Pioneering a Framework for Robust Telemedicine Technology Assessment (Telemechron Study)
Abstract
1. Introduction
1.1. The Importance of the Telemedicine Technology Assessment
1.2. From One Health to Key Performance Indicators: An Examination of Telemedicine Technology Assessment Trends
1.3. The Vital Role and the Need of a Framework in Telemedicne Technology Assessment
1.4. The Telemechron Study and the Role of the Istituto Superiore di Sanità
- –
- In WP3, IRCCS Maugeri of Lumezzane concentrates on patients with chronic heart failure and comorbidities.
- –
- In WP4, the Trento Company directs its attention to patients with type 2 diabetes mellitus.
- –
- WP2, led by ISS, serves as a bridge between diverse telemedicine experiences. Its primary focus is on facing technological assessment issues, including: (a) Examining the effectiveness, clinical safety, organizational, technological, and financial aspects of telemedicine services designed and implemented by the three healthcare entities. (b) Elaborating and validating a set of multidimensional indicators for clinical governance.
1.5. Purpose of the Study
2. Methods
2.1. The Structure of the Framework for the Telemedicine Technology Assessment
- –
- Captures key information about the telemedicine initiative, including the official name, responsible healthcare company, reference region, operational unit, manager, work team, and types of telemedicine services offered;
- –
- Provides an overview of the organizational structure, key personnel, and the nature of services offered.
- –
- Conducts a SWOT analysis of healthcare before the introduction of the telemedicine service;
- –
- Identifies healthcare needs that the telemedicine service aims to satisfy;
- –
- Describes the functional aspects of the telemedicine service and its integration into patient care;
- –
- Specifies the target population and socio-economic indications;
- –
- Incorporates evidence from the scientific literature supporting the rationale for the telemedicine services;
- –
- Offers a global perspective on the strategic framework of the telemedicine initiative.
- –
- Includes components related to organizational–management and technical–technological requirements;
- –
- Covers economic–financial assessments and procurement strategies;
- –
- Describes the adopted methodology and actors involved in design and implementation;
- –
- Addresses normative and regulatory aspects;
- –
- Focuses on risk management and best practices;
- –
- Outlines crucial elements involved in designing and implementing the telemedicine service.
- –
- Defines criteria and procedures for service activation and management;
- –
- Specifies methodologies and indicators for monitoring service quality;
- –
- Addresses service tariffs and cost-sharing rules;
- –
- Discusses change management and the activation date of the telemedicine service.
- –
- Quantifies the volumes of provided services;
- –
- Provides crucial details related to the adoption phase of the telemedicine service.
- –
- Identifies dimensions and indicators for service evaluation, including expectations and KPIs for assessing the effectiveness of the telemedicine application;
- –
- Describes methods for data collection and analysis during service evaluation;
- –
- Presents results of service evaluation;
- –
- Gathers lessons learned, critical success factors, and recommendations for large-scale adoption or transferability of experience.


2.2. The CAWI Tools for the Evaluation of the Proposed Methodology and for the Assessment of the Methodology Impact
- –
- Single-choice questions;
- –
- Multiple-choice questions;
- –
- Evaluation (graded) questions (with a 6-level psychometric scale);
- –
- Likert questions with a 6-level psychometric scale;
- –
- Open-ended questions (in a few cases).
3. Results
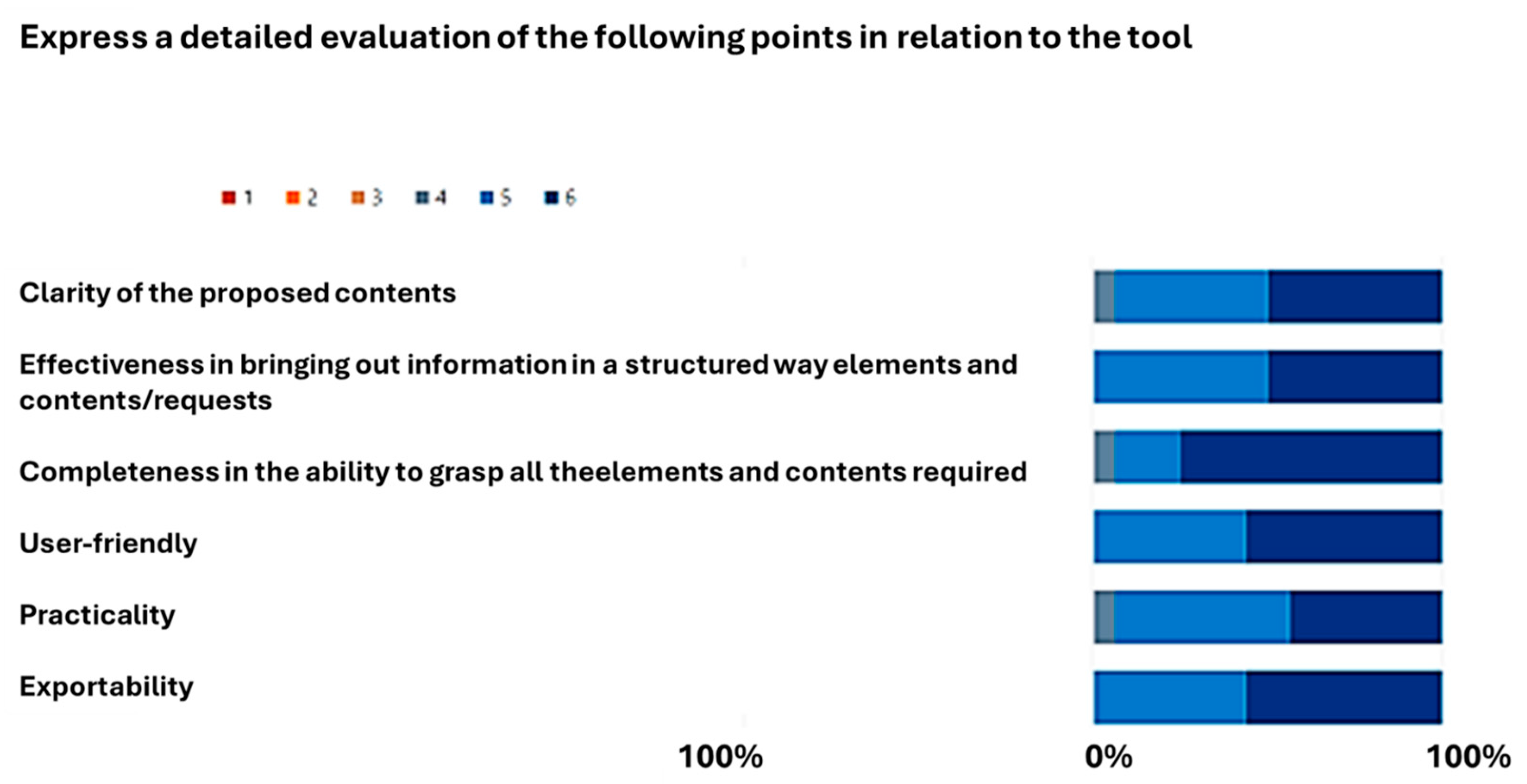
3.1. Evaluation of the Proposed Methodology
3.2. Assessment of Methodology Impact
4. Discussion
4.1. In Focus: Evaluating the Project Report’s Value and Contributions
4.2. Work in Progress
4.3. Suggestions for Future Investigation
4.4. Limitations
4.5. Takeway Message
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scott, P.; Kuziemsky, C.; Zhu, X.; Nøhr, C.; Ammenwerth, E.; Kukhareva, P.; Peute, L.; Marcilly, R. One Health: Insights from Organizational & Social, Technology Assessment and Human Factors Perspectives. Yearb. Med. Inform. 2023, 32, 76–83. [Google Scholar] [CrossRef]
- Welzel, C.; Cotte, F.; Wekenborg, M.; Vasey, B.; McCulloch, P.; Gilbert, S. Holistic Human-Serving Digitization of Health Care Needs Integrated Automated System-Level Assessment Tools. J. Med. Internet Res. 2023, 25, e50158. [Google Scholar] [CrossRef]
- Alami, H.; Rivard, L.; Lehoux, P.; Ahmed, M.A.A.; Fortin, J.-P.; Fleet, R. Integrating environmental considerations in digital health technology assessment and procurement: Stakeholders’ perspectives. Digit. Health 2023, 9, 20552076231219113. [Google Scholar] [CrossRef]
- Kolk, M.Z.H.; Frodi, D.M.; Langford, J.; Meskers, C.J.; Andersen, T.O.; Jacobsen, P.K.; Risum, N.; Tan, H.L.; Svendsen, J.H.; Knops, R.E.; et al. Behavioural digital biomarkers enable real-time monitoring of patient-reported outcomes: A substudy of the multicenter, prospective observational SafeHeart study. Eur. Heart J. Qual. Care Clin. Outcomes 2023, qcad069. [Google Scholar] [CrossRef]
- Brönneke, J.B.; Herr, A.; Reif, S.; Stern, A.D. Dynamic HTA for digital health solutions: Opportunities and challenges for patient-centered evaluation. Int. J. Technol. Assess. Health Care 2023, 39, e72. [Google Scholar] [CrossRef]
- Pearson, S.D.; Singh, P.; Beaudoin, F.; Campbell, J.; Schapiro, L.; Emond, S.K.; Pearson, C. Institute for Clinical and Economic Review—Peterson Health Technology Institute value assessment framework for digital health technologies. J. Comp. Eff. Res. 2023, 12, e230154. [Google Scholar] [CrossRef]
- Hollis, C.; Hall, C.L.; Khan, K.; Le Novere, M.; Marston, L.; Jones, R.; Hunter, R.; Brown, B.J.; Sanderson, C.; Andrén, P.; et al. Online remote behavioural intervention for tics in 9- to 17-year-olds: The ORBIT RCT with embedded process and economic evaluation. Health Technol. Assess. 2023, 27, 1–120. [Google Scholar] [CrossRef]
- Main, C.; Haig, M.; Chavez, D.; Kanavos, P. Assessing the Value of Provider-Facing Digital Health Technologies Used in Chronic Disease Management: Toward a Value Framework Based on Multistakeholder Perceptions. Med. Decis. Mak. 2024, 44, 28–41. [Google Scholar] [CrossRef]
- Goetz, G.; Jeindl, R.; Panteli, D.; Busse, R.; Wild, C. Digital Health Applications (DiHA): Approaches to develop a reimbursement process for the statutory health insurance in Austria. Health Policy Technol. 2023, 12, 100780. [Google Scholar] [CrossRef]
- Mezei, F.; Horváth, K.; Pálfi, M.; Lovas, K.; Ádám, I.; Túri, G. International practices in health technology assessment and public financing of digital health technologies: Recommendations for Hungary. Front. Public Health 2023, 11, 1197949. [Google Scholar] [CrossRef]
- Agnail, R.; Balas, E.A.; Heboyan, V.; Silva, J.; Coughlin, S.; Beltrame, F.; De Leo, G. Design and development of a Telemedicine Assessment Toolkit (TAT) for the assessment of audiovisual telemedicine encounters. J. Telemed. Telecare 2023, 1357633X231194381. [Google Scholar] [CrossRef]
- Malvehy, J.; Dreno, B.; Barba, E.; Dirschka, T.; Fumero, E.; Greis, C.; Gupta, G.; Lacarrubba, F.; Micali, G.; Moreno, D.; et al. Smart E-Skin Cancer Care in Europe During and after the COVID-19 Pandemic: A Multidisciplinary Expert Consensus. Dermatol. Pract. Concept. 2023, 13, e2023181. [Google Scholar] [CrossRef]
- Haig, M.; Main, C.; Chávez, D.; Kanavos, P. A Value Framework to Assess Patient-Facing Digital Health Technologies That Aim to Improve Chronic Disease Management: A Delphi Approach. Value Health 2023, 26, 1474–1484. [Google Scholar] [CrossRef]
- Njoku, C.; Hofer, S.G.; Sathyamoorthy, G.; Patel, N.; Potts, H.W. The role of accelerator programmes in supporting the adoption of digital health technologies: A qualitative study of the perspectives of small- and medium-sized enterprises. Digit. Health 2023, 9, 20552076231173303. [Google Scholar] [CrossRef]
- Lipprandt, M.; Klausen, A.D.; Röhrig, R.; DESIREE Study Group. Methodology for the Description of Socio-Technical Systems: A Case Study Approach. Stud. Health Technol. Inform. 2023, 302, 656–660. [Google Scholar] [CrossRef] [PubMed]
- James, J.G.; Park, J.; Oliver, A.; Xie, S.X.; Siderowf, A.; Spindler, M.; Wechsler, L.R.; Tropea, T.F. Linked Patient and Provider Impressions of Outpatient Teleneurology Encounters. Neurol. Clin. Pract. 2023, 13, e200159. [Google Scholar] [CrossRef]
- Myung, J.-E.; Strachan, L.; Shin, J.; Yim, J.; Lee, S.-S. Reimbursement Coverage Decision Making for Digital Health Technologies in South Korea: Does It Fit the Value Framework Used in Traditional Medical Technologies? Value Health Reg. Issues 2023, 36, 27–33. [Google Scholar] [CrossRef]
- Brenner, M.; Weir, A.; McCann, M.; Doyle, C.; Hughes, M.; Moen, A.; Ingvar, M.; Nauwelaerts, K.; Turk, E.; McCabe, C. Development of the key performance indicators for digital health interventions: A scoping review. Digit. Health 2023, 9, 20552076231152160. [Google Scholar] [CrossRef] [PubMed]
- Papavero, S.C.; Fracasso, A.; Ramaglia, P.; Cicchetti, A.; de Belvis, A.G.; Ferrara, F.M. Telemedicine Has a Social Impact: An Italian National Study for the Evaluation of the Cost-Opportunity for Patients and Caregivers and the Measurement of Carbon Emission Savings. Telemed. e-Health 2023, 29, 1252–1260. [Google Scholar] [CrossRef]
- Baltaxe, E.; Hsieh, M.; Roca, J.; Cano, I. The Assessment of Medical Device Software Supporting Health Care Services for Chronic Patients in a Tertiary Hospital: Overarching Study. J. Med. Internet Res. 2023, 25, e40976. [Google Scholar] [CrossRef]
- Baroni, M.P.; Jacob, M.F.A.; Rios, W.R.; Fandim, J.V.; Fernandes, L.G.; Chaves, P.I.; Fioratti, I.; Saragiotto, B.T. The state of the art in telerehabilitation for musculoskeletal conditions. Arch. Physiother. 2023, 13, 1. [Google Scholar] [CrossRef]
- Giansanti, D.; Morelli, S.; Macellari, V. Telemedicine technology assessment part II: Tools for a quality control system. Telemed. e-Health 2007, 13, 130–140. [Google Scholar] [CrossRef]
- Giansanti, D.; Morelli, S.; Macellari, V. Telemedicine technology assessment part I: Setup and validation of a quality control System. Telemed. e-Health 2007, 13, 118–129. [Google Scholar] [CrossRef]
- Mackintosh, N.; Terblanche, M.; Maharaj, R.; Xyrichis, A.; Franklin, K.; Keddie, J.; Larkins, E.; Maslen, A.; Skinner, J.; Newman, S.; et al. Telemedicine with clinical decision support for critical care: A systematic review. Syst. Rev. 2016, 5, 176. [Google Scholar] [CrossRef]
- Doupi, P. Evolving Health IT Systems Evaluation: The Convergence of Health Informatics and HTA. Stud. Health Technol. Inform. 2016, 222, 220–236. [Google Scholar]
- Ekeland, A.G.; Grøttland, A. Assessment of Mast in European Patient-Centered Telemedicine Pilots. Int. J. Technol. Assess. Health Care 2015, 31, 304–311. [Google Scholar] [CrossRef]
- Giansanti, D.; Morelli, S.; Maccioni, G.; Guerriero, L.; Bedini, R.; Pepe, G.; Colombo, C.; Borghi, G.; Macellari, V. A web based health technology assessment in tele-echocardiography: The experience within an Italian project. Alcohol Alcohol. 2009, 45, 392–397. [Google Scholar] [CrossRef]
- Giansanti, G.; Morelli, S.; Bedini, R.; Macellari, V. Uníesperienza Italiana di Controllo di Qualità in Telemedicina: Il Progetto eRMETE; Rapporti ISTISAN 08/23; Istituto Superiore di Sanità: Roma, Italy, 2008. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morelli, S.; Daniele, C.; D’Avenio, G.; Grigioni, M.; Giansanti, D. Pioneering a Framework for Robust Telemedicine Technology Assessment (Telemechron Study). Technologies 2024, 12, 37. https://doi.org/10.3390/technologies12030037
Morelli S, Daniele C, D’Avenio G, Grigioni M, Giansanti D. Pioneering a Framework for Robust Telemedicine Technology Assessment (Telemechron Study). Technologies. 2024; 12(3):37. https://doi.org/10.3390/technologies12030037
Chicago/Turabian StyleMorelli, Sandra, Carla Daniele, Giuseppe D’Avenio, Mauro Grigioni, and Daniele Giansanti. 2024. "Pioneering a Framework for Robust Telemedicine Technology Assessment (Telemechron Study)" Technologies 12, no. 3: 37. https://doi.org/10.3390/technologies12030037
APA StyleMorelli, S., Daniele, C., D’Avenio, G., Grigioni, M., & Giansanti, D. (2024). Pioneering a Framework for Robust Telemedicine Technology Assessment (Telemechron Study). Technologies, 12(3), 37. https://doi.org/10.3390/technologies12030037






