Digital Twin Models for Personalised and Predictive Medicine in Ophthalmology
Abstract
1. Introduction
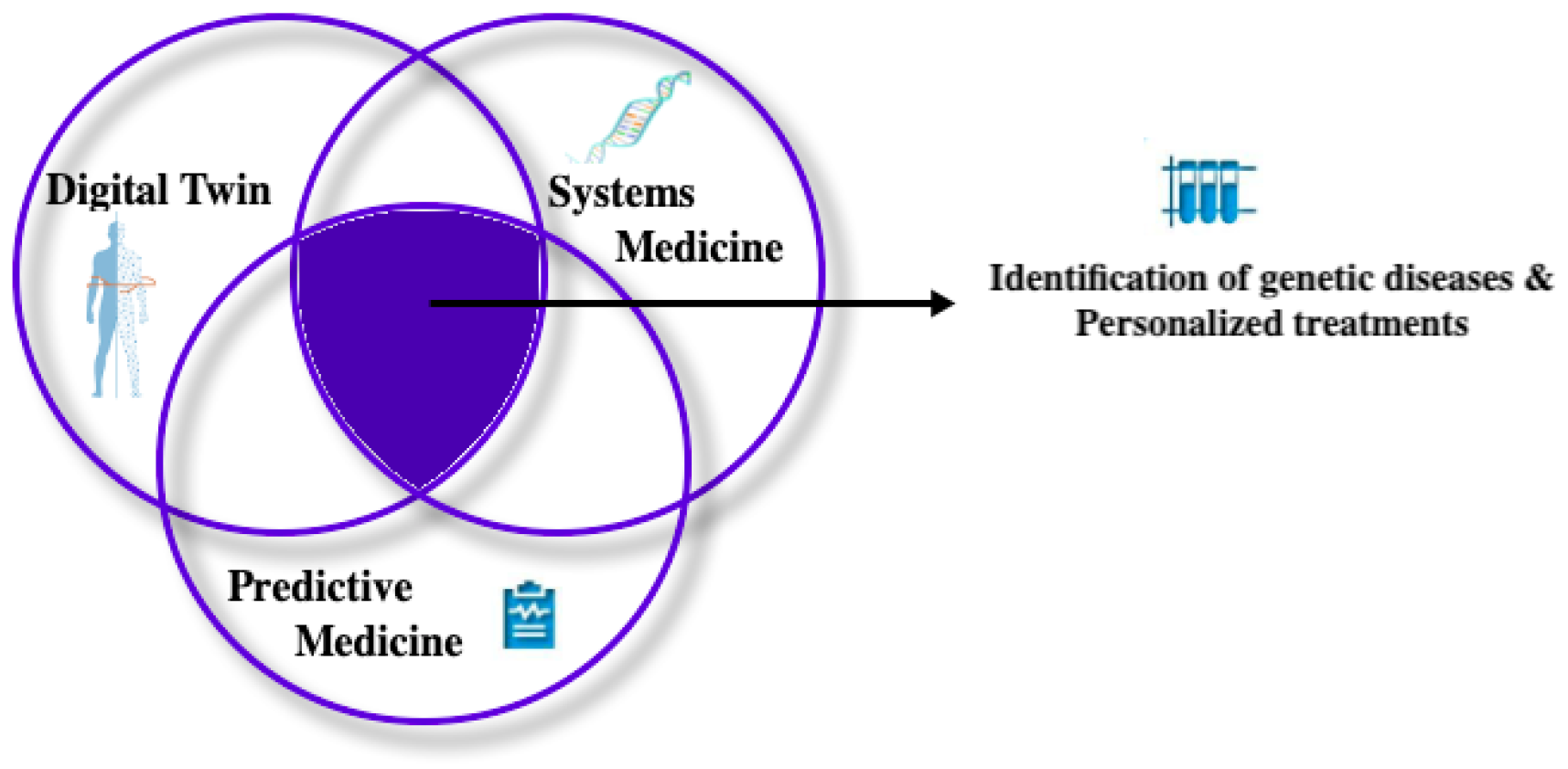
2. Digital Twin, Systems Medicine and Predictive Medicine
2.1. Digital Twin, Systems Medicine and Predictive Medicine in Literature
- Digital Twin
- Son et al. [10]—“A human Digital Twin could show what is happening inside the linked physical twin’s body, making it easier to predict the occurrence of an illness by analyzing the real twin’s personal history and the current context such as location, time, and activity”.
- Ala-Laurinaho et al. [11]—“An emerging approach for disease treatment and prevention encompassing the use of new diagnostics and therapeutics, targeted to the needs of a patient based on their own genetic, biomarker, phenotypic, physical, or psychosocial characteristics”.
- Bruynseels et al. [12]—“Human Digital Twins—the assumption that one is in possession of a data magnifying glass, that gives a detailed account of the molecular, phenotypic, and lifestyle history of persons”.
- Shengli [13]—“A human Digital Twin is a copy or counterpart in cyberspace of a real person in our physical world. It is the digital description of you in a digital manner on a computer or server in the cloud. The model analyzes the timely data, historic data, the data from your relatives, and obtains insight from these data by Cloud Computing, Deep Learning, etc.”.
- Systems Medicine
- Benson [15]—“Systems medicine encompasses the application of theoretical strategies through iterative and reciprocal exchanges of inputs between physicians, biologists, pharmacologists, bioinformaticians, and mathematicians in the fields of medical concepts, study, and practice”.
- Cascante et al. [16]—“The driving force behind the eventual improvement of patient outcomes is, therefore, the recurrent interaction between bedside examinations, experimental models, and statistical analyses”.
- Bousquet et al. [17]—“Systems Medicine is the latest definition of pediatric allergic diseases over a systematic translational approach involving detection, diagnosis, prevention, and therapy”.
- Mayer et al. [18]—“System-based modeling also resulted from heterogeneous and daunting conditions, such as irritable bowel disease”.
- Hood and Flores [19]—“Systems Medicine is a systemic approach to medicine and health that identifies all the components of a system and the interactions between them. Thus, the complex processes of the human body are characterized by interactions at the level of structural and functional organization”.
2.2. Digital Twin, Systems Medicine and Predictive Medicine in Ophthalmology
- Congenital glaucoma is a rare eye condition (optic neuropathy) that predominantly affects males. This disease may manifest during the neonatal or early infantile period and is characterized by symptoms such as sensitivity to light and ocular discomfort. Primary congenital glaucoma (PCG) is an autosomal recessive disorder largely attributed to mutations in CYP1B1, and to a lesser extent, in LTBP2, TEK, MYOC, and FOXC1 [22].
- Primary open-angle glaucoma (POAG) [23] is the most common form of glaucoma and is associated with increased intraocular pressure (IOP) (>21 mmHg) and an open iridocorneal angle (35°–45°). Another manifestation is central retinal venous occlusion (the stoppage of circulation in the central vein of the retina or in one of its branches); heredity is one of the factors that favor the appearance of this pathology. In [24], Sears et al. highlighted that mutations in each of the three genes, myocilin (MYOC), optineurin (OPTN), and TANK binding kinase 1 (TBK1), can cause primary open-angle glaucoma (POAG).
- Primary closed-angle glaucoma (PCAG) is determined by an anatomical predisposition of the eyeball, which leads to partial or total obstruction of the drainage of the aqueous humor by blocking the angle at the periphery of the iris.
- Secondary open-angle glaucoma can be associated with intraocular tumors and eye hemorrhages, or it can be caused by treatment with cortisone and post-laser procedures.
- Secondary closed-angle glaucoma can be caused by pupillary block, which is determined by intumescent cataract, or it can occur without pupillary block, as in neovascular post-inflammatory glaucoma.
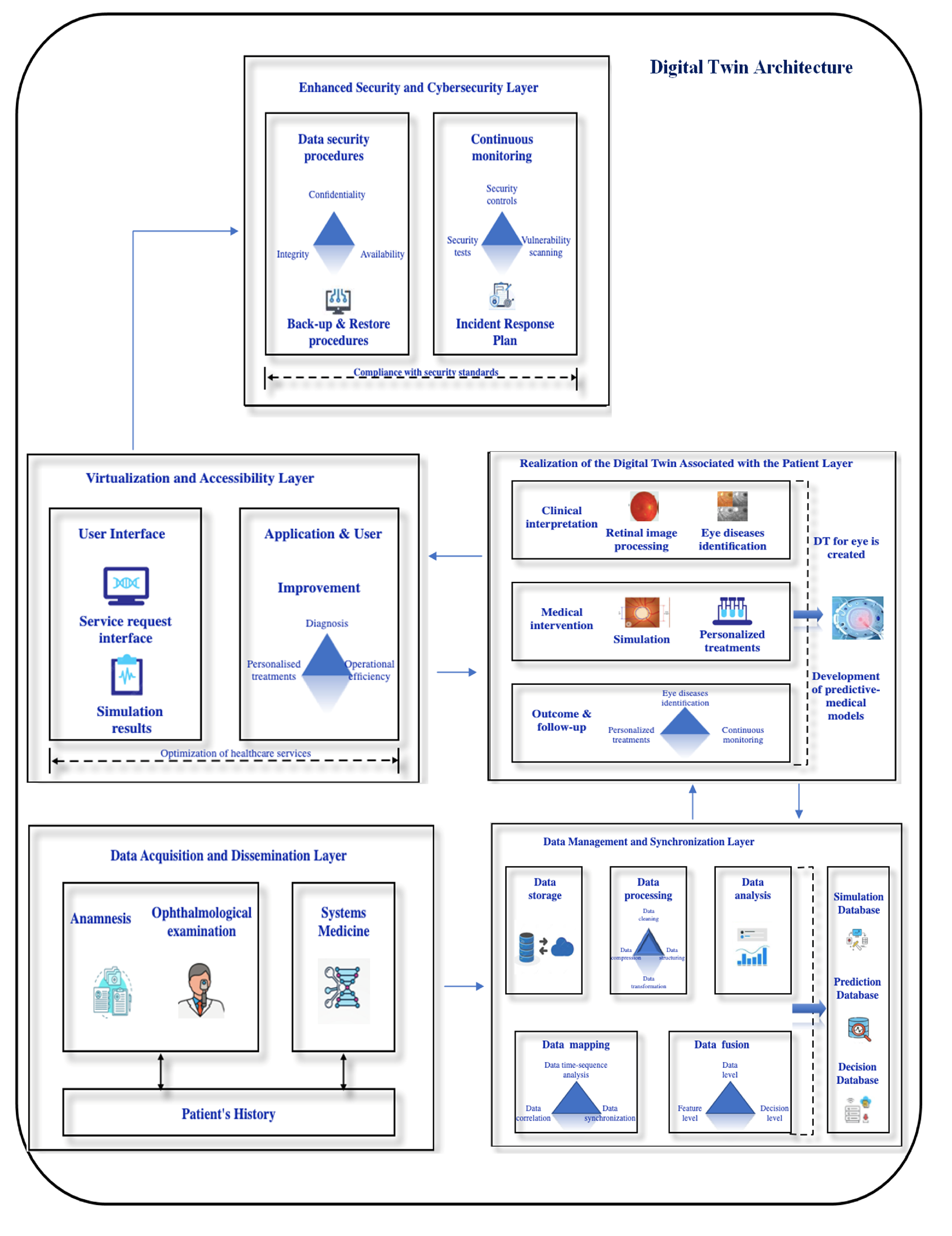
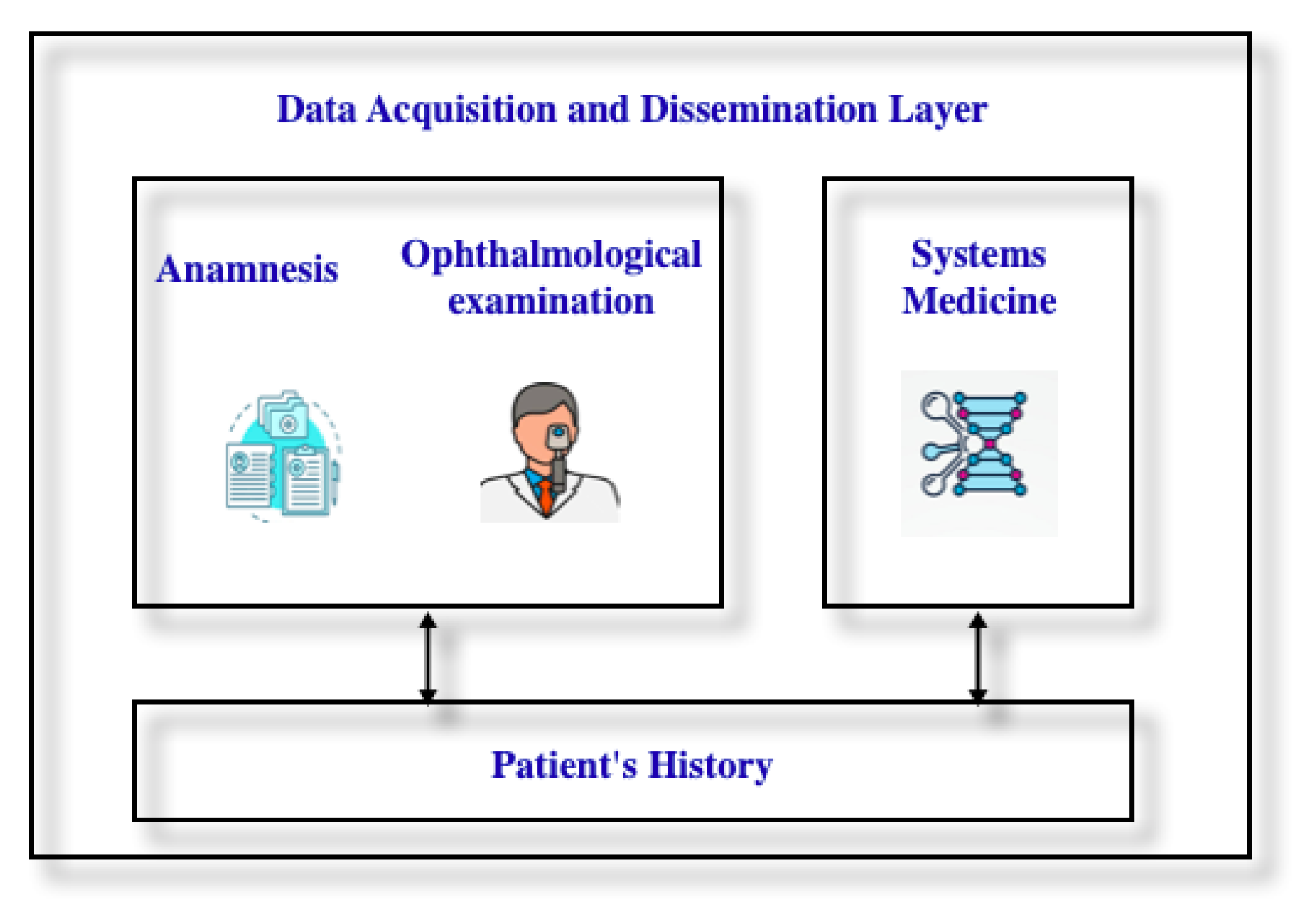
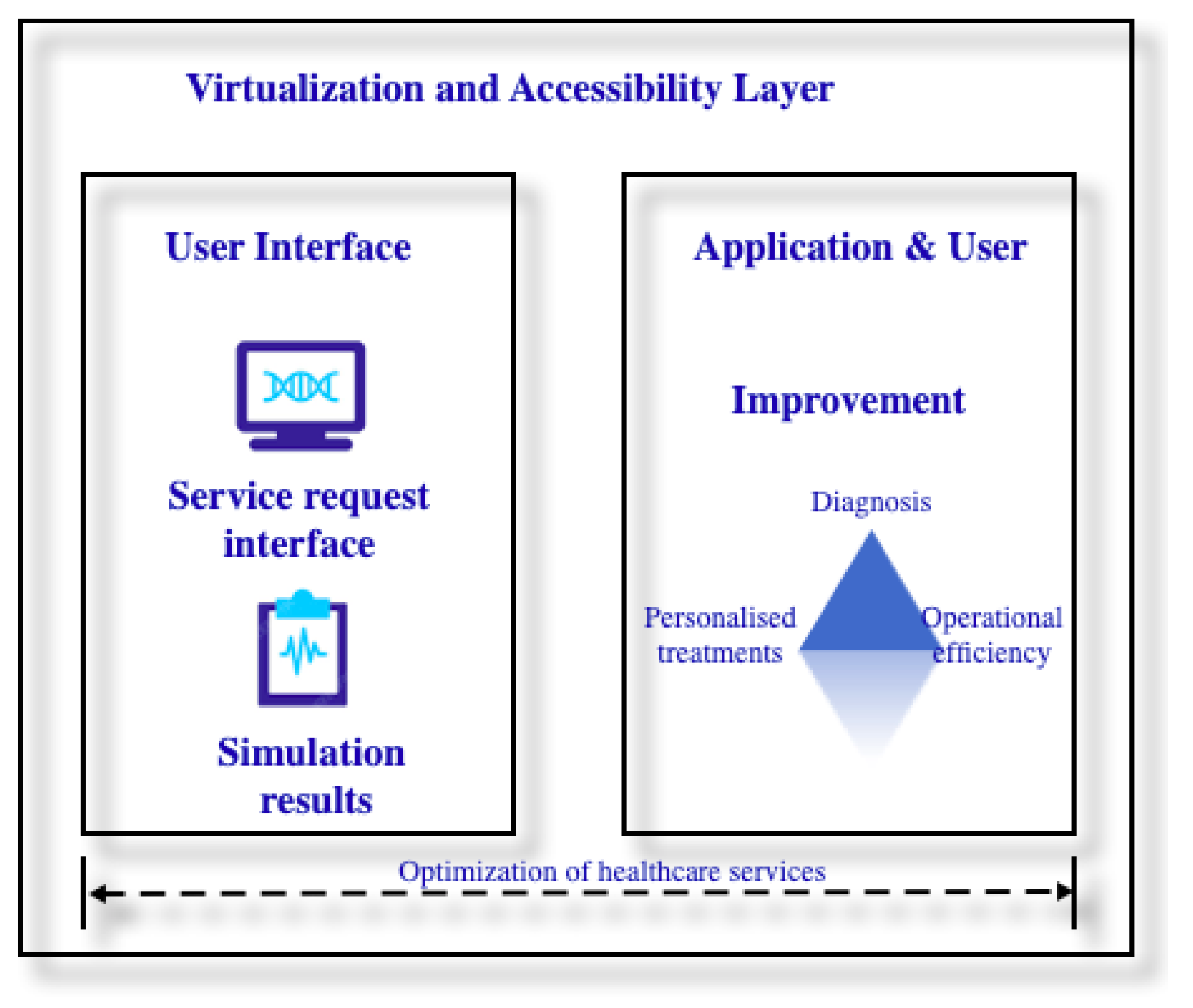
3. Architectural Framework for Digital Twin, Risks and Vulnerabilities
3.1. An Architectural Framework for Digital Twin in Ophthalmology
- If the IOP has not increased compared to the previous examination conducted no more than six months ago, the patient will be advised to undergo another ophthalmological examination after six months.
- If the IOP has not increased compared to the previous examination conducted more than six months ago, the patient will be advised to undergo an eye examination after 12 months.
- If the IOP has increased compared to the previous examination, the patient will be advised to undergo an eye exam after 1–2 months.
- If the IOP has not increased compared to the previous examination, but there has been progression in other aspects, an ophthalmological examination will be recommended after 1–2 months.
- If the IOP has not increased compared to the previous examination and there has been no progression in other aspects, an ophthalmological examination will be recommended after 3–6 months.
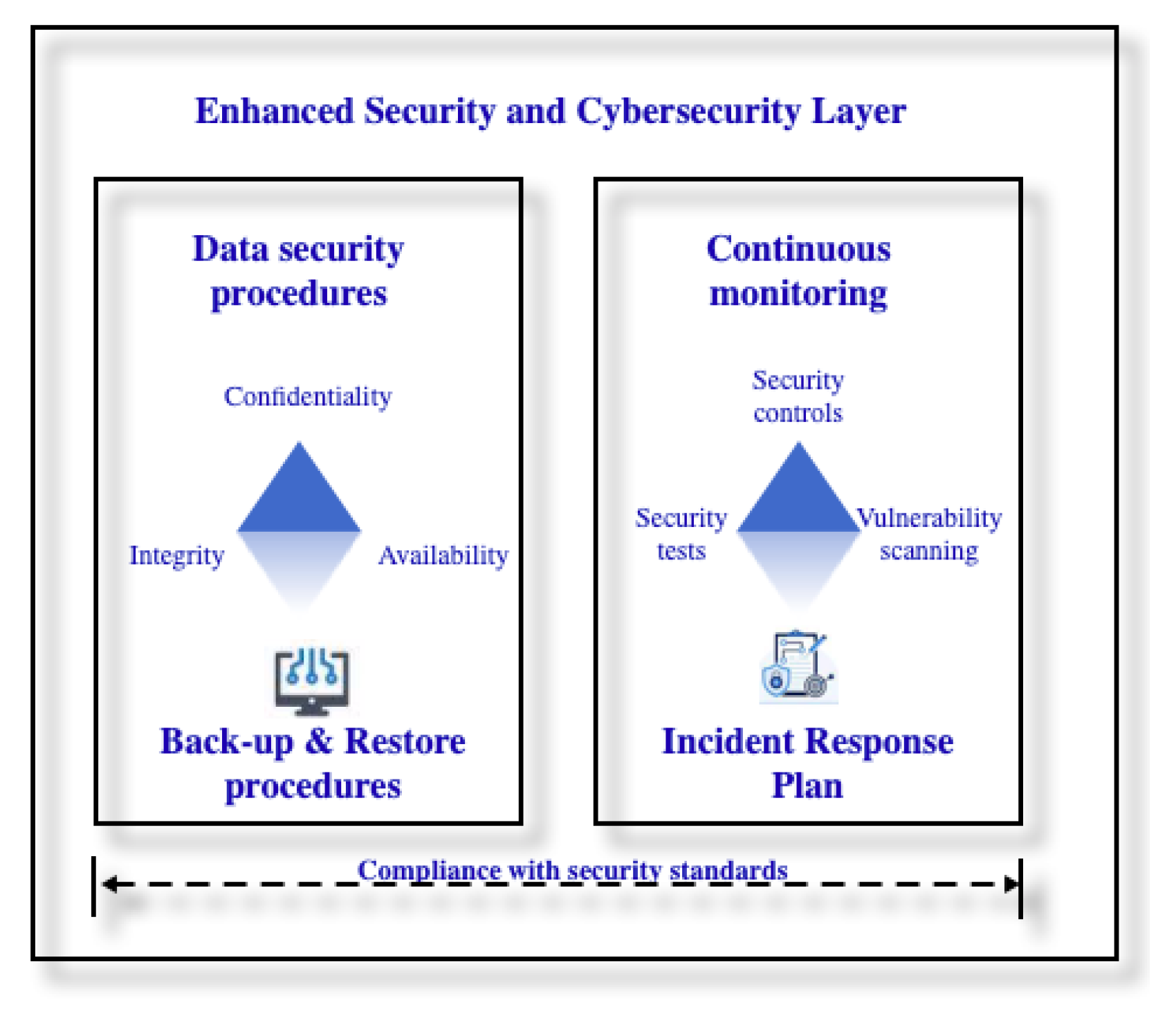
3.2. Risks and Vulnerabilities at the Digital Twin Level
- The risks of doctor reputation and patient health in Digital Twin for glaucoma detection
- Cybersecurity challenges and research frontiers for Digital Twin
4. Materials and Methods
4.1. Datasets
4.2. Convolutional Neural Network Architecture
- Training—80% of the dataset was used to train the network.
- Validation—10% of the dataset was used for fine-tuning the model’s hyperparameters and preventing overfitting during the training phase. This helps assess the model’s performance on unseen data during training. Unfortunately, the dataset only contains 500 samples, so we compromised and used only 10% for validation to avoid underfitting.
- Testing—10% of the dataset was used to test the model. This serves as an independent dataset, not used during training and validation, and is exclusively used after model training to evaluate its performance on entirely new, unseen data.
5. Results
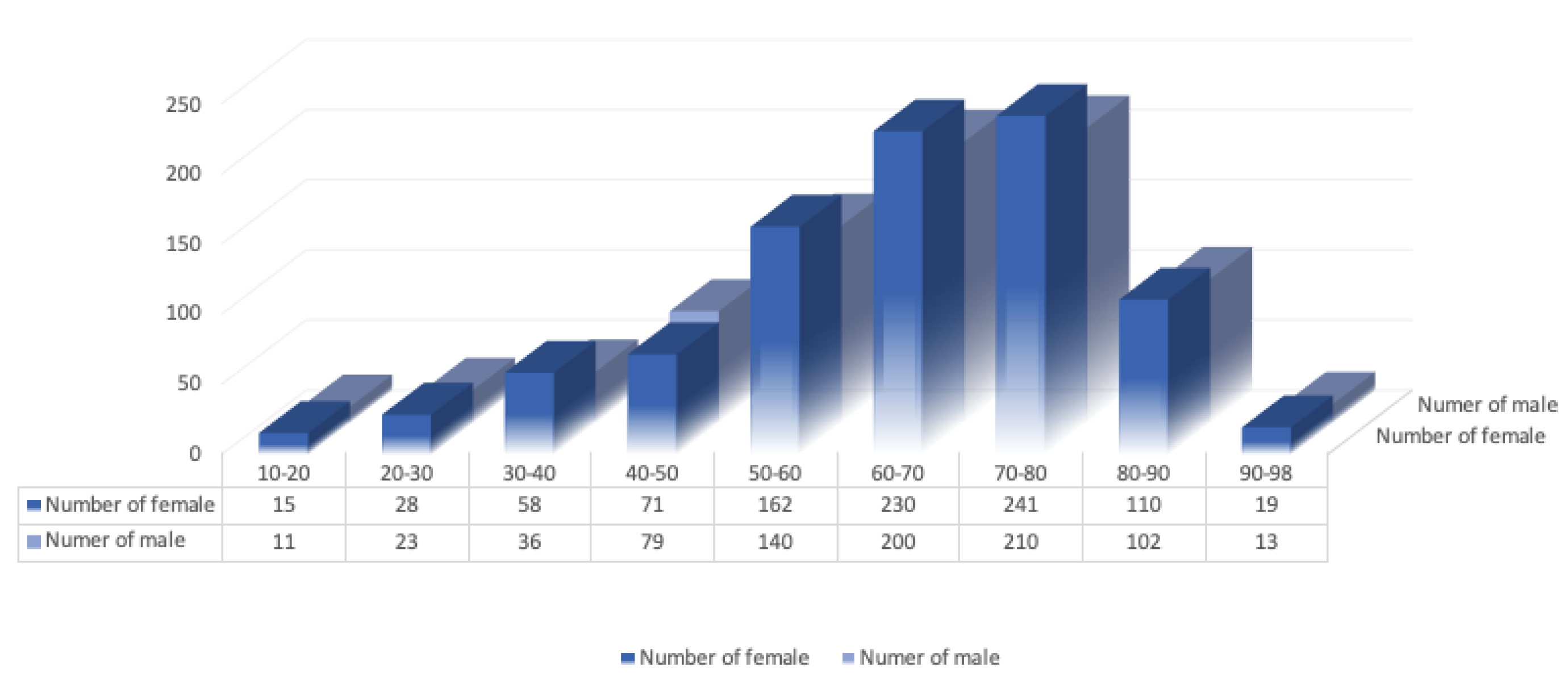
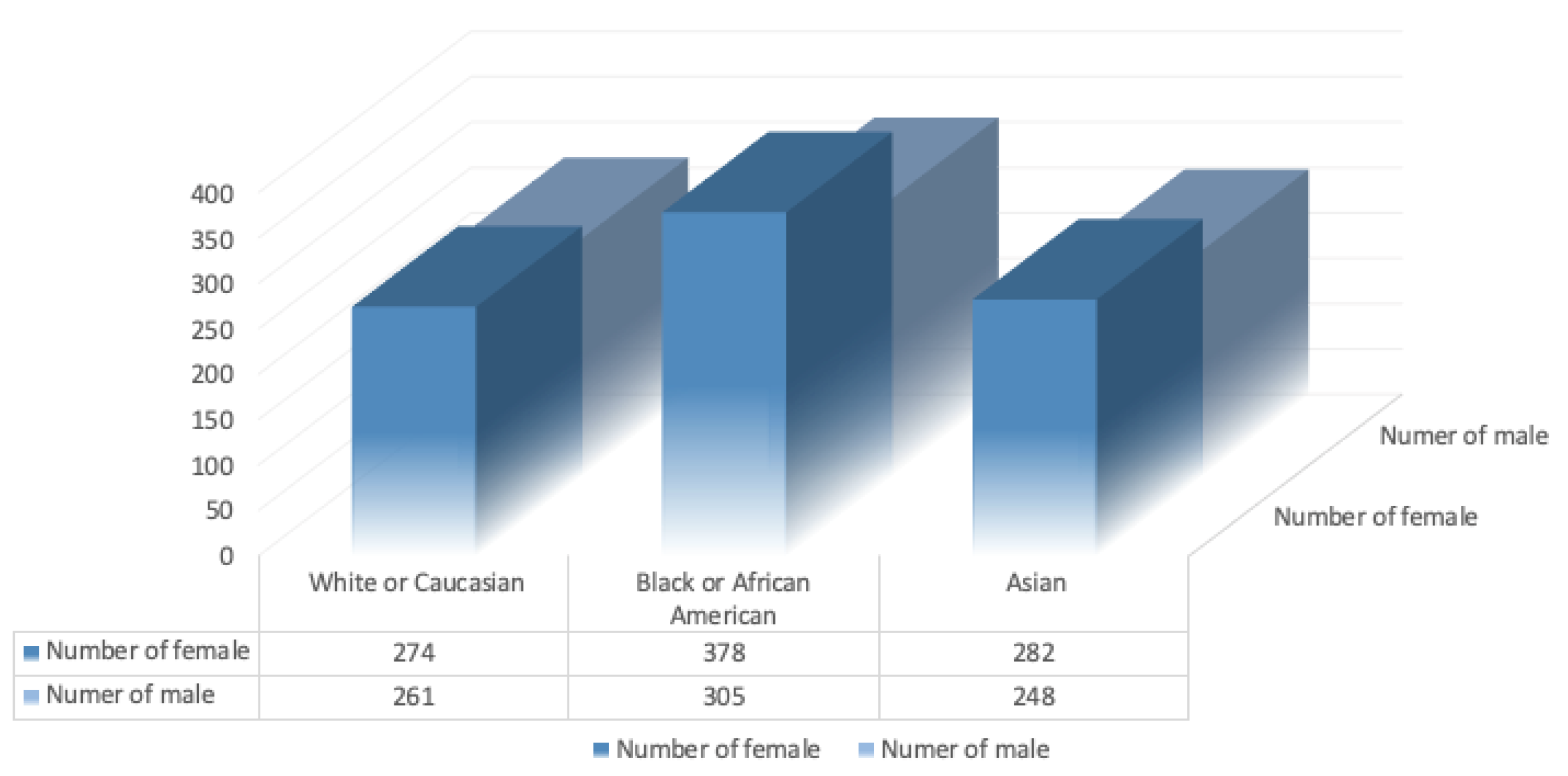
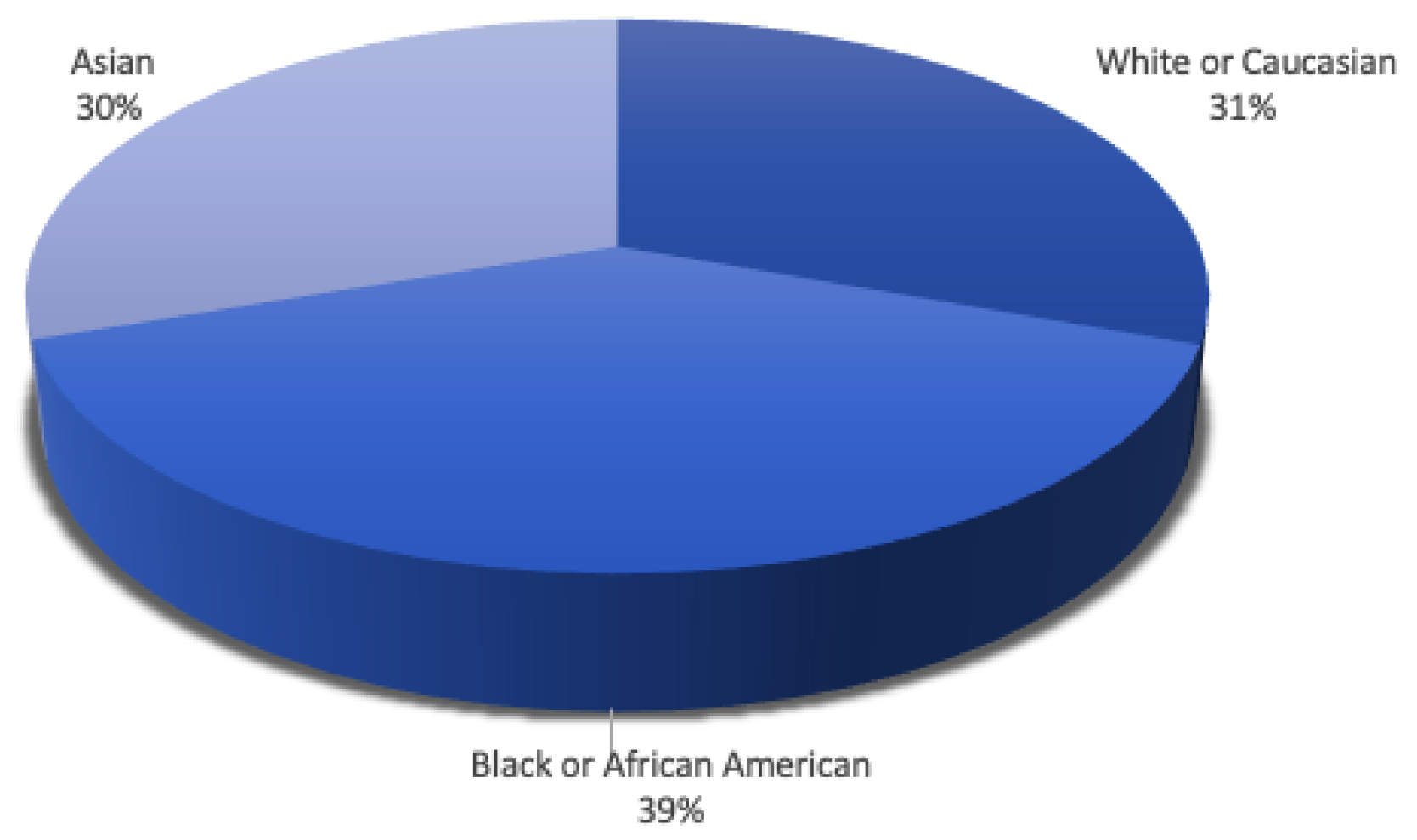
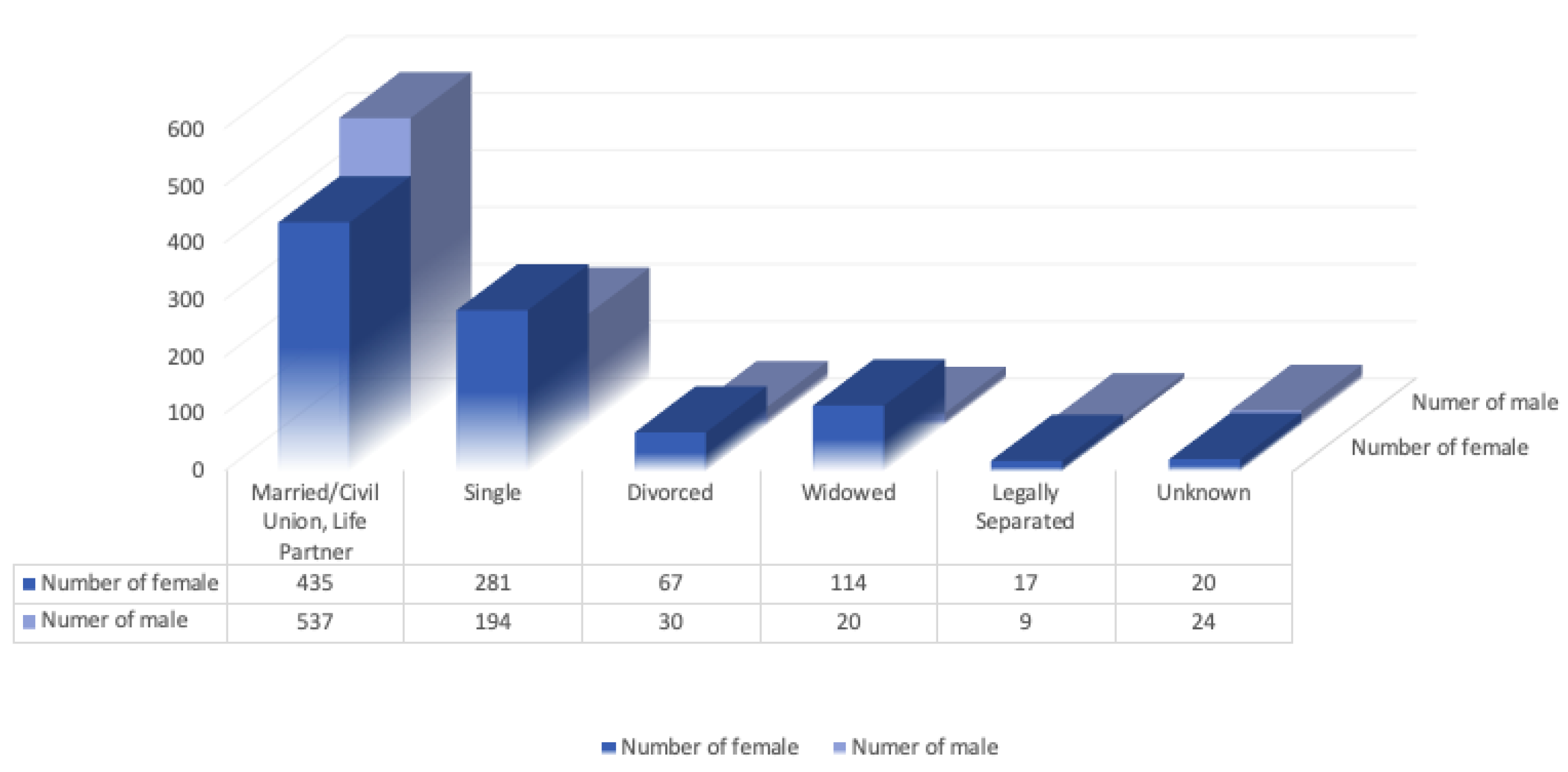
5.1. Statistics on the Occurrence of Glaucoma Based on Demographic Factors
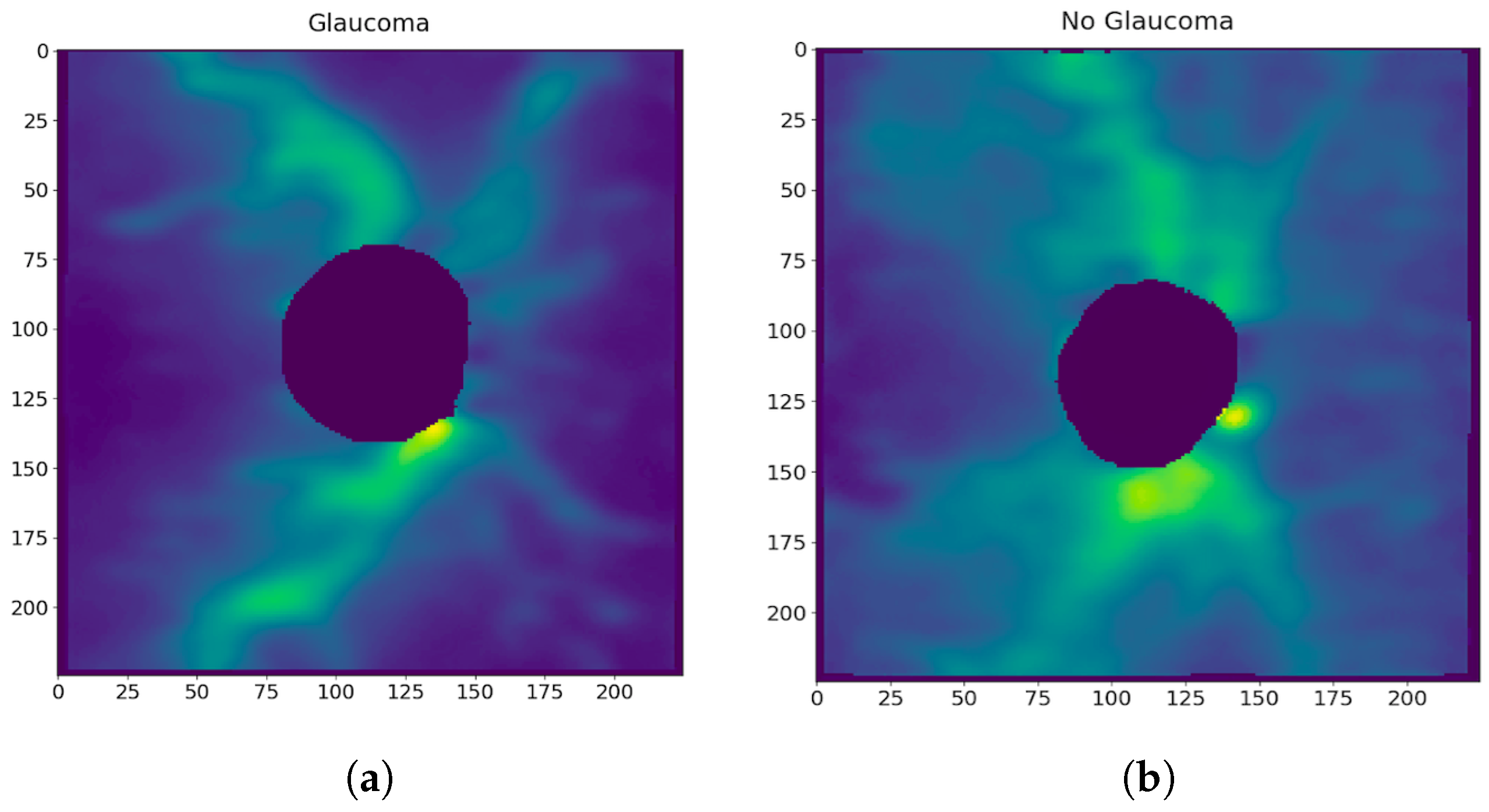
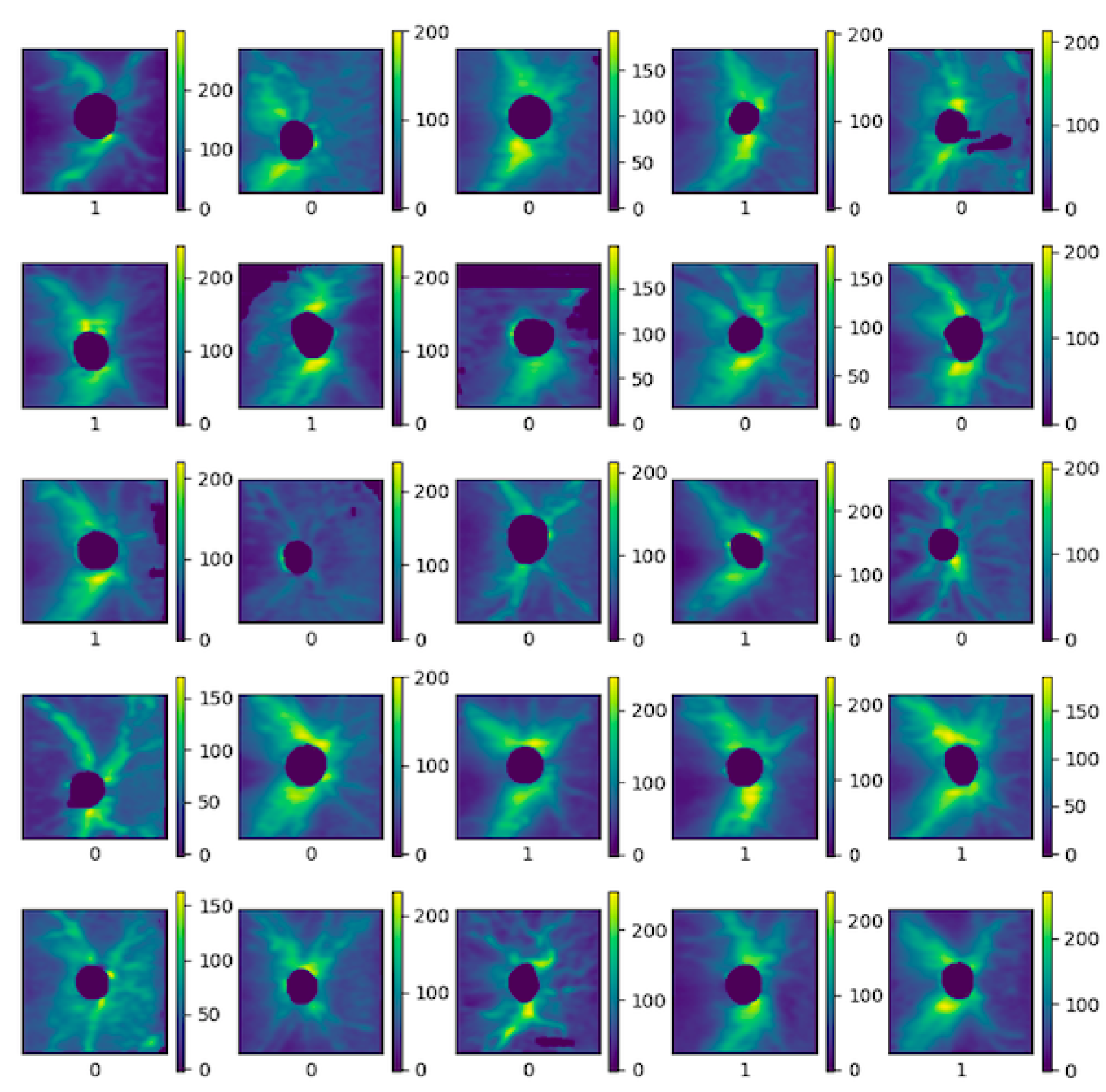
5.2. Training the Neural Network for Glaucoma Detection
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization, Blindness and Vision Impairment. Available online: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment (accessed on 17 January 2024).
- VanDerHorn, E.; Mahadevan, S. Digital Twin: Generalization, characterization and implementation. Decis. Support Syst. 2021, 145, 113524. [Google Scholar] [CrossRef]
- Grieves, M. Digital Twin: Manufacturing Excellence through Virtual Factory Replication; White Paper; Michael W. Grieves, LLC.: Cocoa Beach, FL, USA, 2014; pp. 1–7. [Google Scholar]
- Flores, M.; Glusman, G.; Brogaard, K.; Price, N.D.; Hood, L. P4 medicine: How systems medicine will transform the healthcare sector and society. Pers. Med. 2013, 10, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Valet, G.K.; Tárnok, A. Cytomics in predictive medicine. Cytom. Part B Clin. Cytom. J. Int. Soc. Anal. Cytol. 2003, 53, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Vasco, P.; Moscatelli, F.; La Torre, M.E.; Valenzano, A.; Monda, V.; Cibelli, G.; de Stefano, M.I.; Marsala, G.; Dalia, C.; Bassi, P.; et al. Role of Technology Innovation in Telemedicine: Focus on Sport Nutrition. Appl. Sci. 2023, 13, 4837. [Google Scholar] [CrossRef]
- Manickam, P.; Mariappan, S.A.; Murugesan, S.M.; Hansda, S.; Kaushik, A.; Shinde, R.; Thipperudraswamy, S.P. Artificial intelligence (AI) and internet of medical things (IoMT) assisted biomedical systems for intelligent healthcare. Biosensors 2022, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Costin, H.N.; Sanei, S. Intelligent biosignal processing in wearable and implantable sensors. Biosensors 2022, 12, 396. [Google Scholar] [CrossRef]
- Cellina, M.; Cè, M.; Alì, M.; Irmici, G.; Ibba, S.; Caloro, E.; Fazzini, D.; Oliva, G.; Papa, S. Digital Twins: The New Frontier for Personalized Medicine? Appl. Sci. 2023, 13, 7940. [Google Scholar] [CrossRef]
- Son, S.; Kwon, D.; Lee, J.; Yu, S.; Jho, N.S.; Park, Y. On the design of a privacy-preserving communication scheme for cloud-based digital twin environments using blockchain. IEEE Access 2022, 10, 75365–75375. [Google Scholar] [CrossRef]
- Ala-Laurinaho, R.; Autiosalo, J.; Nikander, A.; Mattila, J.; Tammi, K. Data link for the creation of digital twins. IEEE Access 2020, 8, 228675–228684. [Google Scholar] [CrossRef]
- Bruynseels, K.; Santoni de Sio, F.; Van den Hoven, J. Digital twins in health care: Ethical implications of an emerging engineering paradigm. Front. Genet. 2018, 9, 31. [Google Scholar] [CrossRef]
- Shengli, W. Is human digital twin possible? Comput. Methods Programs Biomed. Update 2021, 1, 100014. [Google Scholar] [CrossRef]
- Ayers, D.; Day, P.J. Systems medicine: The application of systems biology approaches for modern medical research and drug development. Mol. Biol. Int. 2015, 2015, 698169. [Google Scholar] [CrossRef] [PubMed]
- Benson, M. Clinical implications of omics and systems medicine: Focus on predictive and individualized treatment. J. Intern. Med. 2016, 279, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Cascante, M.; Boros, L.G.; Comin-Anduix, B.; de Atauri, P.; Centelles, J.J.; Lee, P.W.N. Metabolic control analysis in drug discovery and disease. Nat. Biotechnol. 2002, 20, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Anto, J.M.; Akdis, M.; Auffray, C.; Keil, T.; Momas, I.; Postma, D.S.; Valenta, R.; Wickman, M.; Cambon-Thomsen, A.; et al. Paving the way of systems biology and precision medicine in allergic diseases: The Me DALL success story: Mechanisms of the Development of ALL ergy; EU FP 7-CP-IP; Project No: 261357; 2010–2015. Allergy 2016, 71, 1513–1525. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Labus, J.S.; Tillisch, K.; Cole, S.W.; Baldi, P. Towards a systems view of IBS. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 592–605. [Google Scholar] [CrossRef]
- Hood, L.; Flores, M. A personal view on systems medicine and the emergence of proactive P4 medicine: Predictive, preventive, personalized and participatory. New Biotechnol. 2012, 29, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Tarnok, A.; Valet, G.K. Cytomics in predictive medicine. In Proceeding of the Advanced Biomedical and Clinical Diagnostic Systems II, San Jose, CA, USA, 24–29 January 2004; SPIE: Bellingham, WA, USA, 2004; Volume 5318, pp. 12–22. [Google Scholar]
- Dada, T.; Verma, S.; Gagrani, M.; Bhartiya, S.; Chauhan, N.; Satpute, K.; Sharma, N. Ocular and Systemic Factors Associated with Glaucoma. J. Curr. Glaucoma Pract. 2022, 16, 179. [Google Scholar]
- Chakrabarti, S.; Kabra, M.; Pyatla, G.; Rathi, S.; Mandal, A.K.; Senthil, S.; Kaur, I.; Khanna, R.C.; Khanna, H. Genetic Interactions of Crystallins with Known Primary Congenital Glaucoma Genes. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5148. [Google Scholar]
- Available online: https://www.omicsdi.org/dataset/ega/phs000238.v1.p1 (accessed on 17 January 2024).
- Sears, N.C.; Boese, E.A.; Miller, M.A.; Fingert, J.H. Mendelian genes in primary open angle glaucoma. Exp. Eye Res. 2019, 186, 107702. [Google Scholar] [CrossRef]
- Alcaraz, C.; Lopez, J. Digital twin: A comprehensive survey of security threats. IEEE Commun. Surv. Tutorials 2022, 24, 1475–1503. [Google Scholar] [CrossRef]
- Qi, Q.; Tao, F.; Hu, T.; Anwer, N.; Liu, A.; Wei, Y.; Wang, L.; Nee, A.Y. Enabling technologies and tools for digital twin. J. Manuf. Syst. 2021, 58, 3–21. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, S.; Cheng, H. An application framework of digital twin and its case study. J. Ambient. Intell. Humaniz. Comput. 2019, 10, 1141–1153. [Google Scholar] [CrossRef]
- Haleem, M.S.; Han, L.; Van Hemert, J.; Li, B. Automatic extraction of retinal features from colour retinal images for glaucoma diagnosis: A review. Comput. Med. Imaging Graph. 2013, 37, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Bellazzi, R.; Zupan, B. Predictive data mining in clinical medicine: Current issues and guidelines. Int. J. Med. Inform. 2008, 77, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Prum, B.E.; Rosenberg, L.F.; Gedde, S.J. American Academy of Ophthalmology, Primary Open-Angle Glaucoma; American Academy of Ophthalmology: San Francisco, CA, USA, 2016. [Google Scholar]
- Liu, Y.; Zhang, L.; Yang, Y.; Zhou, L.; Ren, L.; Wang, F.; Liu, R.; Pang, Z.; Deen, M.J. A novel cloud-based framework for the elderly healthcare services using digital twin. IEEE Access 2019, 7, 49088–49101. [Google Scholar] [CrossRef]
- Cannon, D.L. CISA—Certified Information Systems Auditor Study Guide; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Chapple, M. CISM Certified Information Security Manager Study Guide; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Harvard Glaucoma Fairness with 3300 Samples (Harvard-GF3300). Available online: https://ophai.hms.harvard.edu/datasets/harvard-gf3300/ (accessed on 17 January 2024).
- Harvard Glaucoma Fairness with 3300 Samples (Harvard-GF3300). Available online: https://github.com/Harvard-Ophthalmology-AI-Lab/Harvard-GF (accessed on 17 January 2024).
- Harvard Glaucoma Detection with 500 Samples (Harvard-GD500). Available online: https://ophai.hms.harvard.edu/datasets/harvard-gd500/ (accessed on 17 January 2024).
- Harvard Glaucoma Detection with 500 Samples (Harvard-GD500). Available online: https://drive.google.com/drive/folders/1-8NIRenXBy8sNxWUV8DMV6HNu2VX5H4u (accessed on 17 January 2024).
- Sample, P.A.; Girkin, C.A.; Zangwill, L.M.; Jain, S.; Racette, L.; Becerra, L.M.; Weinreb, R.N.; Medeiros, F.A.; Wilson, M.R.; De León-Ortega, J.; et al. The African descent and glaucoma evaluation study (ADAGES): Design and baseline data. Arch. Ophthalmol. 2009, 127, 1136–1145. [Google Scholar] [CrossRef]
- Shi, M.; Lokhande, A.; Fazli, M.S.; Sharma, V.; Tian, Y.; Luo, Y.; Pasquale, L.R.; Elze, T.; Boland, M.V.; Zebardast, N.; et al. Artifact-Tolerant Clustering-Guided Contrastive Embedding Learning for Ophthalmic Images in Glaucoma. IEEE J. Biomed. Health Inform. 2023, 27, 4329–4340. [Google Scholar] [CrossRef]







| Stage | The Patient’s Acquired Data |
|---|---|
| Information about the patient |
|
| Data acquisition from the ophthalmological examination |
|
| Genetic Information |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iliuţă, M.-E.; Moisescu, M.-A.; Caramihai, S.-I.; Cernian, A.; Pop, E.; Chiş, D.-I.; Mitulescu, T.-C. Digital Twin Models for Personalised and Predictive Medicine in Ophthalmology. Technologies 2024, 12, 55. https://doi.org/10.3390/technologies12040055
Iliuţă M-E, Moisescu M-A, Caramihai S-I, Cernian A, Pop E, Chiş D-I, Mitulescu T-C. Digital Twin Models for Personalised and Predictive Medicine in Ophthalmology. Technologies. 2024; 12(4):55. https://doi.org/10.3390/technologies12040055
Chicago/Turabian StyleIliuţă, Miruna-Elena, Mihnea-Alexandru Moisescu, Simona-Iuliana Caramihai, Alexandra Cernian, Eugen Pop, Daniel-Ioan Chiş, and Traian-Costin Mitulescu. 2024. "Digital Twin Models for Personalised and Predictive Medicine in Ophthalmology" Technologies 12, no. 4: 55. https://doi.org/10.3390/technologies12040055
APA StyleIliuţă, M.-E., Moisescu, M.-A., Caramihai, S.-I., Cernian, A., Pop, E., Chiş, D.-I., & Mitulescu, T.-C. (2024). Digital Twin Models for Personalised and Predictive Medicine in Ophthalmology. Technologies, 12(4), 55. https://doi.org/10.3390/technologies12040055








