Impact of Removing Race Coefficient from Glomerular Filtration Rate Estimation Equations on Antidiabetics Among Black Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
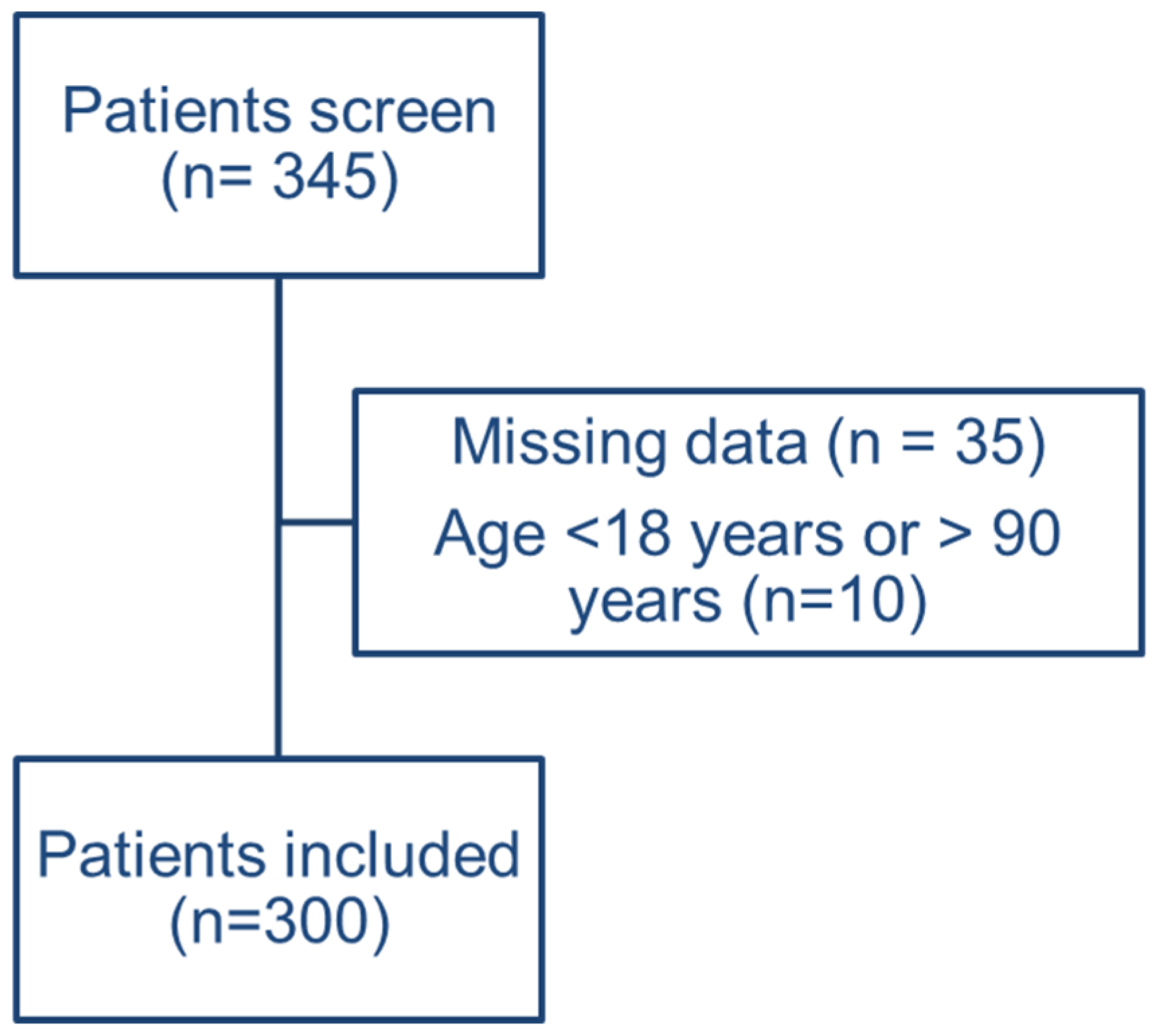
2.2. Study Population
2.3. Data Collection
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
3.1. Demographics
3.2. Kidney Function Assessment
3.3. Differences Between Various Estimation Equations
3.4. Antidiabetic Dosing and Eligibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matzke, G.R.; Aronoff, G.R.; Atkinson, A.J., Jr.; Bennett, W.M.; Decker, B.S.; Eckardt, K.U.; Golper, T.; Grabe, D.W.; Kasiske, B.; Keller, F.; et al. Drug dosing consideration in patients with acute and chronic kidney disease—A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011, 80, 1122–1137. [Google Scholar] [PubMed]
- Rungkitwattanakul, D.; Chaijamorn, W.; Han, E.; Aldhaeefi, M. Kidney Function Assessment in African American Patients: A Narrative Review for Pharmacists. Pharmacy 2022, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A More Accurate Method To Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef]
- Eneanya, N.D.; Boulware, L.E.; Tsai, J.; Bruce, M.A.; Ford, C.L.; Harris, C.; Morales, L.S.; Ryan, M.J.; Reese, P.P.; Thorpe, R.J.; et al. Health inequities and the inappropriate use of race in nephrology. Nat. Rev. Nephrol. 2022, 18, 84–94. [Google Scholar] [CrossRef]
- Bukabau, J.B.; Sumaili, E.K.; Cavalier, E.; Pottel, H.; Kifakiou, B.; Nkodila, A.; Makulo, J.R.R.; Mokoli, V.M.; Zinga, C.V.; Longo, A.L.; et al. Performance of glomerular filtration rate estimation equations in Congolese healthy adults: The inopportunity of the ethnic correction. PLoS ONE 2018, 13, e0193384. [Google Scholar] [CrossRef]
- Bukabau, J.B.; Yayo, E.; Gnionsahé, A.; Monnet, D.; Pottel, H.; Cavalier, E.; Nkodila, A.; Makulo, J.R.R.; Mokoli, V.M.; Lepira, F.B.; et al. Performance of creatinine- or cystatin C–based equations to estimate glomerular filtration rate in sub-Saharan African populations. Kidney Int. 2019, 95, 1181–1189. [Google Scholar] [CrossRef]
- Delgado, C.; Baweja, M.; Crews, D.C.; Eneanya, N.D.; Gadegbeku, C.A.; Inker, L.A.; Mendu, M.L.; Miller, W.G.; Moxey-Mims, M.M.; Roberts, G.V.; et al. A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am. J. Kidney Dis. 2022, 79, 268–288.e1. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Brown, D.L.; Masselink, A.J.; Lalla, C.D. Functional Range of Creatinine Clearance for Renal Drug Dosing: A Practical Solution to the Controversy of Which Weight to Use in the Cockcroft-Gault Equation. Ann. Pharmacother. 2013, 47, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Gault, H. Prediction of Creatinine Clearance from Serum Creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C–Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.Q.; Nolin, T.D. Pragmatic Use of Kidney Function Estimates for Drug Dosing: The Tide Is Turning. Adv. Chronic Kidney Dis. 2018, 25, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Vlasschaert, C.; Thibodeau, S.; Parmar, M.S. De-indexed estimated glomerular filtration rates: A simple step towards improving accuracy of drug dosing of renally excreted medications in moderate to severe obesity. Nephrology 2020, 25, 29–31. [Google Scholar] [CrossRef]
- Hahr, A.J.; Molitch, M.E. Management of Diabetes Mellitus in Patients With CKD: Core Curriculum 2022. Am. J. Kidney Dis. 2022, 79, 728–736. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Merchant, P.; Gadegbeku, C.; Mehdi, A.; Thomas, G.; Nakhoul, G.; Taliercio, J. The new GFR equations: How will eliminating the race coefficient affect Black patients? Cleve. Clin. J. Med. 2023, 90, 685–691. [Google Scholar] [CrossRef]
- Peter, W.L.S.; Bzowyckyj, A.S.; Anderson-Haag, T.; Awdishu, L.; Blackman, M.; Bland, A.; Chan, E.; Chmielewski, C.; Delgado, C.; Eyler, R.; et al. Moving forward from Cockcroft-Gault creatinine clearance to race-free estimated glomerular filtration rate to improve medication-related decision-making in adults across healthcare settings: A consensus of the National Kidney Foundation Workgroup for Implementation of Race-Free eGFR-Based Medication-Related Decisions. Am. J. Health Syst. Pharm. 2024, Online ahead of print. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- The EMPA-KIDNEY Collaborative Group; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef] [PubMed]
- The EMPA-KIDNEY Collaborative Group; Herrington, W.G.; Staplin, N.; Agrawal, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; et al. Long-Term Effects of Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2025, 392, 777–787. [Google Scholar] [CrossRef]
- Miller, J.; Knorr, J.P. Impact of Removing the Race Coefficient in Renal Function Estimate Equations on Drug Dosage Recommendations. Ann. Pharmacother. 2022, 56, 44–51. [Google Scholar] [CrossRef]
- Butrovich, M.A.; Qin, J.; Xue, X.; Ivy, S.P.; Nolin, T.D.; Beumer, J.H. Impact of the 2021 CKD-EPI equation on anticancer pharmacotherapy in black and non-black cancer patients. Cancer Lett. 2024, 586, 216679. [Google Scholar] [CrossRef]
- Teaford, H.R.; Barreto, J.N.; Vollmer, K.J.; Rule, A.D.; Barreto, E.F. Cystatin C: A Primer for Pharmacists. Pharmacy 2020, 8, 35. [Google Scholar] [CrossRef]
- Barreto, E.F.; Rule, A.D.; Murad, M.H.; Kashani, K.B.; Lieske, J.C.; Erwin, P.J.; Steckelberg, J.M.; Gajic, O.; Reid, J.M.; Kane-Gill, S.L. Prediction of the Renal Elimination of Drugs With Cystatin C vs Creatinine: A Systematic Review. Mayo Clin. Proc. 2019, 94, 500–514. [Google Scholar] [CrossRef]
| Parameter | DTC Cohort (n = 300) | Simulated Patients (n = 10,000) |
|---|---|---|
| Age, years | 63.8 ± 15.7 | 65.2 ± 12.7 |
| Female sex, n | 64 | 5000 |
| Weight, kg | 88.0 ± 25.8 | 89.9 ± 20.8 |
| Height, cm | 166.2 ± 11.6 | 186.2 ± 18.6 |
| Body mass index, kg/m2 | 32.19 ± 12.1 | 33.49 ± 12.1 |
| Body surface area, m2 | 2.0 ± 0.3 | 2.0 ± 0.4 |
| SCr, mg/dL | 1.14 ± 0.8 | 1.09 ± 0.8 |
| Parameter | DTC Cohort (n = 300) | Simulated Patients (n = 10,000) | p Value |
|---|---|---|---|
| eCrCl (actual body weight), mL/min | 93.1 ± 52.2 | 114.9 ± 59.2 | 0.09 |
| eCrCl (ideal body weight), mL/min | 63.5 ± 32.1 | 78.6 ± 34.9 | 0.008 |
| eCrCl (adjusted body weight), mL/min | 93.1 ± 52.2 | 114.9 ± 59.2 | 0.12 |
| eGFR CKD-EPI 2009, mL/min/1.73 m2 | 81.8 ± 32.3 | 86.7 ± 42.5 | 0.52 |
| eGFR CKD-EPI 2021, mL/min/1.73 m2 | 75.0 ± 28.7 | 76.6 ± 34.9 | 0.68 |
| De-indexed eGFR CKD-EPI 2009, mL/min | 94.1 ± 39.5 | 100.1 ± 52.3 | 0.25 |
| De-indexed eGFR CKD-EPI 2021, mL/min | 86.2 ± 35.1 | 88.4 ± 43.3 | 0.23 |
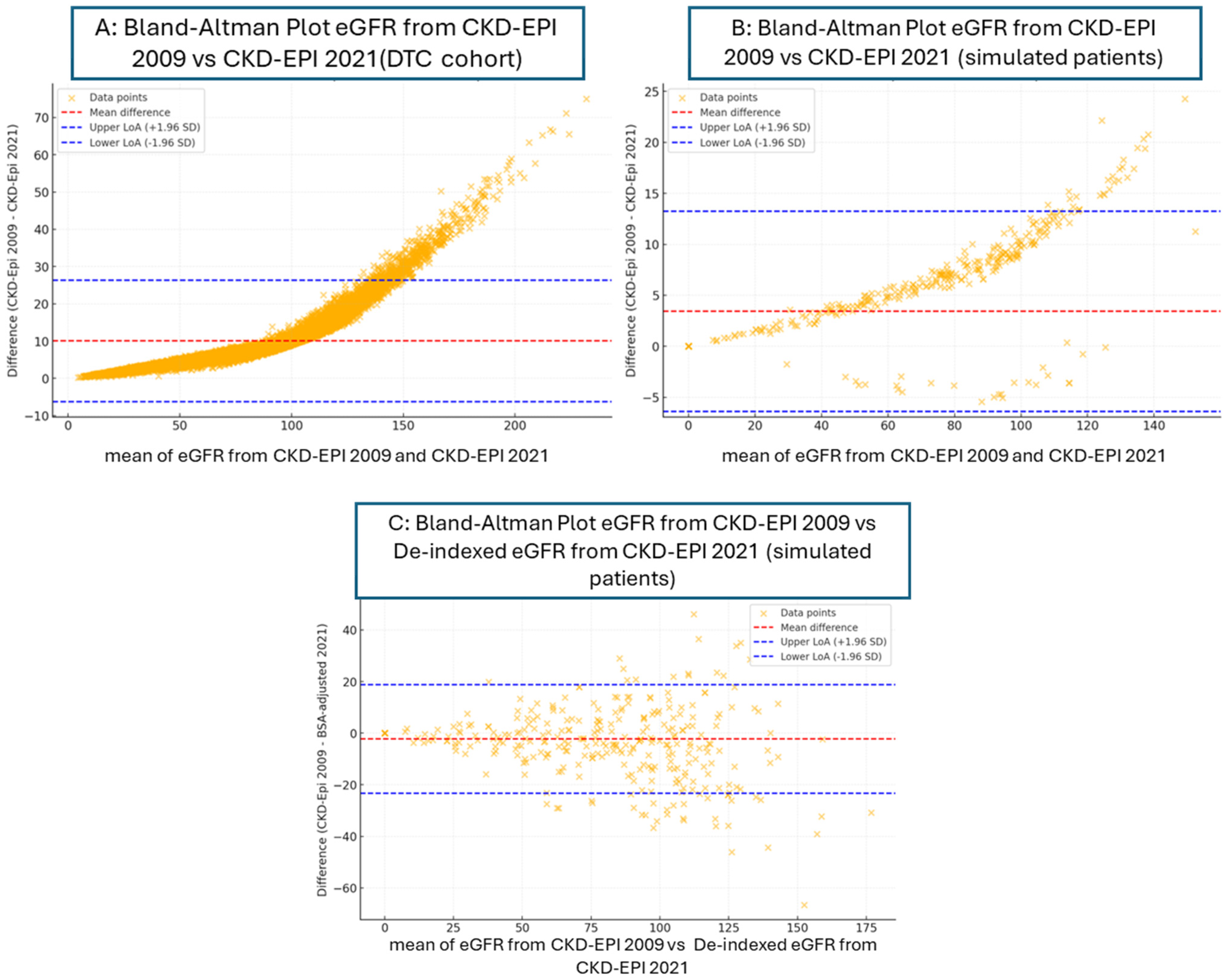
| Bland–Altman Plot | eGFR from CKD-EPI 2009 vs. CKD-EPI 2021 | eGFR from CKD-EPI 2009 vs. CKD-EPI 2021 | eGFR from CKD-EPI 2009 vs. De-Indexed eGFR from CKD-EPI 2021 |
|---|---|---|---|
| DTC Cohort (n = 300) | Simulated Patients (n = 10,000) | Simulated Patients (n = 10,000) | |
| Mean difference (bias) | 10.06 | 3.43 | −2.24 |
| Upper limit of agreement (LoA) | 26.38 | 13.24 | 18.83 |
| Lower limit of agreement (LoA) | −6.37 | −6.37 | −23.31 |
| Comparison | Mean Difference | Standard Deviation | Cohen’s d (Effect Size) | Effect Size Magnitude |
|---|---|---|---|---|
| Simulation Cohort (n = 10,000) | ||||
| eGFR from CKD-EPI 2009 vs. CKD-EPI 2021 | 3.43 | 5.0 | 0.685 | Medium |
| eGFR from CKD-EPI 2009 vs. de-indexed eGFR from CKD-EPI 2021 | −2.24 | 10.7 | −0.208 | Small |
| Antidiabetics | Degree of Kidney Impairment and Dosing Recommendation |
|---|---|
| Sulfonylureas | |
| Gliclazide | Avoid use when eGFR < 40 mL/min/1.73 m2 |
| Biguanides | |
| Metformin | eGFR < 45 mL/min/1.73 m2, maximum dose is 1000 mg/d eGFR < 30 mL/min/1.73 m2, do not continue |
| DPP-4 inhibitors | |
| Sitagliptin | GFR > 50 mL/min/1.73 m2: 100 mg daily GFR 30–50 mL/min/1.73 m2: 50 mg daily GFR < 30 mL/min/1.73 m2: 25 mg daily |
| Saxagliptin | GFR > 50 mL/min/1.73 m2: 5 mg daily GFR ≤ 50 mL/min/1.73 m2: 2.5 mg daily |
| Alogliptin | GFR > 50 mL/min/1.73 m2: 25 mg daily GFR 30–50 mL/min/1.73 m2: 12.5 mg daily GFR < 30 mL/min/1.73 m2: 6.25 mg daily |
| GLP-1 agonists | |
| Exenatide | GFR < 30 mL/min/1.73 m2: not recommended |
| Lixisenatide | eGFR < 15 mL/min/1.73 m2: not recommended |
| SGLT2 inhibitors | |
| Canagliflozin | eGFR 30 ≤ 60 mL/min/1.73 m2: max dose 100 mg once daily eGFR < 30 mL/min/1.73 m2: not recommended |
| Dapagliflozin | eGFR < 25 mL/min/1.73 m2: not recommended |
| Empagliflozin | eGFR < 30 mL/min/1.73 m2: not recommended |
| Ertugliflozin | eGFR < 45 mL/min/1.73 m2: not recommended |
| Mineralocorticoid receptor antagonist | |
| Finerenone | eGFR < 25 mL/minute/1.73 m2: not recommended |
| GFR Cut Point (mL/min/1.73 m2) | Creatinine Clearance (mL/min) | CKD-EPI 2009 (mL/min/1.73 m2) | CKD-EPI 2021 (mL/min/1.73 m2) | De-Indexed CKD-EPI 2009 (mL/min) | De-Indexed CKD-EPI 2021 (mL/min) | ||
|---|---|---|---|---|---|---|---|
| Actual BW | Adjusted BW | Ideal BW | |||||
| DTC cohort (n = 300) | |||||||
| 50 | 97.67 | 80.00 | 64.67 | 80.00 | 79.00 | 81.67 | 85.00 |
| 45 | 99.33 | 84.00 | 68.33 | 85.33 | 82.67 | 89.00 | 86.00 |
| 30 | 99.67 | 93.00 | 87.67 | 92.33 | 92.00 | 93.67 | 91.67 |
| 25 | 100.00 | 94.67 | 91.33 | 95.33 | 94.33 | 96.67 | 95.33 |
| 20 | 100.00 | 98.33 | 94.00 | 97.33 | 96.67 | 97.00 | 96.67 |
| Simulated patients (n =10,000) | |||||||
| 50 | 77.02 | 77.02 | 61.11 | 75.59 | 72.13 | 79.94 | 76.79 |
| 45 | 80.82 | 80.82 | 66.58 | 79.43 | 76.26 | 83.46 | 80.58 |
| 30 | 92.10 | 92.10 | 83.95 | 90.97 | 89.01 | 92.76 | 91.42 |
| 25 | 95.07 | 95.07 | 89.26 | 94.00 | 92.83 | 95.61 | 94.51 |
| 20 | 97.29 | 97.29 | 93.88 | 96.71 | 95.86 | 97.57 | 97.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rungkitwattanakul, D.; Evans, E.; Brown, E.; Patterson Jr., K.; Chaijamorn, W.; Charoensareerat, T.; Belrhiti, S.; Nwaogwugwu, U.; Mere, C. Impact of Removing Race Coefficient from Glomerular Filtration Rate Estimation Equations on Antidiabetics Among Black Patients. Pharmacy 2025, 13, 52. https://doi.org/10.3390/pharmacy13020052
Rungkitwattanakul D, Evans E, Brown E, Patterson Jr. K, Chaijamorn W, Charoensareerat T, Belrhiti S, Nwaogwugwu U, Mere C. Impact of Removing Race Coefficient from Glomerular Filtration Rate Estimation Equations on Antidiabetics Among Black Patients. Pharmacy. 2025; 13(2):52. https://doi.org/10.3390/pharmacy13020052
Chicago/Turabian StyleRungkitwattanakul, Dhakrit, Ebony Evans, Ewanna Brown, Kent Patterson Jr., Weerachai Chaijamorn, Taniya Charoensareerat, Sanaa Belrhiti, Uzoamaka Nwaogwugwu, and Constance Mere. 2025. "Impact of Removing Race Coefficient from Glomerular Filtration Rate Estimation Equations on Antidiabetics Among Black Patients" Pharmacy 13, no. 2: 52. https://doi.org/10.3390/pharmacy13020052
APA StyleRungkitwattanakul, D., Evans, E., Brown, E., Patterson Jr., K., Chaijamorn, W., Charoensareerat, T., Belrhiti, S., Nwaogwugwu, U., & Mere, C. (2025). Impact of Removing Race Coefficient from Glomerular Filtration Rate Estimation Equations on Antidiabetics Among Black Patients. Pharmacy, 13(2), 52. https://doi.org/10.3390/pharmacy13020052






