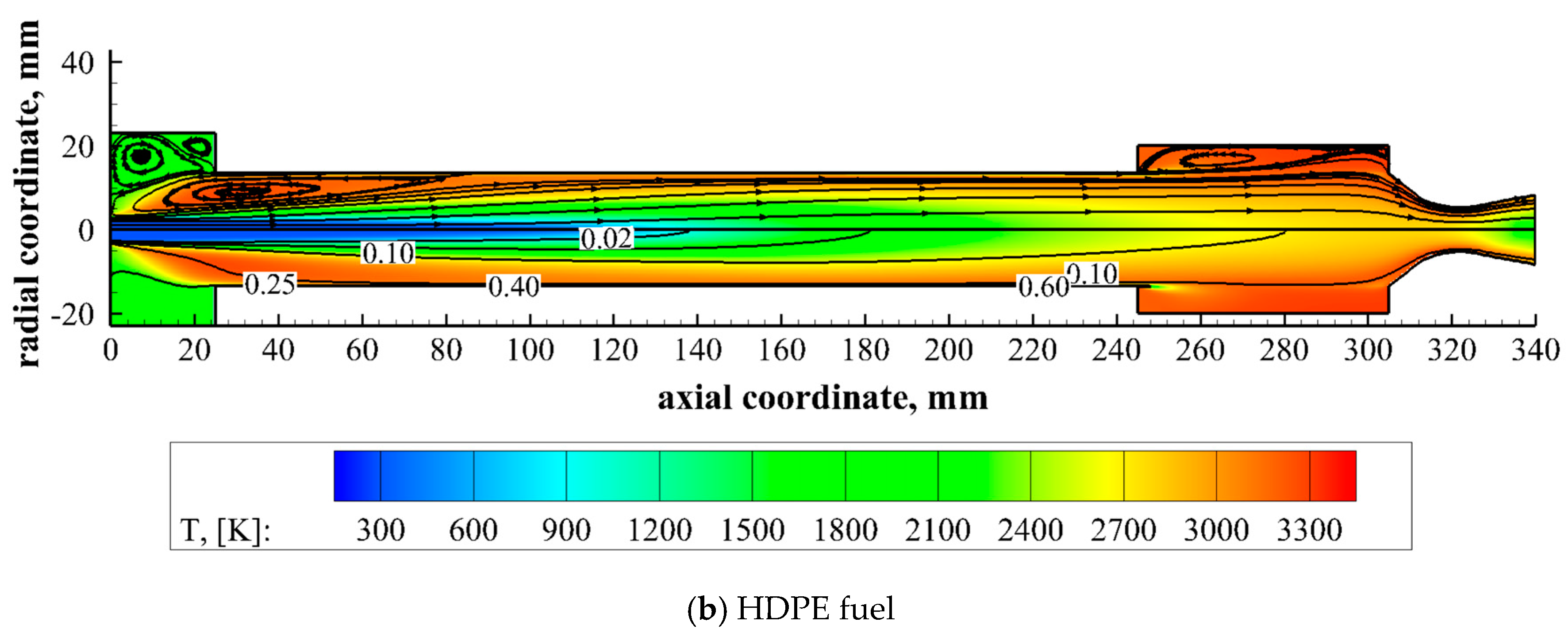
The theoretical model formulation is completed by assigning the boundary conditions at the interface between the gaseous flow region and the solid fuel wall, which can properly describe the fuel consumption mechanism. The fuel surface is an inlet boundary along which the fuel mass flux, the temperature and the mixture fraction depend on the regression rate that is an unknown to be determined.
Now it is necessary to separate the case of classical polymers from the one of liquefying fuels. In fact, while regardless of the type of fuel, the gas/surface interface treatment relies on local mass, energy and mean mixture-fraction balances. In the former case, an additional pyrolysis rate equation is needed for modeling the regression rate, whereas, in the latter case, a different formulation is introduced to account for the entrainment of liquid paraffin from the unstable melt layer forming along the fuel surface, which is the dominant consumption mechanism.
3.3.1. Classical Non-Liquefying Fuels
In the case of pyrolyzing fuels, since no material is removed from the surface in a condensed phase (neither solid, such as in the case of fuel loaded with metal particles; nor liquid, as in the case of paraffin wax analyzed later), the mass conservation at the gas-solid interface imposes that
where
is the gas density at the wall and
is the normal-to-wall velocity component due to the pyrolysis products injection;
is the solid fuel density and
is the local regression rate.
The energy balance at the gas-solid interface, taking into account the convective heat transfer from the gas to the fuel surface, the steady heat conduction into the solid, and neglecting the radiation, leads to the following relationship between the convective heat flux to the wall,
, and the regression rate [
29]
where
is the coordinate normal to the surface oriented from solid to gas,
is the gas thermal conductivity,
is the solid heat capacity per unit mass,
is the so-called heat of pyrolysis,
Tw is the fuel surface temperature, and
Ta is its initial temperature (which is assumed to be equal to the one of the external surface of the fuel). The term in brackets at the right-hand side represents the effective heat of gasification of the fuel, which accounts for the heat radially conducted into the solid grain, further than for the heat of pyrolysis. Note that Equation (25), by properly defining the heat of pyrolysis, implicitly takes into account the heat transfer to the wall by the species diffusion [
30].
The fuel pyrolysis is, finally, modeled with the following Arrhenius-type equation [
25]:
where
is the pre-exponential factor,
is the activation energy and
is the universal gas constant.
The values of the constants appearing in Equations (25) and (26) considered for the HDPE fuel grains analyzed in this work are summarized in
Table 3. The density, specific heat and heat of pyrolysis are taken from the work in Reference [
53], while the values of the pre-exponential factor and the activation energy from Reference [
25] are obtained by modifying the activation energy to match the surface temperature commonly observed in polymeric hybrid fuels (which is around 800 K) [
54].
A dedicated treatment of the mean mixture-fraction boundary condition at the fuel wall is needed as well. The fuel regression rate is typically low in hybrid rockets; therefore, on the one hand, the normal convection of the fuel at the grain surface is relatively weak compared to the gas convection in the cells near the boundary. On the other hand, the diffusive flux plays a dominant role in the mixture-fraction transport near the grain surface where the species concentrations rapidly change, so that a steep mixture-fraction gradient at the fuel wall is present. As a consequence, the simple Dirichlet boundary condition,
, on the gas-fuel interface (which is equivalent to imposing that the diffusion coefficient is equal to zero in the cells close to the fuel inlet boundary) is not adequate. In fact, first, it would imply a non-exact evaluation of the gradients in this zone, and, in particular, of the heat flux to the wall (which, for Equation (25), would produce a flawed regression rate), and, second, an extra mixture fraction would be diffused into the flow affecting the global oxidizer to fuel ratio and the chemical equilibrium properties, which eventually would lead to an incorrect estimation of the characteristic exhaust velocity and chamber pressure. The correct solution to this problem is considering an additional equation for the mean mixture-fraction balance at the gas-solid interface [
55], which, by rearranging Equation (15) at the wall, can be expressed as
According to Equation (27), the total mass flux entering the gaseous domain due to the solid fuel regression, which appears on the right-hand side of the equation and represents the production term, is partially balanced by the convection and partially by the diffusion of the fuel mass fraction.
The balance of the mixture-fraction variance, , at the wall is instead ignored and it is imposed to be zero.
Note that Equations (25)–(27), upon substitution of Equation (24) into Equation (27) for the wall mass flux, constitute a system of three algebraic equations for the three unknowns—regression rate, the surface temperature and the mixture fraction at the wall—which need the computation of the flowfield at each iterative step to be solved.
The required level of mesh refinement near the grain surface allows for the resolution of the viscous sub-layer, with the following boundary conditions for the turbulent kinetic energy and the specific dissipation rate, respectively:
where
is the friction velocity.
Both the source terms appearing in Equations (1), (15) and (20) are identically zero:
As we will see in the forthcoming section, they are instead calculated in the modeling of the paraffin-wax fuel combustion.
3.3.2. Liquefying Fuels
The boundary conditions for the turbulent quantities applied in this case are equal to those described above for polymeric fuels.
The regression rate,
, can be, now, assumed composed of two terms: the vaporization fraction,
, that is generated by the liquid thermal decomposition and later vaporization into the gas stream, and the entrainment fraction,
, that is related to the mechanical transfer of the liquid from the surface melt layer
A scheme of this peculiar fuel consumption mechanism is shown in
Figure 2 in the case of the supercritical regime: part of the molten fuel on the solid surface undergoes pyrolysis and part leaves the surface in the form of a supercritical fluid that is entrained in the gas stream and burns farther from the wall. More precisely, the molten paraffin wax will behave like a dense fluid which, for simplicity, we call “liquid” and assume having the same properties as paraffin in the liquid state.
A set of equations is, thus, needed for the calculation of the regression rate and its two components, along with the resulting fuel mass flow rates, whose solution is to be incorporated in the fluid dynamic computation. As in the previous case, the mass, energy and mixture-fraction balances at the gas/fuel surface boundary are formulated, with a difference that an additional equation for the calculation of the entrainment component of the fuel mass flow rate is required.
Following the arguments in References [
7,
54], by coupling the energy balance equations at both the liquid-solid and gas-liquid interfaces (see
Figure 2), at the steady state, the following relationship of the total surface heat flux with the total and the vaporization regression rate is obtained
where
is the solid fuel density,
and
are the specific heats of the solid and liquid fuels (which are considered independent from the temperature here), respectively,
is the fuel melting temperature, and
and
are the fuel heat of the fusion and the heat of pyrolysis, respectively.
Equation (31) represents the fact that the total heat flux transferred from the combusting gases to the fuel surface must be equal to the sum of heat conducted into the liquid layer and the heat required for the pyrolysis of the fuel vaporized fraction (the overall term in the square brackets and last term on the right-hand side of Equation (31), respectively). Note that, for the finite thickness of the liquid layer, the conductive heat flux at the liquid-gas interface is not equal to , which one might accidentally expect by analogy with the heat conducted in the solid appearing in Equation (25).
In the subcritical regime, the wall phenomena are governed by the evaporation process and
(
being the heat of vaporization) and the wall temperature is equal to the vaporization temperature; whereas, in supercritical conditions, pyrolysis takes place on the surface. In the absence of clear paraffin pyrolysis data, for the sake of simplicity, and also in the supercritical case, the heat of vaporization reported in Reference [
7] has been employed by neglecting the heat required for the thermal degradation of paraffin into gaseous ethylene monomers. The wall temperature has a significant influence on the fuel regression rate as it affects both the heat flux and the term
appearing in the wall energy balance, Equation (31). Compared to a purely pyrolyzing polymer, due to the effect of the entrainment, the surface temperature is reduced [
54]; however, this parameter can be hardly determined and. in Reference [
56], a sensitivity analysis was performed for which the value of 675 K is the one allowing for the best fit of the experimental data. An isothermal boundary condition is set all over the fuel wall.
It is worth noting that regardless of the definition of the wall temperature and enthalpy (either vaporization or pyrolysis) entailing the vaporization fraction of the regression rate, Equation (31) produces a total regression rate that is marginally sensitive to the value of the enthalpy because. If the vaporization regression rate is decreased for a larger vaporization enthalpy, the total heat flux to the wall tends to rise and so does the entrainment fraction. This sort of balance is further explained later.
Material constants appearing in Equation (31) are listed in
Table 4.
According to the approach described in Reference [
7], the following semiempirical relationship has been considered for modeling the entrainment component of the fuel regression rate as a function of the rocket chamber operating conditions and of an entrainment parameter that lumps the liquid fuel properties together:
where
is the total mass flux in the local section of the grain port and
is the entrainment factor depending on the physical properties of the selected fuel, primarily on the fuel liquid viscosity, and on the average gas density in the chamber as
Equation (33) is derived from a theoretical assessment of the surface liquid-layer fluid dynamic stability; the main result is that the susceptibility of a given fuel to the instability increases with decreasing viscosity and surface tension of the melt layer; the entrainment component of fuel regression rate is, therefore, roughly inversely proportional to viscosity at the characteristic temperature of the layer, while it depends directly on the dynamic pressure. A parametric analysis of the effect of the entrainment parameter on the regression rate components is reported in Reference [
56]. From this analysis, the value of 2.1 × 10
−13 m
8.5s
0.5/kg
3 has been identified for the best fit of the experimental data in the reference Test P4. The latter value, considering the different densities (average gas density in Test P4 is 1.62 kg/m
3) is in good agreement with the one reported in Reference [
7].
Once Equations (30)–(32) are combined, given the heat flux to the wall and the total mass flux, the three components of the fuel regression rate can be calculated. The fuel mass fluxes associated with the vaporization and entrainment components, respectively, are obtained as follows
Vaporization and entrainment components are handled differently.
The vaporization component is treated equally to the case of pyrolyzing fuels, considering the mass and mixture-fraction balance equations at the grain wall, given by
This allows for correctly taking the blocking effect on the heat transfer to the wall into account, while, as explained in the previous section, Equation (37) is needed to ensure the mixture fraction has a global balance.
The entrainment mass flux does not contribute to the blocking effect, thus, a specific treatment is adopted for the introduction of the entrainment component into the computational domain. For the sake of simplicity, we assume that despite the entrained paraffin initially in the liquid phase immediately gasifies because of the large combustion heat release. The local entrainment contribution is uniformly assigned as a mass production term in the local volume of the grain port corresponding to the surface cell of length
through which the fuel mass enters the fluid domain,
:
In order to satisfy the species balance, an equal production term is assigned also for the mean mixture fraction. Finally, the energy required by the pyrolysis of the liquid fuel mass flow rate is taken into account by assigning a corresponding negative energy source term in the same volume:
Additionally, in this case, as the heat flux to the surface and the total mass flux needed for the calculation of the regression rate are outputs of the flowfield resolution, which, in turn, depends on the regression rate itself, an iterative procedure is needed for the problem solution.