Direct In-Situ Capture, Separation and Visualization of Biological Particles with Fluid-Screen in the Context of Venus Life Finder Mission Concept Study
Abstract
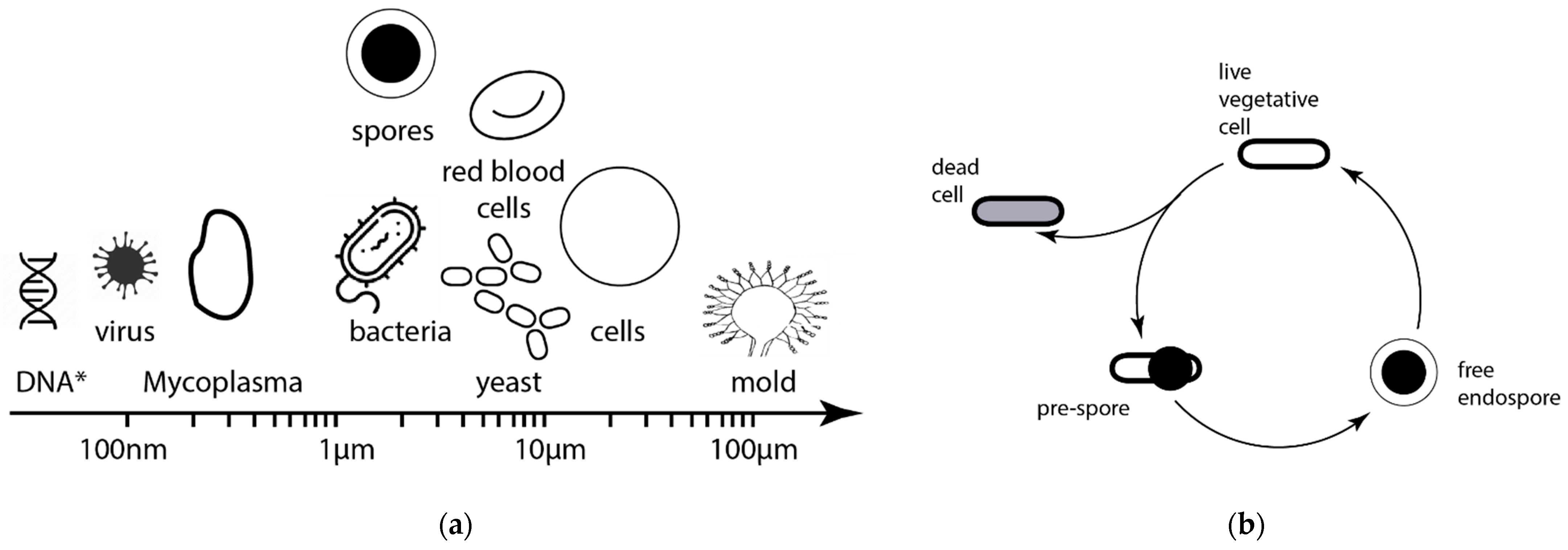
1. Introduction
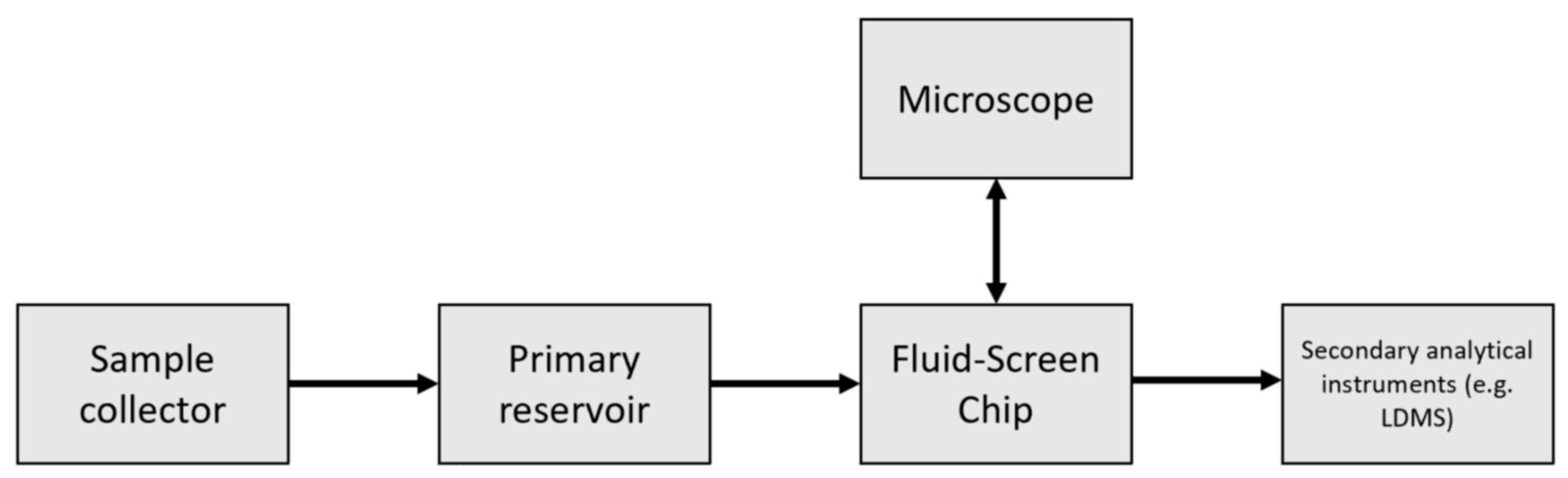
Fluid-Screen Technology Overview
2. Motivation for Utilization of Fluid-Screen System in the Exploration of Venus’ Clouds
3. Fluid-Screen Chip Configurations for Venus Life Finder VAIHL Mission
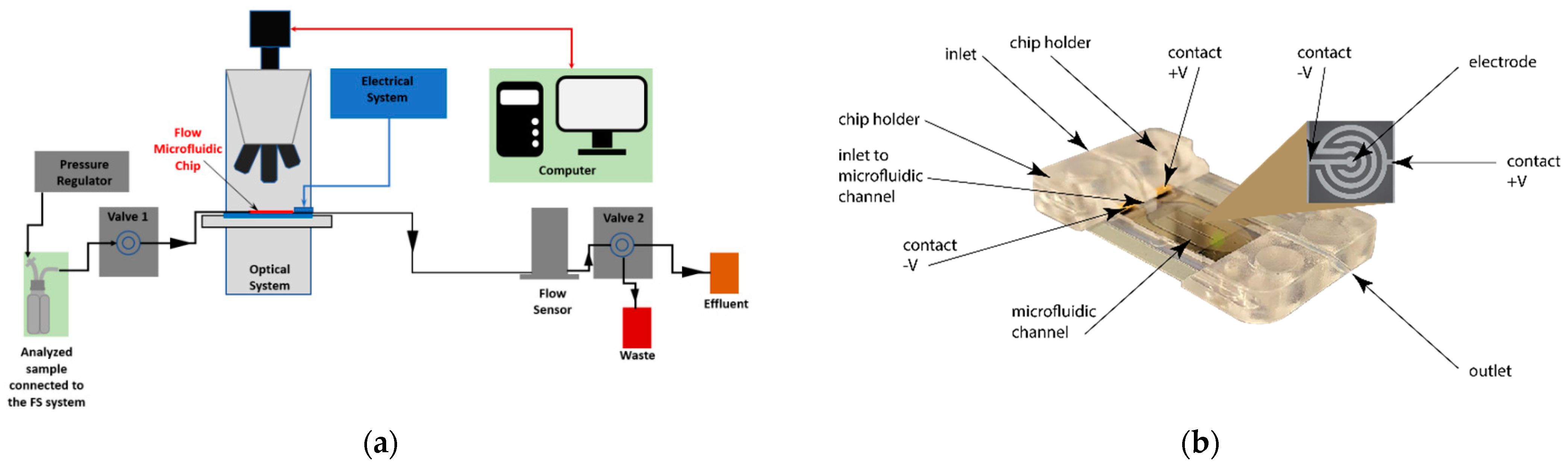
3.1. Fluid-Screen Chip Characteristics
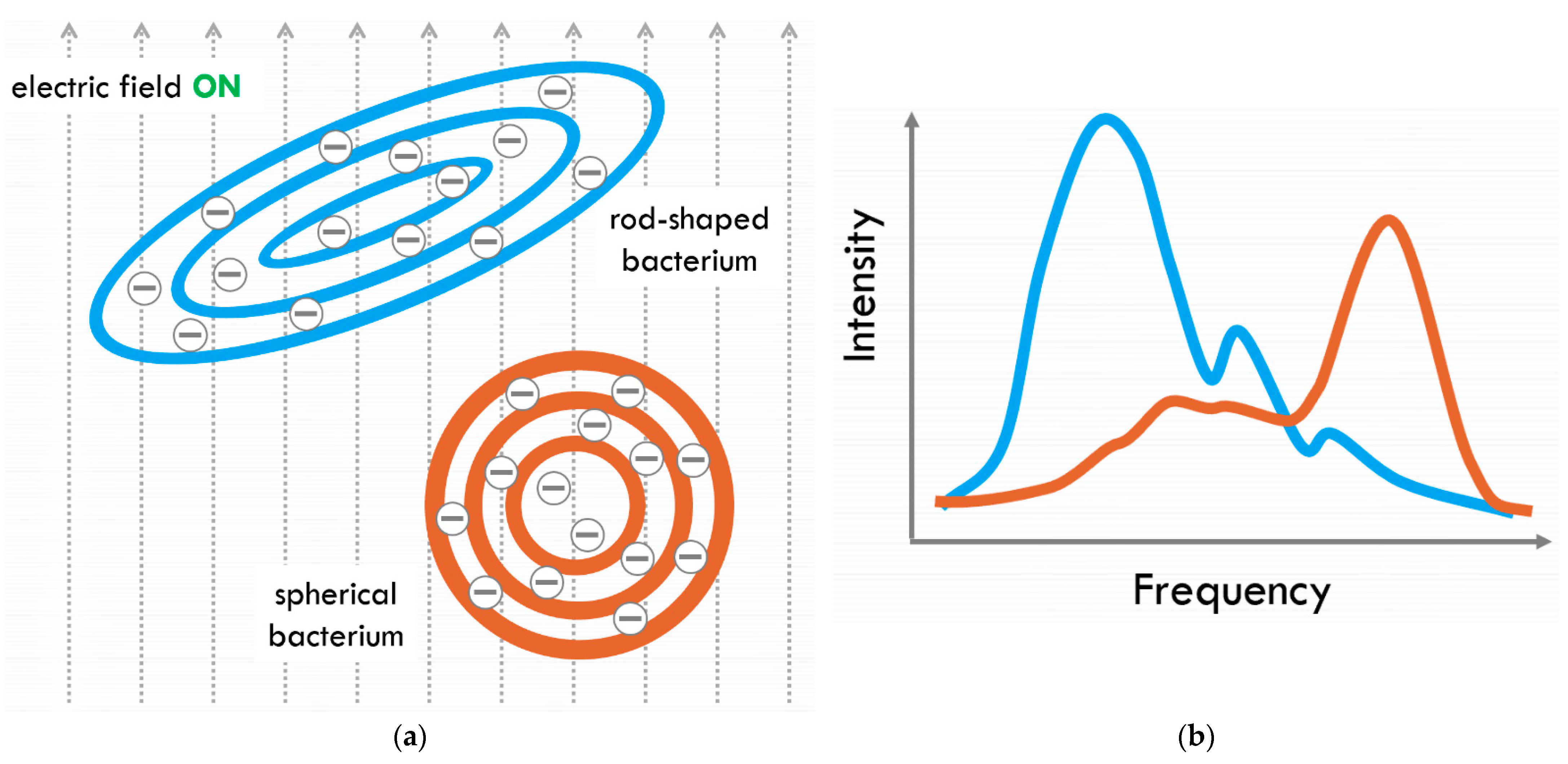
3.2. Predicted Scientific Outcome for Venus VAIHL Mission
4. Challenges and Path Forward
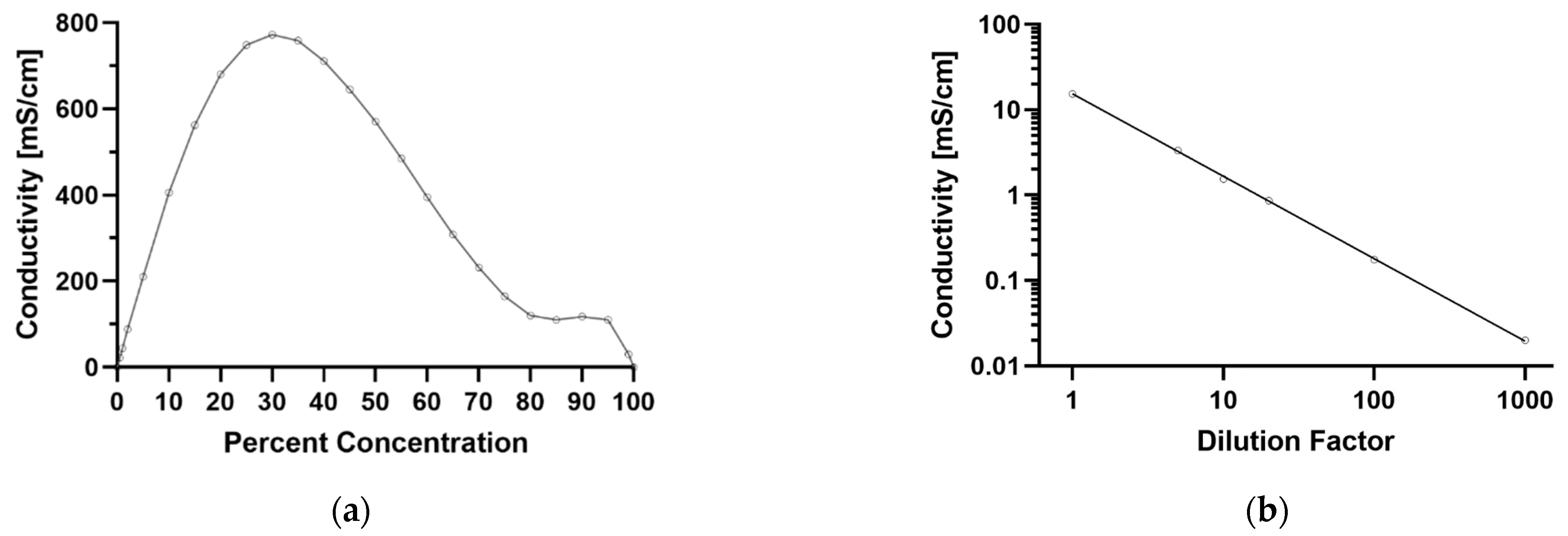
4.1. Dielectrophoretic Capture of Particles in Concentrated Sulfuric Acid
4.2. Miniaturization and the Overall Engineering Adaptation for Venus and Other Space Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pohl, H.A.; Hawk, I. Separation of living and dead cells by dielectrophoresis. Science 1966, 152, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Sher, L.D. Dielectrophoresis in lossy dielectric media. Nature 1968, 220, 695–696. [Google Scholar] [CrossRef]
- Voldman, J. Electrical forces for microscale cell manipulation. Annu. Rev. Biomed. Eng. 2006, 8, 425–454. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.E.; Rohani, A.; Farmehini, V.; Swami, N.S. Microbial analysis in dielectrophoretic microfluidic systems. Anal. Chim. Acta 2017, 966, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chang, H.; Neuzil, P. DEP-on-a-chip: Dielectrophoresis applied to microfluidic platforms. Micromachines 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Sarno, B.; Heineck, D.; Heller, M.J.; Ibsen, S. Dielectrophoresis: Developments and applications from 2010 to 2020. Electrophoresis 2021, 42, 539–564. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.-F.; Chen, T.-Y.; Lu, R.-J.; Wu, H.-W. Rapid identification of bacteria utilizing amplified dielectrophoretic force-assisted nanoparticle-induced surface-enhanced Raman spectroscopy. Nanoscale Res. Lett. 2014, 9, 324. [Google Scholar] [CrossRef]
- Schröder, U.-C.; Ramoji, A.; Glaser, U.; Sachse, S.; Leiterer, C.; Csaki, A.; Hübner, U.; Fritzsche, W.; Pfister, W.; Bauer, M. Combined dielectrophoresis—Raman setup for the classification of pathogens recovered from the urinary tract. Anal. Chem. 2013, 85, 10717–10724. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Wang, X.-B.; Becker, F.F.; Gascoyne, P.R.C. Differential analysis of human leukocytes by dielectrophoretic field-flow-fractionation. Biophys. J. 2000, 78, 2680–2689. [Google Scholar] [CrossRef]
- Chang, S.; Cho, Y.-H. A continuous size-dependent particle separator using a negative dielectrophoretic virtual pillar array. Lab Chip 2008, 8, 1930–1936. [Google Scholar] [CrossRef]
- Huang, Y.; Holzel, R.; Pethig, R.; Wang, X.-B. Differences in the AC electrodynamics of viable and non-viable yeast cells determined through combined dielectrophoresis and electrorotation studies. Phys. Med. Biol. 1992, 37, 1499. [Google Scholar] [CrossRef]
- Abd Rahman, N.; Ibrahim, F.; Yafouz, B. Dielectrophoresis for biomedical sciences applications: A review. Sensors 2017, 17, 449. [Google Scholar] [CrossRef] [PubMed]
- Kwizera, E.A.; Sun, M.; White, A.M.; Li, J.; He, X. Methods of generating dielectrophoretic force for microfluidic manipulation of bioparticles. ACS Biomater. Sci. Eng. 2021, 7, 2043–2063. [Google Scholar] [CrossRef] [PubMed]
- Mohd Maidin, N.N.; Buyong, M.R.; Rahim, A.R.; Mohamed, M.A. Dielectrophoresis applications in biomedical field and future perspectives in biomedical technology. Electrophoresis 2021, 42, 2033–2059. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.U.; Petkowski, J.J.; Weber, R.E.; Krajnik, B.; Stemplewski, S.; Panek, M.; Dziubak, T.; Mrozińska, P.; Piela, A.; Lo, S.L.; et al. Fluid-Screen Dielectrophoretic Microbial Capture, Separation and Detection I: Theoretical Study. Nanotechnology, 2022; submitted. [Google Scholar]
- Weber, M.U.; Petkowski, J.J.; Weber, R.E.; Krajnik, B.; Stemplewski, S.; Panek, M.; Dziubak, T.; Mrozińska, P.; Piela, A.; Reed, M.A. Fluid-Screen Dielectrophoretic Microbial Capture, Separation and Detection II: Experimental Study. Nanotechnology, 2022; submitted. [Google Scholar]
- Weber, R.E.; Petkowski, J.J.; Michaels, B.; Wisniewski, K.; Piela, A.; Antoszczyk, S.; Weber, M.U. Fluid-Screen as a Real Time Dielectrophoretic Method for Universal Microbial Capture. Sci. Rep. 2021, 11, 22222. [Google Scholar] [CrossRef]
- Kwak, T.J.; Jung, H.; Allen, B.D.; Demirel, M.C.; Chang, W.-J. Dielectrophoretic separation of randomly shaped protein particles. Sep. Purif. Technol. 2021, 262, 118280. [Google Scholar] [CrossRef]
- Camacho-Alanis, F.; Ros, A. Protein dielectrophoresis and the link to dielectric properties. Bioanalysis 2015, 7, 353–371. [Google Scholar] [CrossRef]
- Mohamad, A.S.; Hamzah, R.; Hoettges, K.F.; Hughes, M.P. A dielectrophoresis-impedance method for protein detection and analysis. AIP Adv. 2017, 7, 15202. [Google Scholar] [CrossRef]
- Sonnenberg, A.; Marciniak, J.Y.; Krishnan, R.; Heller, M.J. Dielectrophoretic isolation of DNA and nanoparticles from blood. Electrophoresis 2012, 33, 2482–2490. [Google Scholar] [CrossRef]
- Song, Y.; Sonnenberg, A.; Heaney, Y.; Heller, M.J. Device for dielectrophoretic separation and collection of nanoparticles and DNA under high conductance conditions. Electrophoresis 2015, 36, 1107–1114. [Google Scholar] [CrossRef]
- Seager, S.; Petkowski, J.J.; Carr, C.E.; Grinspoon, D.; Ehlmann, B.; Saikia, S.J.; Agrawal, R.; Buchanan, W.; Weber, M.U.; French, R. Venus Life Finder Mission Study. arXiv 2021, arXiv:2112.05153. [Google Scholar]
- Seager, S.; Petkowski, J.J.; Carr, C.E.; Grinspoon, D.H.; Ehlmann, B.L.; Saikia, S.J.; Agrawal, R.; Buchanan, W.P.; Weber, M.U.; French, R.; et al. Venus Life Finder Missions Motivation and Summary. Aerospace 2022, 9, 385. [Google Scholar] [CrossRef]
- Agrawal, R.; Buchanan, W.P.; Arora, A.; Girija, A.P.; de Jong, M.; Seager, S.; Petkowski, J.J.; Saikia, S.J.; Carr, C.E.; Grinspoon, D.H.; et al. Mission Architecture to Characterize Habitability of Venus Cloud Layers via an Aerial Platform. Aerospace 2022, 9, 359. [Google Scholar] [CrossRef]
- Buchanan, W.P.; de Jong, M.; Agrawal, R.; Petkowski, J.J.; Arora, A.; Saikia, S.J.; Seager, S.; Longuski, J. Aerial Platform Design Options for a Life-Finding Mission at Venus. Aerospace 2022, 9, 363. [Google Scholar] [CrossRef]
- Seager, S.; Petkowski, J.J.; Carr, C.E.; Saikia, S.J.; Agrawal, R.; Buchanan, W.P.; Grinspoon, D.H.; Weber, M.U.; Klupar, P.; Worden, S.P.; et al. Venus Life Finder Habitability Mission: Motivation, Science Objectives, and Instrumentation. Aerospace, 2022; in review. [Google Scholar]
- Knollenberg, R.G.; Hunten, D.M. Clouds of Venus: A preliminary assessment of microstructure. Science 1979, 205, 70–74. [Google Scholar] [CrossRef]
- Knollenberg, R.G. A reexamination of the evidence for large, solid particles in the clouds of Venus. Icarus 1984, 57, 161–183. [Google Scholar] [CrossRef]
- Knollenberg, R.G.; Hunten, D.M. The microphysics of the clouds of Venus: Results of the Pioneer Venus particle size spectrometer experiment. J. Geophys. Res. Space Phys. 1980, 85, 8039–8058. [Google Scholar] [CrossRef]
- Knollenberg, R.G.; Hunten, D.M. Clouds of Venus: Particle size distribution measurements. Science 1979, 203, 792–795. [Google Scholar] [CrossRef]
- Knollenberg, R.G. Clouds and hazes. Nature 1982, 296, 18. [Google Scholar] [CrossRef]
- Knollenberg, R.; Travis, L.; Tomasko, M.; Smith, P.; Ragent, B.; Esposito, L.; McCleese, D.; Martonchik, J.; Beer, R. The clouds of Venus: A synthesis report. J. Geophys. Res. Space Phys. 1980, 85, 8059–8081. [Google Scholar] [CrossRef]
- Bains, W.; Petkowski, J.J.; Rimmer, P.B.; Seager, S. Production of Ammonia Makes Venusian Clouds Habitable and Explains Observed Cloud-Level Chemical Anomalies. Proc. Natl. Acad. Sci. USA 2021, 118, e2110889118. [Google Scholar] [CrossRef]
- Beegle, L.; Bhartia, R.; White, M.; DeFlores, L.; Abbey, W.; Wu, Y.-H.; Cameron, B.; Moore, J.; Fries, M.; Burton, A. SHERLOC: Scanning habitable environments with Raman & luminescence for organics & chemicals. In Proceedings of the 2015 IEEE Aerospace Conference, Big Sky, MT, USA, 7–14 March 2015; pp. 1–11. [Google Scholar]
- Bhartia, R.; Beegle, L.W.; DeFlores, L.; Abbey, W.; Hollis, J.R.; Uckert, K.; Monacelli, B.; Edgett, K.S.; Kennedy, M.R.; Sylvia, M. Perseverance’s Scanning Habitable Environments with Raman and Luminescence for Organics and Chemicals (SHERLOC) investigation. Space Sci. Rev. 2021, 217, 58. [Google Scholar] [CrossRef]
- Ligterink, N.F.W.; Kipfer, K.A.; Gruchola, S.; Boeren, N.J.; Keresztes Schmidt, P.; de Koning, C.P.; Tulej, M.; Wurz, P.; Riedo, A. The ORIGIN Space Instrument for Detecting Biosignatures and Habitability Indicators on a Venus Life Finder Mission. Aerospace 2022, 9, 312. [Google Scholar] [CrossRef]
- Knapczyk-Korczak, J.; Szewczyk, P.K.; Ura, D.P.; Bailey, R.J.; Bilotti, E.; Stachewicz, U. Improving water harvesting efficiency of fog collectors with electrospun random and aligned Polyvinylidene fluoride (PVDF) fibers. Sustain. Mater. Technol. 2020, 25, e00191. [Google Scholar] [CrossRef]
- Fernandez, D.M.; Torregrosa, A.; Weiss-Penzias, P.S.; Zhang, B.J.; Sorensen, D.; Cohen, R.E.; McKinley, G.H.; Kleingartner, J.; Oliphant, A.; Bowman, M. Fog water collection effectiveness: Mesh intercomparisons. Aerosol Air Qual. Res. 2018, 18, 270–283. [Google Scholar] [CrossRef]
- Schemenauer, R.S.; Joe, P.I. The collection efficiency of a massive fog collector. Atmos. Res. 1989, 24, 53–69. [Google Scholar] [CrossRef]
- Shi, W.; van der Sloot, T.W.; Hart, B.J.; Kennedy, B.S.; Boreyko, J.B. Harps Enable Water Harvesting under Light Fog Conditions. Adv. Sustain. Syst. 2020, 4, 2000040. [Google Scholar] [CrossRef]
- Baumgardner, D.; Fisher, T.; Newton, R.; Roden, C.; Zmarzly, P.; Seager, S.; Petkowski, J.J.; Carr, C.E.; Špaček, J.; Benner, S.A.; et al. Deducing the Composition of Venus Cloud Particles with the Autofluorescence Nephelometer (AFN). Aerospace 2022, 9, 492. [Google Scholar] [CrossRef]
- French, R.; Mandy, C.; Hunter, R.; Mosleh, E.; Sinclair, D.; Beck, P.; Seager, S.; Petkowski, J.J.; Carr, C.E.; Grinspoon, D.H.; et al. Rocket Lab Mission to Venus. Aerospace 2022, 9, 445. [Google Scholar] [CrossRef]
- Weber, M. AC Kinetics System for Universal Bacterial Capture. Ph.D. Thesis, Yale University, New Haven, CT, USA, 2017. [Google Scholar]
- McMahon, S.; Cosmidis, J. False biosignatures on Mars: Anticipating ambiguity. J. Geol. Soc. Lond. 2022, 179. [Google Scholar] [CrossRef]
- Schulze-Makuch, D.; Grinspoon, D.H.; Abbas, O.; Irwin, L.N.; Bullock, M.A. A sulfur-based survival strategy for putative phototrophic life in the Venusian atmosphere. Astrobiology 2004, 4, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Darling, H.E. Conductivity of sulfuric acid solutions. J. Chem. Eng. Data 1964, 9, 421–426. [Google Scholar] [CrossRef]
- Weast, R.C. (Ed.) CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 1989; Volume 70, ISBN 0849304857. [Google Scholar]
- Zhou, Y.; Xu, X.; Wei, Y.; Cheng, Y.; Guo, Y.; Khudyakov, I.; Liu, F.; He, P.; Song, Z.; Li, Z. A widespread pathway for substitution of adenine by diaminopurine in phage genomes. Science 2021, 372, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Kirnos, M.D.; Khudyakov, I.Y.; Alexandrushkina, N.I.; Vanyushin, B.F. 2-Aminoadenine is an adenine substituting for a base in S-2L cyanophage DNA. Nature 1977, 270, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Pezo, V.; Jaziri, F.; Bourguignon, P.-Y.; Louis, D.; Jacobs-Sera, D.; Rozenski, J.; Pochet, S.; Herdewijn, P.; Hatfull, G.F.; Kaminski, P.-A. Noncanonical DNA polymerization by aminoadenine-based siphoviruses. Science 2021, 372, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, D.; Garcia, P.S.; Lagune, M.; Loc’h, J.; Haouz, A.; Taib, N.; Röthlisberger, P.; Gribaldo, S.; Marlière, P.; Kaminski, P.A. A third purine biosynthetic pathway encoded by aminoadenine-based viral DNA genomes. Science 2021, 372, 516–520. [Google Scholar] [CrossRef]
- Davies, P.C.W.; Benner, S.A.; Cleland, C.E.; Lineweaver, C.H.; McKay, C.P.; Wolfe-Simon, F. Signatures of a Shadow Biosphere. Astrobiology 2009, 9, 241–249. [Google Scholar] [CrossRef]



| Region | Altitude (km) | Temperature (K) | Pressure (atm) | Cloud Particle Properties | ||
|---|---|---|---|---|---|---|
| Average Num. Density (n cm−3) | Mean Diameter (µm) | Cloud Particle H2SO4 Concentration | ||||
| Layers above upper haze | 100–110 | 100% H2SO4 | ||||
| Upper haze | 70–90 | 225–190 | 0.04–0.0004 | 500 | 0.4 | 70% H2SO4 30% H2O |
| Upper cloud | 56.5–70 | 286–225 | 0.5–0.04 | (1) −1500 (2) −50 | Bimodal 0.4 and 2.0 | liquid 80% H2SO4 20% H2O |
| Middle cloud | 50.5–56.5 | 345–286 | 1.0–0.5 | (1) −300 (2) −50 (3) −10 | Trimodal 0.3, 2.5 and 7.0 | liquid 90% H2SO4 10% H2O |
| Lower cloud | 47.5–50.5 | 367–345 | 1.5–1.0 | (1) −1200 (2) −50 (3) −50 | Trimodal 0.4, 2.0 and 8.0 | liquid 98% H2SO4 2% H2O (or fumic H2SO4) |
| Lower haze | 31–47.5 | 482–367 | 9.5–1.5 | 2–20 | 0.2 | |
| Pre-cloud layers | 46 and 47.5 | 378 and 367 | 1.8–1.5 | 50 and 150 | Bimodal 0.3 and 2.0 | |
| Instrument Characteristics | Fluid-Screen Chip |
|---|---|
| Mass (kg) | 1–10 |
| Volume (cm3) | 3000 |
| Power (W) (average/peak) | 1.5–30 |
| Data volume (per measurement and total) | 100 kb–2.4 mb per image, max 20 images per second * |
| Data sampling time | <1 s per image * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, R.E.; Petkowski, J.J.; Weber, M.U. Direct In-Situ Capture, Separation and Visualization of Biological Particles with Fluid-Screen in the Context of Venus Life Finder Mission Concept Study. Aerospace 2022, 9, 692. https://doi.org/10.3390/aerospace9110692
Weber RE, Petkowski JJ, Weber MU. Direct In-Situ Capture, Separation and Visualization of Biological Particles with Fluid-Screen in the Context of Venus Life Finder Mission Concept Study. Aerospace. 2022; 9(11):692. https://doi.org/10.3390/aerospace9110692
Chicago/Turabian StyleWeber, Robert E., Janusz J. Petkowski, and Monika U. Weber. 2022. "Direct In-Situ Capture, Separation and Visualization of Biological Particles with Fluid-Screen in the Context of Venus Life Finder Mission Concept Study" Aerospace 9, no. 11: 692. https://doi.org/10.3390/aerospace9110692
APA StyleWeber, R. E., Petkowski, J. J., & Weber, M. U. (2022). Direct In-Situ Capture, Separation and Visualization of Biological Particles with Fluid-Screen in the Context of Venus Life Finder Mission Concept Study. Aerospace, 9(11), 692. https://doi.org/10.3390/aerospace9110692







