Recent Development of Corrosion Inhibitors: Types, Mechanisms, Electrochemical Behavior, Efficiency, and Environmental Impact
Abstract
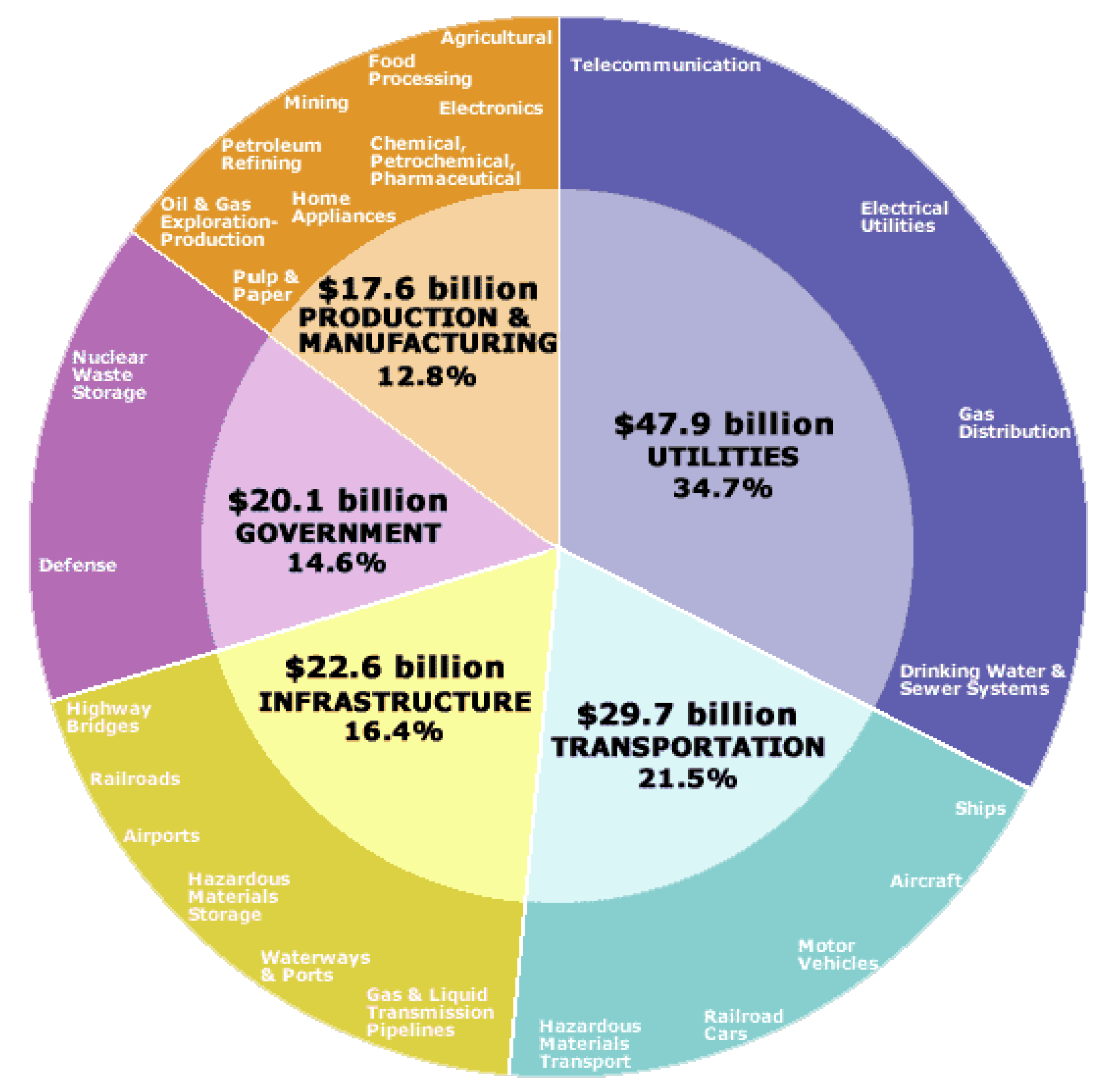
1. Introduction
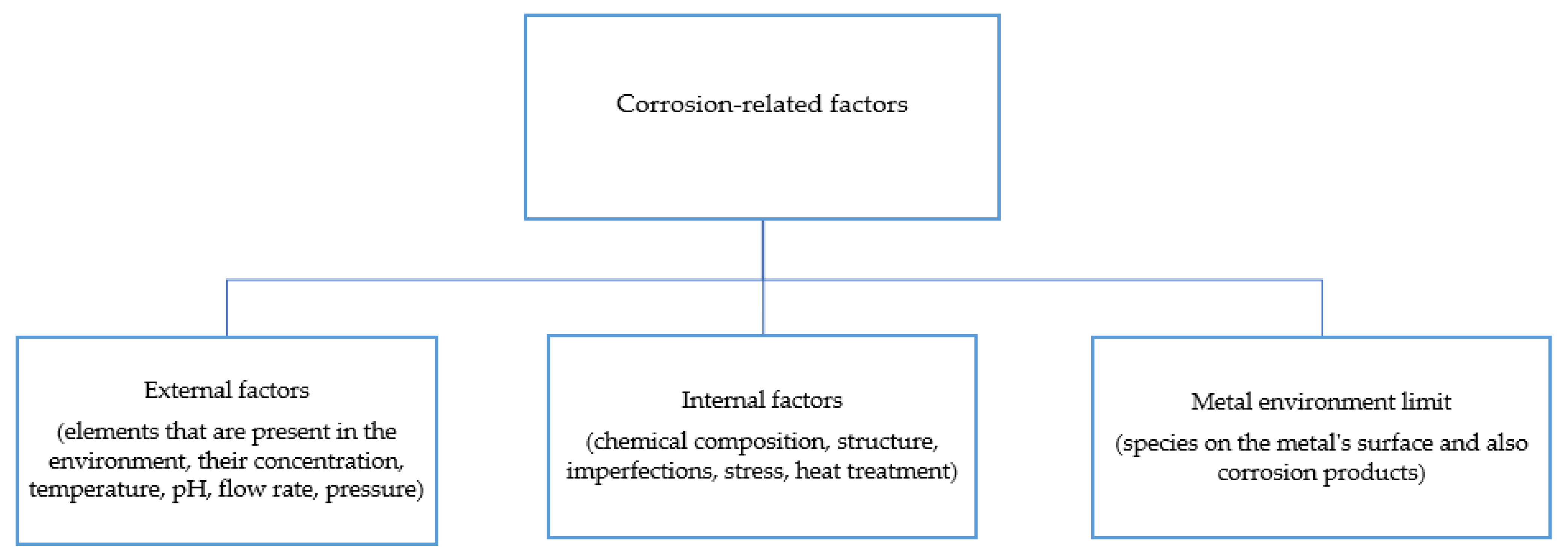
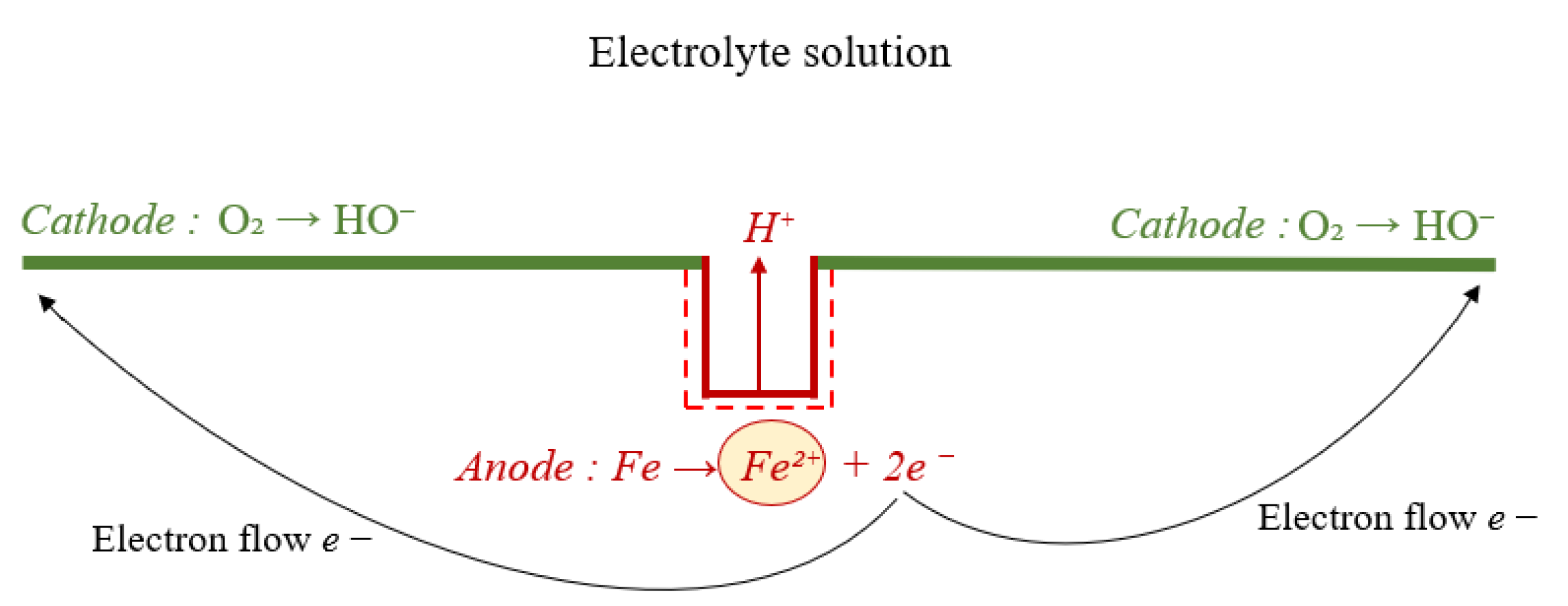
2. Corrosion Basics
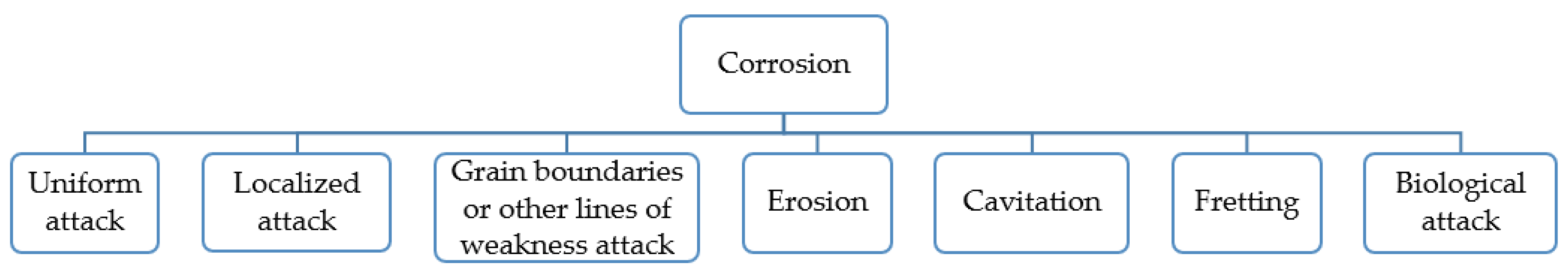
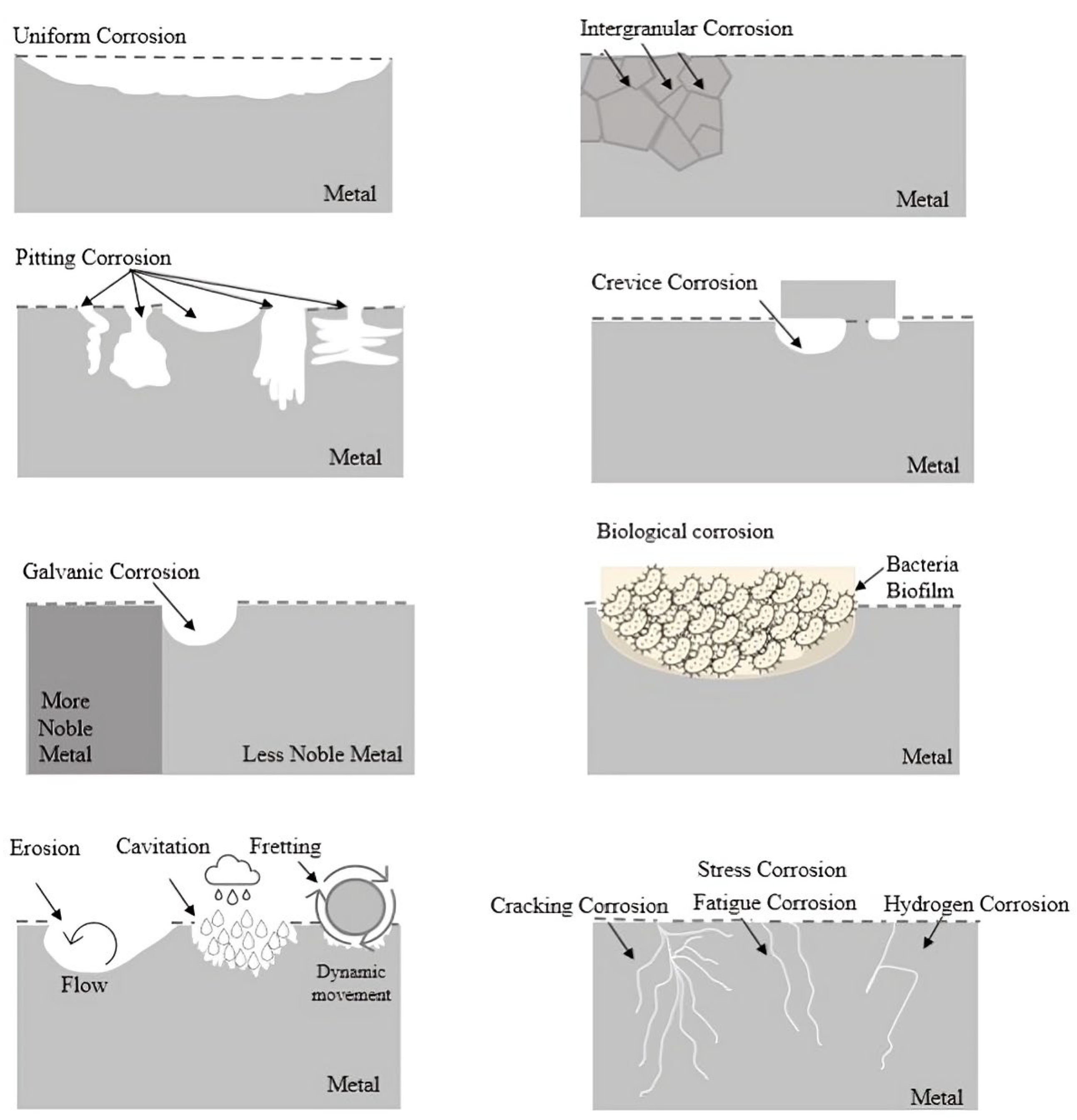
3. Corrosion Types
3.1. Uniform Corrosion
3.2. Localized Attack
3.2.1. Crevice Corrosion
3.2.2. Pitting Corrosion
3.3. Grain Boundaries
3.3.1. Galvanic Corrosion
3.3.2. Stress Corrosion
3.3.3. Intergranular Corrosion
3.3.4. Erosion Corrosion
3.3.5. Cavitation Corrosion
3.3.6. Fretting Corrosion
3.4. Biological Corrosion
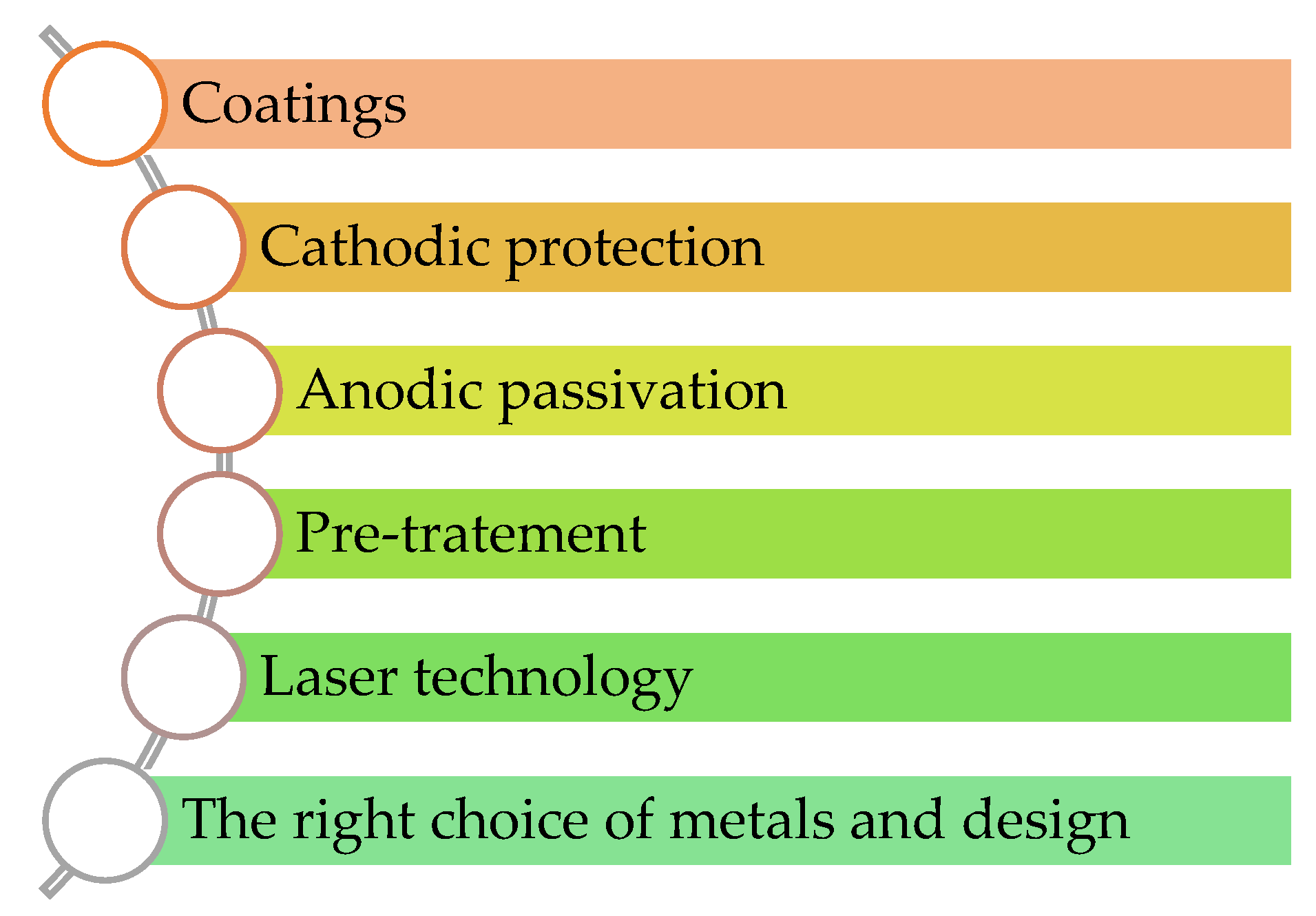
4. Techniques for Corrosion Prevention
4.1. Coatings
- ▪
- Organic;
- ▪
- Inorganic.
4.2. Cathodic Protection
- ▪
- Impressed current— this method uses a direct power supply feeding systems, where the positive pole is coupled to an anode. The anode can be either inert (such as silicon-cast iron, graphite, or platinized and activated titanium) or soluble (such as iron).
- ▪
- Galvanic or sacrificial anodes—galvanic or sacrificial anodes are created by coupling a less noble metal, such as iron or magnesium, with the substrate to provide cathodic protection. This process is used to protect various materials, including copper alloys, stainless steels, steel in seawater, and structures in soil or freshwater environments [46]. More reactive metals, like magnesium (Mg), aluminum (Al), and zinc (Zn), are often used as sacrificial anodes, because they corrode faster than the protected metal, thereby offering cathodic protection for metals such as steel and aluminum alloys [52].
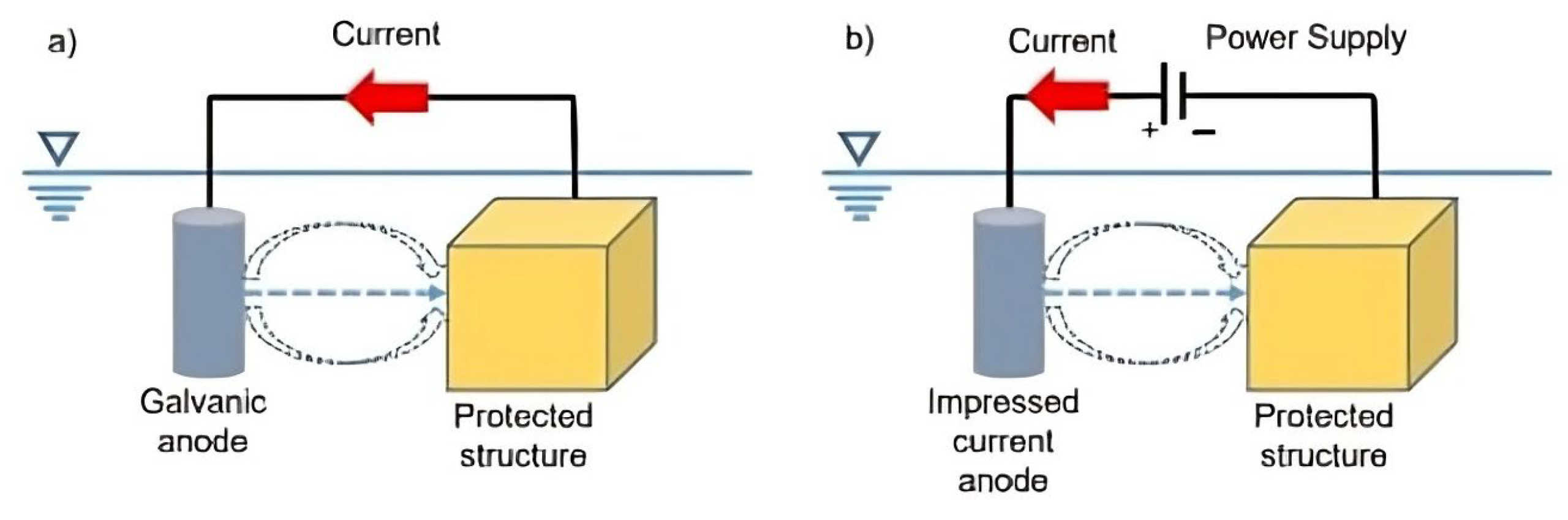
4.3. Anodic Passivation
4.4. Pre-Treatment
- -
- Improving the metallic substrate’s resistance to corrosion;
- -
- Creation of a suitable surface for the subsequent layers of the coating system.
- Mechanical cleaning: This process rapidly removes the exterior layers of a material, including methods such as grinding, blasting, and machining. It is effective regardless of the surface’s microstructure or chemical composition.
- Chemical pre-treatment: Chemical solutions used for pre-treatment are categorized into alkaline and acidic solutions. These chemicals help can eliminate grease, oil residues, and other contaminants left from manufacturing or mechanical pre-treatment. Organic solvents, such as ethanol and acetone, are commonly used in scientific research for this purpose.
- Laser technology: Due to its high automation, selectivity, efficiency, and environmental friendliness, laser surface texturing has gained popularity in recent years for preparing functional surfaces [61]. Laser processing combines surface texturing with laser chemical modification, making it a popular method for material surface treatment [62]. When a solid surface is irradiated with a laser, it develops nano- and microscale structures, which reduce the surface energy of the substrate or enable specific interactions between the surface and the fouling materials [63]. Additionally, laser surface modification, involving melting and annealing materials, is being explored for improving corrosion resistance in metallic alloys. This process homogenizes microstructures, dissolves intermetallic particles, and extends solid solubility through fast cooling rates, all without altering the surface’s chemical composition [64]. Laser cladding is another advanced process where particles are heated using a defocused laser beam, creating a metallurgical bond between the substrate and clad layer. Factors such as laser power, beam size, scanning velocity, cladding geometry, dilution rate, melt pool profile, aspect ratio, and clad layer thickness influence the coating’s quality [65]. The technique selective laser melting (SLM) creates metal objects with high surface roughness, which can limit their direct application without further processing [66]. Laser shock peening (LSP) is a new surface treatment method designed to reduce metal corrosion. It involves applying high-energy-density laser pulses to create a cavity on the target surface, inducing deep mechanical residual compressive stresses that enhance corrosion resistance [67].
4.5. Relevant Design and Sustainable Material Selection
5. Corrosion Inhibitors
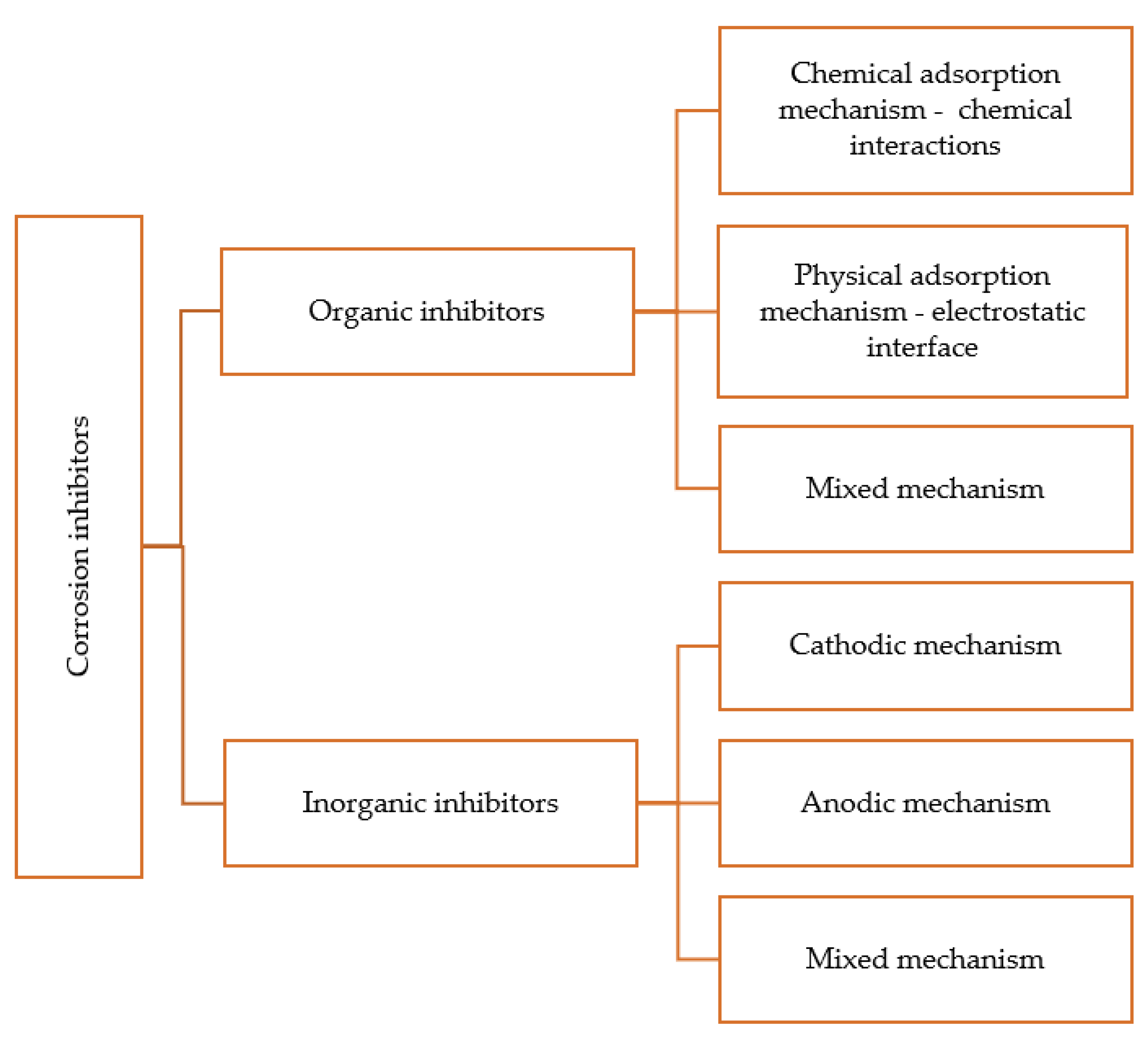
5.1. Classification of Corrosion Inhibitors
5.1.1. Anodic Corrosion Inhibitors or Chemical Passivators
5.1.2. Cathodic Corrosion Inhibitors
5.1.3. Mixed Corrosion Inhibitors
- (a)
- Chemisorbed films, where lone pair of electrons is donated to a central adsorption atom in a functional group;
- (b)
- Electrostatic adsorption films, which rely on electrostatic interactions;
- (c)
- Precipitation and/or complex films, which result from reactions between dissolved metal ion and organic inhibitor molecules.
5.1.4. Organic Inhibitors
- Synthetic organic corrosion inhibitors:
- -
- 1,2,3-triazole derivatives serve as corrosion inhibitors for metal surfaces. Unprotonated molecules, such as lone pairs from oxygen and nitrogen atoms and π-electrons from the benzene ring, can occupy the metal surface’s empty iron orbitals, resulting in the chemisorption phenomenon. The triazole derivatives interact with the metal surface through donor–acceptor interactions between the -N=N-, -C=N-, and (-C=C-) π-electrons of the benzene rings and the empty orbitals of the metal atoms [100].
- -
- Quinoline and its derivatives act as corrosion inhibitors in acidic media for mild steel. Through coordination bonding, quinoline derivatives with polar substituents, such as hydroxyl (-OH), methoxy (-OMe), amino -NH2), and nitro (-NO2), among others, efficiently adsorb and form highly stable chelating complexes with surface metallic atoms. Quinoline is expected to interact strongly with metallic surfaces due to its high electron density (10 π- and 2 non-bonding electrons). Using a donor–acceptor mechanism, organic compounds interact with metal surfaces throughs the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), which relate to the compounds’ ability to donate and accept electrons [101,102].
- -
- Oxalamide (Carbamothiol) derivatives act as corrosion inhibitors for copper in 3.5% NaCl [103].
- -
- Imidazoline derivatives act as corrosion inhibitors and surface-active agents for steel alloy in 1 M H2SO4 and HCl [104].
- -
- Imidazolone, an eco-friendly corrosion inhibitor for mild steel [105], is a mixed-type inhibitor, and follows the Langmuir adsorption model, forming heterogeneous films on the mild steel surface.
- -
- Quinazolinone derivatives act as mixed-type inhibitors for mild steel in a 1.0 M HCl medium. The Langmuir isotherm suggests that the dissolution of mild steel is an endothermic process and that these inhibitors function through physical adsorption on metallic surfaces [106].
- -
- Amino acid derivatives—novel benzimidazole derivatives incorporating three amino acids (tryptophan, tyrosine, and histidine) were designed for acidic solutions. The Langmuir adsorption model was followed, showing the presence of both chemisorption and physisorption processes [107].
- -
- Surfactants. Surfactants are surface-active substances with inherent amphiphilicity, containing both hydrophobic and hydrophilic groups. They serve as effective organic corrosion inhibitors, especially in protecting metals from corrosion. This protection is achieved through adsorption, driven the coordination or electrostatic electron-donating functions of the hydrophilic group [108].
- Ionic liquids
- Drugs
- Natural organic corrosion inhibitors
- Biopolymers
- Amino Acids
- Plant extracts
- Inorganic inhibitors
- Hybrid inhibitors (inorganic–organic) corrosion inhibitors
- Film-forming corrosion inhibitors work by creating a protective barrier or blocking layer made of a substance other than the inhibitor itself. Calcium and zinc salts are known to inhibit cathodic film formation, while benzoate is a typical example of an anodic film-forming inhibitor. These inhibitors act specifically at the cathode or anode [197].
- Vapor-phase/volatile-phase corrosion inhibitors function by releasing protective vapors that form a thin film over the metal surface to prevent corrosion.
- Nanotechnology corrosion inhibitors
- Self-healing coatings
- Antifouling corrosion inhibitors
- (a)
- Adding polymerizable agents to repair defects in the polymeric coating matrix;
- (b)
- Using corrosion inhibitors to prevent areas from corroding.
- Super-hydrophobic coatings
6. Mechanism of Action of Organic Corrosion Inhibitors
7. Interactions of Corrosion Inhibitors with Metal Surface; Molecular Modeling
- Chemical and electronic key configuration of corrosion inhibitors: This includes factors such as acidic and basic properties, molecular volume, lone pair electrons, dipole effects, etc. All these properties can influence the availability of inhibitor molecules to interact with the surface [230].
- Bonding of inhibitor with the metal surface.
- Surface configuration in terms of rugosity and chemistry.
- Solvent influence: This mainly concerns the competition between inhibitor molecules and solvent molecules for surface sites. Also, the pH of the solvent plays a significant role as it can influence the inhibitor’s structure (whether protonated or non-protonated) and the surface charge based on the point of zero charge (PZC).
- Effect of anodic and cathodic areas: Various types of reactions and phenomena can occur at the solid surface (Figure 11).
- Effects of electrode potential: Polarization of the dual system, the inhibitor metal surface, can occur when the solid surface is exposed to different chemical species.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NACE Study Estimates Global Cost of Corrosion. Available online: https://www.rustbullet.com/cost-of-corrosion/?srsltid=AfmBOoor60Cy-USkAyUAObxGazC-ThrGmbPpliLgqczvovNQZCWmvT-Q (accessed on 1 January 2025).
- Verma, C.; Ebenso, E.; Abdellaziz, B.; Hussain, C.M. Recent developments in Sustainable Corrosion Inhibitors: Design, Performance, and industrial scale applications. Mater. Adv. 2021, 2, 3806–3850. [Google Scholar] [CrossRef]
- Yang, L. 1—Introduction. In Techniques for Corrosion Monitoring; Yang, L., Ed.; Woodhead Publishing: Sawston, UK, 2008; pp. 1–5. [Google Scholar]
- NACE International. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study (IMPACT); NACE International: Huston, TX, USA, 2016. [Google Scholar]
- Koch, G. Cost of corrosion. In Trends in Oil and Gas Corrosion Research and Technologies; Woodhead Publishing: Sawston, UK, 2017; pp. 3–30. [Google Scholar]
- Balangao, J.K. Corrosion of Metals: Factors, Types and Prevention Strategies. J. Chem. Health Risks 2024, 14, 79–87. [Google Scholar]
- Alkadir Aziz, I.A.; Annon, I.A.; Abdulkareem, M.H.; Hanoon, M.M.; Alkaabi, M.H.; Shaker, L.M.; Alamiery, A.A.; Wan Isahak, W.N.; Takriff, M.S. Insights into Corrosion Inhibition Behavior of a 5-Mercapto-1,2,4-triazole Derivative for Mild Steel in Hydrochloric Acid Solution: Experimental and DFT Studies. Lubricants 2021, 9, 122. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Yousif, E.; Isahak, W.N.R.W.; Al-Azzawi, W.K. A Review of Inorganic Corrosion Inhibitors: Types, Mechanisms, and Applications. Tribol. Ind. 2023, 45, 313–339. [Google Scholar] [CrossRef]
- Dawood, M.A.; Alasady, Z.M.K.; Abdulazeez, M.S.; Ahmed, D.S.; Sulaiman, G.M.; Kadhum, A.A.H.; Shaker, L.M.; Alamiery, A.A. The corrosion inhibition effect of a pyridine derivative for low carbon steel in 1 M HCl medium: Complemented with antibacterial studies. Int. J. Corros. Scale Inhib. 2021, 10, 1766–1782. [Google Scholar]
- Verma, C.; Quraishi, M.; Rhee, K.Y. Electronic Effect vs. Molecular Size Effect: Experimental and Computational based Designing of Potential Corrosion Inhibitors. Chem. Eng. J. 2021, 430, 132645. [Google Scholar] [CrossRef]
- Abtan, N.; Al-Hamid, M.; Kadhim, L.; Sayyid, F.; Noori, F.; Kadum, A.; Al-Amiery, A.; Al-Azzawi, W. Unlocking the Power of 4-Acetamidoantipyrine: A Promising Corrosion Inhibitor for Preserving Mild Steel in Harsh Hydrochloric Acid Environments. Prog. Color Color. Coat. 2023, 17, 85–96. [Google Scholar] [CrossRef]
- Nnoka, M.; Alaso Jack, T.; Szpunar, J. Effects of different microstructural parameters on the corrosion and cracking resistance of pipeline steels: A review. Eng. Fail. Anal. 2024, 159, 108065. [Google Scholar] [CrossRef]
- Tait, W.S. Chapter 27—Controlling Corrosion of Chemical Processing Equipment. In Handbook of Environmental Degradation of Materials, 3rd ed.; Kutz, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 583–600. [Google Scholar]
- Kim, Y.-J.; Bahn, C.B.; Baek, S.H.; Choi, W.; Song, G.D. Crevice chemistry and corrosion in high temperature water: A review. Nucl. Eng. Technol. 2024, 56, 3112–3122. [Google Scholar] [CrossRef]
- Qasim, A.; Khan, M.S.; Lal, B.; Shariff, A.M. A perspective on dual purpose gas hydrate and corrosion inhibitors for flow assurance. J. Pet. Sci. Eng. 2019, 183, 106418. [Google Scholar] [CrossRef]
- Aljibori, H.; Al-Amiery, A.; Isahak, W. Advancements in Corrosion Prevention Techniques. J. Bio- Tribo-Corros. 2024, 10, 78. [Google Scholar] [CrossRef]
- Mahmoodian, D.M. Reliability and Maintainability of In-Service Pipelines; Gulf Professional Publishing: Huston, TX, USA, 2018. [Google Scholar]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control; Elsevier: Burlington, MA, USA, 2006. [Google Scholar] [CrossRef]
- Harsimran, S.; Santosh, K.; Rakesh, K. Overview of Corrosion and its Control: A Critical Review. Proc. Eng. Sci. 2021, 3, 13–24. [Google Scholar] [CrossRef]
- Nogara, J.; Zarrouk, S.J. Corrosion in geothermal environment: Part 1: Fluids and their impact. Renew. Sustain. Energy Rev. 2018, 82, 1333–1346. [Google Scholar] [CrossRef]
- Fontana, M.G.; Greene, N.D.; Klerer, J. Corrosion Engineering. J. Electrochem. Soc. 1968, 115, 142C. [Google Scholar] [CrossRef]
- Zade, G.S.; Patil, K.D. Advances in Corrosion-Resistant Coatings. In Functional Coatings; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 110–152. [Google Scholar]
- Liu, Q.; Qian, J.; Barker, R.; Wang, C.; Neville, A.; Pessu, F. Application of Double Loop Electrochemical Potentio-kinetic Reactivation for characterizing the intergranular corrosion susceptibility of stainless steels and Ni-based alloys in solar nitrate salts used in CSP systems. Eng. Fail. Anal. 2021, 129, 105717. [Google Scholar] [CrossRef]
- Yang, Z.; Li, L.; Qiao, Y.; Li, C.; Zhang, L.; Cui, J.; Ren, D.; Ji, H.; Zheng, Y. Cavitation erosion-corrosion properties of as-cast TC4 and LPBF TC4 in 0.6 mol/L NaCl solution: A comparison investigation. Ultrason. Sonochem. 2024, 108, 106947. [Google Scholar] [CrossRef]
- Li, L.; Nie, S.; Li, C.; Chen, X.; Qiao, Y.; Ma, R.; Chen, Z.; Zhang, L.; Cui, J. Study on cavitation erosion-corrosion behavior of CoCrFeNiMoCu0.1 high entropy alloy in 3.5 wt% NaCl solution. Ultrason. Sonochem. 2024, 110, 107021. [Google Scholar] [CrossRef]
- Reclaru, L.; Brooks, R.A.; Zuberbühler, M.; Eschler, P.Y.; Constantin, F.; Tomoaia, G. Evaluation of taper joints with combined fatigue and crevice corrosion testing: Comparison to human explanted modular prostheses. Mater. Sci. Eng. C 2014, 34, 69–77. [Google Scholar] [CrossRef]
- Schijve, J. Fretting Corrosion. In Fatigue of Structures and Materials; Springer: Dodrecht, The Netherlands, 2001. [Google Scholar]
- Royhman, D.; Pourzal, R.; Hall, D.; Lundberg, H.J.; Wimmer, M.A.; Jacobs, J.; Hallab, N.J.; Mathew, M.T. Fretting-corrosion in hip taper modular junctions: The influence of topography and pH levels—An in-vitro study. J. Mech. Behav. Biomed. Mater. 2021, 118, 104443. [Google Scholar] [CrossRef]
- Pal, M.; Lavanya, M. Microbial Influenced Corrosion: Understanding Bioadhesion and Biofilm Formation. J. Bio- Tribo-Corros. 2022, 8, 76. [Google Scholar] [CrossRef]
- Gonçalves, M.C.; Margarido, F. Materials for Construction and Civil Engineering: Science, Processing, and Design. In Corrosion; Springer International Publishing: Cham, Switzerland, 2015; pp. 679–716. [Google Scholar]
- Hansen, D. Metal Corrosion in the Human Body: The Ultimate Bio-Corrosion Scenario. Electrochem. Soc. Interface 2008, 17, 31–34. [Google Scholar] [CrossRef]
- Liu, D.; Jia, R.; Xu, D.; Yang, H.; Zhao, Y.; Khan, M.s.; Huang, S.; Wen, J.; Yang, K.; Gu, T. Biofilm inhibition and corrosion resistance of 2205-Cu duplex stainless steel against acid producing bacterium Acetobacter aceti. J. Mater. Sci. Technol. 2019, 35, 2494–2502. [Google Scholar] [CrossRef]
- Lou, Y.; Chang, W.; Cui, T.; Qian, H.; Huang, L.; Ma, L.; Hao, X.; Zhang, D. Microbiologically influenced corrosion inhibition of carbon steel via biomineralization induced by Shewanella putrefaciens. npj Mater. Degrad. 2021, 5, 59. [Google Scholar] [CrossRef]
- Lekbach, Y.; Liu, T.; Li, Y.; Moradi, M.; Dou, W.; Xu, D.; Smith, J.A.; Lovley, D.R. Microbial corrosion of metals: The corrosion microbiome. Adv. Microb. Physiol. 2021, 78, 317–390. [Google Scholar] [CrossRef]
- Lekbach, Y.; Li, Z.; Xu, D.; El Abed, S.; Dong, Y.; Liu, D.; Gu, T.; Koraichi, S.I.; Yang, K.; Wang, F. Salvia officinalis extract mitigates the microbiologically influenced corrosion of 304L stainless steel by Pseudomonas aeruginosa biofilm. Bioelectrochemistry 2019, 128, 193–203. [Google Scholar] [CrossRef]
- Zhou, E.; Li, H.; Yang, C.; Wang, J.; Xu, D.; Zhang, D.; Gu, T. Accelerated corrosion of 2304 duplex stainless steel by marine Pseudomonas aeruginosa biofilm. Int. Biodeterior. Biodegrad. 2018, 127, 1–9. [Google Scholar] [CrossRef]
- Jia, R.; Yang, D.; Abd Rahman, H.B.; Gu, T. Laboratory testing of enhanced biocide mitigation of an oilfield biofilm and its microbiologically influenced corrosion of carbon steel in the presence of oilfield chemicals. Int. Biodeterior. Biodegrad. 2017, 125, 116–124. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A. Hybrid Nanomaterials as Next-Generation Corrosion Inhibitors for Metals and Alloys. In Innovations and Applications of Hybrid Nanomaterials; Khanna, V., Sharma, P., Mahajan, P., Eds.; IGI Global: Hershey, PA, USA, 2024; pp. 91–117. [Google Scholar]
- Nazeer, A.A.; Madkour, M. Potential use of smart coatings for corrosion protection of metals and alloys: A review. J. Mol. Liq. 2018, 253, 11–22. [Google Scholar] [CrossRef]
- Kumar, P.; Soni, I.; Jayaprakash, G.; Kumar, S.; Rao, S.; Flores-Moreno, R.; Swamirayachar, S. Experimental and theoretical studies of hexylmeythylimidazolium tetrafluoroborate ionic liquid as cathodic corrosion inhibitor for mild steel. Inorg. Chem. Commun. 2022, 146, 110110. [Google Scholar] [CrossRef]
- Zehra, S.; Mobin, M.; Aslam, R. Chapter 2—Corrosion prevention and protection methods. In Eco-Friendly Corrosion Inhibitors; Guo, L., Verma, C., Zhang, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 13–26. [Google Scholar]
- Auepattana-Aumrung, K.; Crespy, D. Self-healing and anticorrosion coatings based on responsive polymers with metal coordination bonds. Chem. Eng. J. 2023, 452, 139055. [Google Scholar] [CrossRef]
- Shaw, B.A.; Kelly, R. What is Corrosion? Electrochem. Soc. Interface 2006, 15, 24–26. [Google Scholar] [CrossRef]
- Olajire, A. Recent advances on organic coating system technologies for corrosion protection of offshore metallic structures. J. Mol. Liq. 2018, 269, 572–606. [Google Scholar] [CrossRef]
- Hayatdavoudi, H.; Rahsepar, M. A mechanistic study of the enhanced cathodic protection performance of graphene-reinforced zinc rich nanocomposite coating for corrosion protection of carbon steel substrate. J. Alloys Compd. 2017, 727, 1148–1156. [Google Scholar] [CrossRef]
- Presuel-Moreno, F.; Jakab, M.A.; Tailleart, N.; Goldman, M.; Scully, J.R. Corrosion-resistant metallic coatings. Mater. Today 2008, 11, 14–23. [Google Scholar] [CrossRef]
- Mondal, K.; Nunez, L.; Downey, C.; Van Rooyen, I. Recent Advances in the Thermal Barrier Coatings for Extreme Environments. Mater. Sci. Energy Technol. 2021, 4, 208–210. [Google Scholar] [CrossRef]
- Hussain, A.; Nuthalapati, S.; Pichumani, M.; Chakra, C. Research progress in organic zinc rich primer coatings for cathodic protection of metals—A comprehensive review. Prog. Org. Coat. 2021, 153, 106040. [Google Scholar] [CrossRef]
- Okokpujie, I.; Tartibu, L.; Musa-Basheer, H.; Adeoye, A. Effect of Coatings on Mechanical, Corrosion and Tribological Properties of Industrial Materials: A Comprehensive Review. J. Bio- Tribo-Corros. 2023, 10, 2. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; You, Z.; Liu, S.; Guan, K.; Wu, R.; Wang, J.; Feng, J. Towards developing Mg alloys with simultaneously improved strength and corrosion resistance via RE alloying. J. Magnes. Alloys 2021, 9, 41–56. [Google Scholar] [CrossRef]
- Pedeferri, P. Cathodic and Anodic Protection. In Corrosion Science and Engineering; Springer: Cham, Switzerland, 2018; pp. 383–422. [Google Scholar]
- Kadhum, A.; Betti, N.; Al-Adili, A.; Shaker, L.; Al-Amiery, A. Limits and developments in organic inhibitors for corrosion of mild steel: A critical review (Part two: 4-aminoantipyrine). Int. J. Corros. Scale Inhib. 2022, 11, 43–63. [Google Scholar] [CrossRef]
- Clematis, D.; Marroccu, A.; Panizza, M.; Barbucci, A. A Critical Analysis on the Current Design Criteria for Cathodic Protection of Ships and Superyachts. Materials 2022, 15, 2645. [Google Scholar] [CrossRef]
- Cheng, L.; Luo, Y.; Ma, S.; Guo, W.; Xiaohui, W. Corrosion resistance of inorganic zinc-rich coating reinforced by Ni-coated coal fly ash. J. Alloys Compd. 2019, 786, 791–797. [Google Scholar] [CrossRef]
- Silva, R.; Alemán, C.; Ferreira, C.A.; Armelin, E.; Ferreira, J.; Meneguzzi, Á. Smart Paint for anodic protection of steel. Prog. Org. Coat. 2014, 78, 116–123. [Google Scholar] [CrossRef]
- Edeleanu, C. Corrosion Control by Anodic Protection. Platin. Met. Rev. 1960, 4, 86–91. [Google Scholar] [CrossRef]
- Cowley, W.E.; Robinson, F.P.A.; Kerrich, J.E. Anodic Protection for the Control of Corrosion Fatigue. Br. Corros. J. 1968, 3, 223–237. [Google Scholar] [CrossRef]
- Paulekat, F.; Gräfen, H.; Kuron, D. Anodic corrosion protection of sulphuric acid plants with regard to the recovery of heat. Mater. Corros. 1982, 33, 254–262. [Google Scholar] [CrossRef]
- Ohtsuka, T. Corrosion Protection of Steels by Conducting Polymer Coating. Int. J. Corros. 2012, 2012, 915090. [Google Scholar] [CrossRef]
- Vaghefinazari, B.; Wierzbicka, E.; Visser, P.; Posner, R.; Arrabal, R.; Matykina, E.; Mohedano, M.; Blawert, C.; Zheludkevich, M.L.; Lamaka, S.V. Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: Part III—Corrosion Inhibitors and Combining Them with Other Protection Strategies. Materials 2022, 15, 8489. [Google Scholar] [CrossRef]
- Liu, C.; Tong, S.; Yue, Y.; Wang, H.; Song, J.; Li, Y.; Wang, Q.; Wang, Z. Laser-based fabrication of superwetting titanium alloy with enhanced corrosion and erosion-corrosion resistance. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133648. [Google Scholar] [CrossRef]
- Emelyanenko, K.A.; Emelyanenko, A.M.; Boinovich, L.B. Laser Obtained Superhydrophobic State for Stainless Steel Corrosion Protection, A Review. Coatings 2023, 13, 194. [Google Scholar] [CrossRef]
- Cholkar, A.; Chatterjee, S.; Richards, C.; McCarthy, É.; Perumal, G.; Regan, F.; Kinahan, D.; Brabazon, D. Biofouling and Corrosion Protection of Aluminum Alloys Through Ultrafast Laser Surface Texturing for Marine Applications. Adv. Mater. Interfaces 2024, 11, 2300835. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; Viejo, F.; Aburas, Z.; Rakhes, M. Laser-induced microstructural modification for corrosion protection. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2010, 224, 1073–1085. [Google Scholar] [CrossRef]
- Krishna, L.R.; Madhavi, Y.; Babu, P.S.; Rao, D.S.; Padmanabham, G. Strategies for corrosion protection of non-ferrous metals and alloys through surface engineering. Mater. Today Proc. 2019, 15, 145–154. [Google Scholar] [CrossRef]
- Macera, L.; Pullini, D.; Boschetto, A.; Bottini, L.; Mingazzini, C.; Falleti, G.L. Sol–Gel Silica Coatings for Corrosion Protection of Aluminum Parts Manufactured by Selective Laser Melting (SLM) Technology. Coatings 2023, 13, 1081. [Google Scholar] [CrossRef]
- Al-Ghezi, M.; Gaaz, T. A mini review on corrosion, inhibitors and mechanism types of mild steel inhibition in an acidic environment. Int. J. Corros. Scale Inhib. 2021, 10, 861–884. [Google Scholar] [CrossRef]
- Hill, R.T.; Ramirez, F.A.; Perez, A.L.; Monty, B.A. Material Selection and Corrosion Control for Topside Process and Utility Piping and Equipment. In Proceedings of the NACE CORROSION 2012, Salt Lake City, UT, USA, 11–15 March 2012. [Google Scholar]
- İpek, M.; Selvi, İ.H.; Findik, F.; Torkul, O.; Cedimoğlu, I.H. An expert system based material selection approach to manufacturing. Mater. Des. 2013, 47, 331–340. [Google Scholar] [CrossRef]
- Vachtsevanos, G.; Natarajan, K.; Rajamani, R.; Sandborn, P. Corrosion Processes: Sensing, Monitoring, Data Analytics, Prevention/Protection, Diagnosis/Prognosis and Maintenance Strategies: Sensing; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Arturson, G. The tragedy of San Juanico—The most severe LPG disaster in history. Burns 1987, 13, 87–102. [Google Scholar] [CrossRef]
- Palanisamy, G. Corrosion Inhibitors; IntechOpen: London, UK, 2019. [Google Scholar]
- Luo, Z.-G.; Zhang, Y.; Wang, H.; Wan, S.; Song, L.-F.; Liao, B.-K.; Guo, X.-P. Modified Nano-Lignin as a Novel Biomass-Derived Corrosion Inhibitor for Enhanced Corrosion Resistance of Carbon Steel. Corros. Sci. 2023, 227, 111705. [Google Scholar] [CrossRef]
- Sowmyashree, A.S.; Somya, A.; Kumar, C.P.; Rao, S. Novel nano corrosion inhibitor, integrated zinc titanate nano particles: Synthesis, characterization, thermodynamic and electrochemical studies. Surf. Interfaces 2021, 22, 100812. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, C. Corrosion inhibition performance of an environmentally friendly water-based nano-inhibitor. Alex. Eng. J. 2023, 70, 495–501. [Google Scholar] [CrossRef]
- Aljibori, H.; Abdulzahra, O.; Adily, A.; Al-Azzawi, W.; Al-Amiery, A.; Kadhum, A. Corrosion inhibition effects of concentration of 2-oxo-3- hydrazonoindoline in acidic solution, exposure period, and temperature. Int. J. Corros. Scale Inhib. 2023, 12, 438–457. [Google Scholar] [CrossRef]
- Verma, C.; Al-Moubaraki, A.; Alfantazi, A.; Rhee, K.Y. Heterocyclic amino acids-based green and sustainable corrosion inhibitors: Adsorption, bonding and corrosion control. J. Clean. Prod. 2024, 446, 141186. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Isahak, W.N.R.W.; Al-Azzawi, W.K. Corrosion Inhibitors: Natural and Synthetic Organic Inhibitors. Lubricants 2023, 11, 174. [Google Scholar] [CrossRef]
- Wonnie Ma, I.A.; Ammar, S.; Kumar, S.S.A.; Ramesh, K.; Ramesh, S. A concise review on corrosion inhibitors: Types, mechanisms and electrochemical evaluation studies. J. Coat. Technol. Res. 2021, 19, 241–268. [Google Scholar] [CrossRef]
- Bastidas, D.M.; Martin, U.; Bastidas, J.M.; Ress, J. Corrosion Inhibition Mechanism of Steel Reinforcements in Mortar Using Soluble Phosphates: A Critical Review. Materials 2021, 14, 6168. [Google Scholar] [CrossRef]
- Mohamed, K.E.M.; Ibrahim, O.H.; El-Bedawy, M.E.; Ali, A.H. Synergistic effect of different Zn salts with sodium octanoate on the corrosion inhibition of carbon steel in cooling water. J. Radiat. Res. Appl. Sci. 2020, 13, 276–287. [Google Scholar] [CrossRef]
- Nishimoto, M.; Muto, I.; Sugawara, Y.; Hara, N. Role of Cerium Ions for Improving Pitting Corrosion Resistance of Sulfide Inclusions in Stainless Steels. In Electrochemical Society Meeting Abstracts; IOP Publishing: Bristol, UK, 2017; Volume MA2017-02, p. 698. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Gharabagh, R.S.; Teimouri, M.; Ahmadzadeh, M.; Hasannejad, H.; Seyfoori, A. Effect of cerium ion addition on corrosion and wear characteristics of plasma electrolytic oxidation coating of CP-Ti. Prot. Met. Phys. Chem. Surf. 2016, 52, 1093–1099. [Google Scholar] [CrossRef]
- Özcan, M.; Dehri, İ.; Erbil, M. Organic sulphur-containing compounds as corrosion inhibitors for mild steel in acidic media: Correlation between inhibition efficiency and chemical structure. Appl. Surf. Sci. 2004, 236, 155–164. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Al-mashhadani, M.H.; Hussain, Z.; Mohammed, S.A.; Yusop, R.M.; Yousif, E. Inhibition of Corrosion: Mechanisms and Classifications an Overview. Al-Qadisiyah J. Pure Sci. 2020, 25, 1–9. [Google Scholar] [CrossRef]
- HosseinpourRokni, M.; Naderi, R.; Soleimani, M.; Kowsari, E.; Pourfath, M. Indirect interactions between the ionic liquid and Cu surface in 0.5 M HCl: A novel mechanism explaining cathodic corrosion inhibition. Corros. Sci. 2023, 216, 111100. [Google Scholar] [CrossRef]
- Nam, N.; Hien, P.; Hoai, N.; Thu, V. A study on the mixed corrosion inhibitor with a dominant cathodic inhibitor for mild steel in aqueous chloride solution. J. Taiwan Inst. Chem. Eng. 2018, 91, 556–569. [Google Scholar] [CrossRef]
- Borbolla, J.; Thangarasu, P.; García-Ochoa, E. A new inhibitor for mild carbon steel: Electrochemical and DFT studies. J. Electroanal. Chem. 2005, 583, 8–16. [Google Scholar] [CrossRef]
- Coelho, L.B.; Lukaczynska-Anderson, M.; Clerick, S.; Buytaert, G.; Lievens, S.; Terryn, H.A. Corrosion inhibition of AA6060 by silicate and phosphate in automotive organic additive technology coolants. Corros. Sci. 2022, 199, 110188. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Chen, X.; Liu, C.; Zhao, J. Synergistic inhibition properties and microstructures of self-assembled imidazoline and phosphate ester mixture for carbon steel corrosion in the CO2 brine solution. J. Mol. Liq. 2022, 357, 119140. [Google Scholar] [CrossRef]
- Palomar, M.E.; Olivares-Xometl, C.O.; Likhanova, N.V.; Pérez-Navarrete, J.-B. Imidazolium, Pyridinium and Dimethyl-Ethylbenzyl Ammonium Derived Compounds as Mixed Corrosion Inhibitors in Acidic Medium. J. Surfactants Deterg. 2011, 14, 211–220. [Google Scholar] [CrossRef]
- Richards, C.A.J.; McMurray, H.N.; Williams, G. Smart-release inhibition of corrosion driven organic coating failure on zinc by cationic benzotriazole based pigments. Corros. Sci. 2019, 154, 101–110. [Google Scholar] [CrossRef]
- Guo, L.; Qi, C.; Zheng, X.; Zhang, R.; Shen, X.; Kaya, S. Toward understanding the adsorption mechanism of large size organic corrosion inhibitors on an Fe(110) surface using the DFTB method. RSC Adv. 2017, 7, 29042–29050. [Google Scholar] [CrossRef]
- Talat, R.; Asghar, M.A.; Tariq, I.; Akhter, Z.; Liaqat, F.; Nadeem, L.; Haider, A.; Ali, S. Evaluating the Corrosion Inhibition Efficiency of Pyridinium-Based Cationic Surfactants for EN3B Mild Steel in Acidic-Chloride Media. Coatings 2022, 12, 1701. [Google Scholar] [CrossRef]
- Fawzy, A.; Abdallah, M.; Zaafarany, I.; Ahmed, S.; Althagafi, I. Thermodynamic, kinetic and mechanistic approach to the corrosion inhibition of carbon steel by new synthesized amino acids-based surfactants as green inhibitors in neutral and alkaline aqueous media. J. Mol. Liq. 2018, 265, 276–291. [Google Scholar] [CrossRef]
- Pour-Ali, S.; Hejazi, S. Tiazofurin drug as a new and non-toxic corrosion inhibitor for mild steel in HCl solution: Experimental and quantum chemical investigations. J. Mol. Liq. 2022, 354, 118886. [Google Scholar] [CrossRef]
- Al-Gorair, A.; Aboubaker Saleh, M.; Alotaibi, M.; Al-Juaid, S.; Abdallah, M.; Wanees, S. Potentiometric and polarization studies on the oxide film repair and retardation of pitting corrosion on indium in an alkaline aqueous solution utilizing some triazole compounds. Inorg. Chem. Commun. 2023, 158, 111497. [Google Scholar] [CrossRef]
- Mehta, R.K.; Yadav, M. Corrosion inhibition properties of expired Broclear medicine and its carbon dot as eco-friendly inhibitors for mild steel in 15% HCl. Mater. Sci. Eng. B 2023, 295, 116566. [Google Scholar] [CrossRef]
- Kokalj, A. Corrosion inhibitors: Physisorbed or chemisorbed? Corros. Sci. 2022, 196, 109939. [Google Scholar] [CrossRef]
- Hrimla, M.; Bahsis, L.; Laamari, M.R.; Julve, M.; Stiriba, S.-E. An Overview on the Performance of 1,2,3-Triazole Derivatives as Corrosion Inhibitors for Metal Surfaces. Int. J. Mol. Sci. 2022, 23, 16. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A.; Ebenso, E.E. Quinoline and its derivatives as corrosion inhibitors: A review. Surf. Interfaces 2020, 21, 100634. [Google Scholar] [CrossRef]
- Dahmani, K.; Galai, M.; Rbaa, M.; Ech-Chebab, A.; Errahmany, N.; Guo, L.; AlObaid, A.A.; Hmada, A.; Warad, I.; Lakhrissi, B.; et al. Evaluating the efficacy of synthesized quinoline derivatives as Corrosion inhibitors for mild steel in acidic environments: An analysis using electrochemical, computational, and surface techniques. J. Mol. Struct. 2024, 1295, 136514. [Google Scholar] [CrossRef]
- Abdel-karim, A.M.; Hussien, H.M.; Shahen, S.; El-Shamy, O.A.A.; Ghayad, I.M.; Saleh, N.M.; El-Sattar, N.E.A.A. Green synthesis of novel bis structure of (Carbamothioyl) oxalamide derivatives as corrosion inhibitors for copper in 3.5% NaCl; experimental and theoretical investigation. J. Mol. Struct. 2024, 1295, 136597. [Google Scholar] [CrossRef]
- Aiad, I.A.; Hafiz, A.A.; El-Awady, M.Y.; Habib, A.O. Some Imidazoline Derivatives as Corrosion Inhibitors. J. Surfactants Deterg. 2010, 13, 247–254. [Google Scholar] [CrossRef]
- Ettahiri, W.; El Moutaouakil Ala Allah, A.; Lazrak, J.; Safir, E.H.; Yadav, K.K.; Hammouti, B.; Obaidullah, A.J.; Rais, Z.; Ramli, Y.; Taleb, M. Synthesis, characterization, theoretical, and experimental evaluation of novel imidazolone—Based compounds as eco-friendly corrosion inhibitors for mild steel. J. Ind. Eng. Chem. 2024, 140, 631–646. [Google Scholar] [CrossRef]
- Touir, R.; Errahmany, N.; Rbaa, M.; Benhiba, F.; Doubi, M.; Kafssaoui, E.H.; Lakhrissi, B. Experimental and computational chemistry investigation of the molecular structures of new synthetic quinazolinone derivatives as acid corrosion inhibitors for mild steel. J. Mol. Struct. 2024, 1303, 137499. [Google Scholar] [CrossRef]
- Yousif, Q.A.; Bedair, M.A.; Fadel, Z.; Al-Odail, F.; Abuelela, A.M. Evaluating the efficacy of newly synthesized amino acid derivatives as corrosion inhibitors in acidic solutions. Inorg. Chem. Commun. 2024, 164, 112454. [Google Scholar] [CrossRef]
- Xiong, Y.; Cao, M. Application of surfactants in corrosion inhibition of metals. Curr. Opin. Colloid Interface Sci. 2024, 73, 101830. [Google Scholar] [CrossRef]
- Wang, T.; Dai, S.; Xiong, Y.; Yan, H.; Zhu, Y. The morpholine surfactants with corrosion inhibition and antibacterial activity: Experiments and theoretical calculations. Colloids Surf. A Physicochem. Eng. Asp. 2024, 700, 134784. [Google Scholar] [CrossRef]
- El-Monem, M.; Farag, A.; Khalil, M.; Migahed, M. Hydroxyethyl cationic surfactants as corrosion inhibitors for S90 steel in produced water: Electrochemical, and computational studies. J. Mol. Struct. 2024, 1314, 138702. [Google Scholar] [CrossRef]
- Yumnam, G.; Adhikari, S.; Pulikkal, A.K.; Rajaraman, P. Impacts of pyridinium gemini surfactants on corrosion inhibition of carbon steel. Surf. Interfaces 2023, 45, 103796. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, S.; Ma, T.; Shi, Y.; Wang, X.; Du, H.; Liu, R.; Han, X. Three anionic surfactants for corrosion inhibition in cobalt CMP: Research on validity and mechanism. Surf. Interfaces 2024, 47, 104202. [Google Scholar] [CrossRef]
- Hameed, R.S.; Aleid, G.; Ragab, H.; Alshafey, H.; Aljuhani, E.; Almhyawi, S. Green synthesis of polymeric surfactants from recycling of plastic waste for applications on steel protection in the petroleum industry. Green Chem. Lett. Rev. 2024, 17, 2379442. [Google Scholar] [CrossRef]
- Ikeuba, A.I.; Essiet, N.; Echem, O.C.; Ezenobi, N.; Okon, E.; Okafor, P.C. A review of the application of ionic liquids as eco-friendly corrosion inhibitors for steel, aluminum, copper and magnesium alloys. J. Ion. Liq. 2024, 4, 100098. [Google Scholar] [CrossRef]
- El-Nagar, R.A.; Khalil, N.A.; Atef, Y.; Nessim, M.I.; Ghanem, A. Evaluation of ionic liquids based imidazolium salts as an environmentally friendly corrosion inhibitors for carbon steel in HCl solutions. Sci. Rep. 2024, 14, 1889. [Google Scholar] [CrossRef]
- Jia, H.; Jia, H.; Wang, Q.; Xu, Y.; Wang, B.; Wang, Q.; Li, X.; Wang, Z.; Lv, K.; Huang, P. Imidazolium-Based Polymeric Ionic Liquids with Short Alkyl Chains as Green Corrosion Inhibitors for Mild Steel in 1 M HCl: Experimental and Theoretical Investigations. Langmuir 2024, 40, 14141–14152. [Google Scholar] [CrossRef]
- Zunita, M.; Kevin, Y.J. Ionic liquids as corrosion inhibitor: From research and development to commercialization. Results Eng. 2022, 15, 100562. [Google Scholar] [CrossRef]
- Cui, L.; Lv, Y.; Dong, Y.; Liao, H.; Wu, S.; Li, X. Benzothiazole-based ionic liquids as environment-friendly and high-efficiency corrosion inhibitors for mild steel in HCl: Experimental and theoretical studies. J. Mol. Liq. 2024, 394, 123769. [Google Scholar] [CrossRef]
- Wanees, S.; Kamel, M.; Ibrahim, M.; Rashwan, S.; Atef, Y.; Gamal Abd Elsadek Ghunime, M. Corrosion inhibition and synergistic effect of ionic liquids and iodide ions on the corrosion of C-steel in formation water associated with crude oil. J. Umm Al-Qura Univ. Appl. Sci. 2023, 10, 107–119. [Google Scholar] [CrossRef]
- Vaszilcsin, N.; Kellenberger, A.; Dan, M.L.; Duca, D.A.; Ordodi, V.L. Efficiency of Expired Drugs Used as Corrosion Inhibitors: A Review. Materials 2023, 16, 5555. [Google Scholar] [CrossRef]
- Lessa, R.C.D.S. Synthetic Organic Molecules as Metallic Corrosion Inhibitors: General Aspects and Trends. Organics 2023, 4, 232–250. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Verma, C.; Quraishi, M.A. Molecular structural aspects of organic corrosion inhibitors: Experimental and computational insights. J. Mol. Struct. 2021, 1227, 129374. [Google Scholar] [CrossRef]
- Oubahou, M.; el Aloua, A.; Benzbiria, N.; Harrari, S.; Takky, D.; Youssef, N.; Zeroual, A.; Wang, S.-F.; Syed, A.; Belghiti, M.E. Electrochemical, Thermodynamic and Computational Investigation of the Use of an Expired Drug as a Sustainable Corrosion Inhibitor for Copper in 0.5 M H2SO4. Mater. Chem. Phys. 2024, 323, 129642. [Google Scholar] [CrossRef]
- Michael, O.; Thompson, A. Experimental Evaluation of Unpreprocessed-Expired Paracetamol Drugs as Corrosion Inhibitor for Mild Steel in Hydrochloric Acid. In Proceedings of the 2023 FUTA SEET Conference, Akure, Nigeria, 7–9 November 2023. [Google Scholar]
- Iorhuna, F. Itraconazole Drug as Corrosion Inhibitor for Aluminium in 0.7 M Hydrochloric Acid Medium. J. Mater. Environ. Sci. 2024, 15, 501–511. [Google Scholar]
- Abd El Maksoud, S.; Fouda, A.E.A.; Badawy, H. Furosemide drug as a corrosion inhibitor for carbon steel in 1.0 M hydrochloric acid. Sci. Rep. 2024, 14, 9052. [Google Scholar] [CrossRef]
- Emmanuel, J. Corrosion protection of mild steel in corrosive media, a shift from synthetic to natural corrosion inhibitors: A review. Bull. Natl. Res. Cent. 2024, 48, 26. [Google Scholar] [CrossRef]
- Salleh, S.Z.; Yusoff, A.H.; Zakaria, S.K.; Taib, M.A.A.; Abu Seman, A.; Masri, M.N.; Mohamad, M.; Mamat, S.; Ahmad Sobri, S.; Ali, A.; et al. Plant extracts as green corrosion inhibitor for ferrous metal alloys: A review. J. Clean. Prod. 2021, 304, 127030. [Google Scholar] [CrossRef]
- El Ibrahimi, B.; Jmiai, A.; Bazzi, L.; El Issami, S. Amino acids and their derivatives as corrosion inhibitors for metals and alloys. Arab. J. Chem. 2020, 13, 740–771. [Google Scholar] [CrossRef]
- Vaidya, N.R.; Aklujkar, P.; Rao, A. Modification of natural gums for application as corrosion inhibitor: A review. J. Coat. Technol. Res. 2021, 19, 223–239. [Google Scholar] [CrossRef]
- Danyliak, M.O.; Zin, I.; Korniy, S. Corrosion inhibition of low-alloy carbon steel by gum Arabic and zinc acetate in neutral chloride-containing environment. J. Ind. Eng. Chem. 2023, 129, 267–277. [Google Scholar] [CrossRef]
- Asaad, M.A.; Huseien, G.F.; Baghban, M.H.; Raja, P.B.; Fediuk, R.; Faridmehr, I.; Alrshoudi, F. Gum Arabic Nanoparticles as Green Corrosion Inhibitor for Reinforced Concrete Exposed to Carbon Dioxide Environment. Materials 2021, 14, 7867. [Google Scholar] [CrossRef]
- Abdallah, M. Guar Gum as Corrosion Inhibitor for Carbon Steel in Sulfuric Acid Solutions. Port. Electrochim. Acta 2004, 22, 161–175. [Google Scholar] [CrossRef]
- Venkatesh, G.; Kamal, C.; Vennila, P.; Kaya, S.; Annaamalai, M.G.L.; Ibrahimi, B.E. Sustainable corrosion inhibitor for steel embedded in concrete by Guar Gum: Electrochemical and theoretical analyses. Appl. Surf. Sci. Adv. 2022, 12, 100328. [Google Scholar] [CrossRef]
- Palumbo, G.; Berent, K.; Proniewicz, E.; Banaś, J. Guar Gum as an Eco-Friendly Corrosion Inhibitor for Pure Aluminium in 1-M HCl Solution. Materials 2019, 12, 2620. [Google Scholar] [CrossRef]
- Biswas, A.; Pal, S.; Udayabhanu, G. Experimental and theoretical studies of xanthan gum and its graft co-polymer as corrosion inhibitor for mild steel in 15% HCl. Appl. Surf. Sci. 2015, 353, 173–183. [Google Scholar] [CrossRef]
- Cao, Y.; Zou, C.; Wang, C.; Chen, W.; Liang, H.; Lin, S. Green corrosion inhibitor of β-cyclodextrin modified xanthan gum for X80 steel in 1 M H2SO4 at different temperature. J. Mol. Liq. 2021, 341, 117391. [Google Scholar] [CrossRef]
- Pokhmurs’kyi, V.; Zin, I.; Tymus’, M.; Korniy, S.; Karpenko, O.; Khlopyk, O.; Korets’ka, N. Inhibition of the Corrosion of Carbon Steel by Xanthan Biopolymer. Mater. Sci. 2020, 55, 522–528. [Google Scholar] [CrossRef]
- Timothy, U.; Umoren, P.; Solomon, M.; Igwe, I.; Umoren, S. An appraisal of the utilization of natural gums as corrosion inhibitors: Prospects, challenges, and future perspectives. Int. J. Biol. Macromol. 2023, 253, 126904. [Google Scholar] [CrossRef]
- Nascimento, R.; Furtado, L.; Guimarães, M.J. Biopolymers as Corrosion Inhibitors: Relative Inhibition Potential of Biopolymers and Grafted Biopolymers. In Grafted Biopolymers as Corrosion Inhibitors: Safety, Sustainability, and Efficiency; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 21–55. [Google Scholar]
- Sundar, S.K.; Solanki, J. Anticorrosion Coating Using Natural Biopolymer. In Functional Coatings: Innovations and Challenges; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 153–167. [Google Scholar]
- Akachar, S.; AitAghzzaf, A.; Zarki, Y.; Idouhli, R.; Azaryouh, L.; El Achaby, M.; Draoui, K. Novel sustainable corrosion inhibitors for enhancing carbon steel corrosion resistance based on clay and lignin biopolymer extracted from raw Alfa fibers. J. Mater. Sci. 2024, 59, 17927–17944. [Google Scholar] [CrossRef]
- Toghan, A.; Alayyafi, A.; Alhussain, H.; Zaki, E.; Khodari, M.; Alqarni, N.; Masoud, E.M.; Eldesoky, A.; Eldesoky, A.; Fawzy, A. Effect of adsorption of two green biopolymers on the corrosion of aluminum in 1.0 M NaCl solution: Physicochemical, spectroscopic, surface and quantum chemical exploration. Int. J. Electrochem. Sci. 2024, 19, 100791. [Google Scholar] [CrossRef]
- Hao, T.; Xu, K.; Zheng, X.; Yao, X.; Li, J.; Yu, Y.; Liu, Z. Hydrogen inhibition of wet Al Li alloy dust collector systems using a composite green biopolymer inhibitor based on chitosan/sodium alginate: Experimental and theoretical studies. Int. J. Biol. Macromol. 2024, 278, 134708. [Google Scholar] [CrossRef]
- Yoko, S.; Yoshishige, M.; Tomoko, T.; Shinichiro, O.; Tomohiko, I.; Hidenori, Y. Comparison of Reducing Sugars as Agent to Retard Corrosion Rates. GEOMATE J. 2024, 26, 133–140. [Google Scholar]
- Rbaa, M.; Dohare, P.; Berisha, A.; Dagdag, O.; Lakhrissi, L.; Galai, M.; Lakhrissi, B.; Touhami, M.E.; Warad, I.; Zarrouk, A. New Epoxy sugar based glucose derivatives as eco friendly corrosion inhibitors for the carbon steel in 1.0 M HCl: Experimental and theoretical investigations. J. Alloys Compd. 2020, 833, 154949. [Google Scholar] [CrossRef]
- Rbaa, M.; Abousalem, A.S.; Rouifi, Z.; Lakhrissi, L.; Galai, M.; Zarrouk, A.; Lakhrissi, B.; Lakhrissi, Y. Selective synthesis of new sugars based on 8-hydroxyquinoline as corrosion inhibitors for mild steel in HCl solution-effect of the saturated hydrocarbon chain: Theoretical and experimental studies. Inorg. Chem. Commun. 2020, 118, 108019. [Google Scholar] [CrossRef]
- Wang, X.; Lei, Y.; Jiang, Z.N.; Zhang, Q.H.; Li, Y.; Liu, H.; Zhang, G. Developing two thiocarbohydrazide modified glucose derivatives as high-efficiency green corrosion inhibitors for carbon steel. Ind. Crops Prod. 2022, 188, 115680. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, N.; Jain, V.; Rai, B. Amino Acids as Copper Corrosion Inhibitors: A Density Functional Theory Approach. Appl. Surf. Sci. 2020, 514, 145905. [Google Scholar] [CrossRef]
- Kasprzhitskii, A.; Lazorenko, G.; Nazdracheva, T.; Yavna, V. Comparative Computational Study of L-Amino Acids as Green Corrosion Inhibitors for Mild Steel. Computation 2021, 9, 1. [Google Scholar] [CrossRef]
- Ben Seddik, N.; Achache, M.; Zarki, Y.; Chraka, A.; Bouchta, D.; Raissouni, I. Computational, theoretical and experimental studies of four amino acids as corrosion inhibitors for brass in 3% NaCl medium. J. Mol. Liq. 2024, 397, 124113. [Google Scholar] [CrossRef]
- Moura, M.J.D.S.; Vasques, R.B.; Magalhães, S.J.D.M.; Almeida Neto, F.W.D.Q.; de Lima Neto, P.; dos Santos, L.P.M.; Florez, M.A.C.; Ribas, G.F.; Medeiros, S.L.S.; Salomão, F.C.C.S.; et al. Assessment of the Amino Acid L-Histidine as a Corrosion Inhibitor for a 1018 Carbon Steel in Aqueous Sodium Chloride Solution. Crystals 2024, 14, 703. [Google Scholar] [CrossRef]
- Toma, D.-I.; Baroi, A.M.; Din, A.; Vizitiu, D.E.; Fierascu, I.; Fierascu, R.C. Grapevine Plant Waste Utilization in Nanotechnology. AgroLife Sci. J. 2024, 13, 203–216. [Google Scholar] [CrossRef]
- Safieh, V.; Gholamhossein, D.; Ali, T. Impact of Kaolin Particle Film on Light Extinction Coefficient and Radiation Use Efficiency of Pistachio (Pistachia vera). AgroLife Sci. J. 2017, 6, 125–131. [Google Scholar]
- Petkova, N.; Denev, P. Evaluation of Fructan Contents in the Taproots of Plants Lactuca serriola L. and Sonchus oleraceus L. Sci. Pap. Ser. D Anim. Sci. 2013, 17, 117–122. [Google Scholar]
- Stoica, F.; Ratu, R.N.; Cara, I.G.; Topa, D.; Jitareanu, G. Exploring the potential of grape pomace powder as a functional ingredient in yogurt. Sci. Pap. Ser. D Anim. Sci. 2024, 67, 558–565. [Google Scholar]
- Dehghani, A.; Ghahremani, P.; Mostafatabar, A.H.; Ramezanzadeh, B. Plant extracts: Probable alternatives for traditional inhibitors for controlling alloys corrosion against acidic media—A review. Biomass Convers. Biorefinery 2022, 14, 7467–7486. [Google Scholar] [CrossRef]
- Sharma, S.; Solanki, A.S.; Thakur, A.; Sharma, A.; Kumar, A.; Sharma, S.K. Phytochemicals as eco-friendly corrosion inhibitors for mild steel in sulfuric acid solutions: A review. Corros. Rev. 2024, 42, 727–742. [Google Scholar] [CrossRef]
- Mzioud, K.; Habsaoui, A.; Rached, S.; Ech-Chihbi, E.; Ouakki, M.; Salghi, R.; Touhami, M. Experimental Investigation and Theoretical Modeling of Copper Corrosion Inhibition by Urginea maritima Essential Oil. Mater. Today Sustain. 2024, 27, 100906. [Google Scholar] [CrossRef]
- Zunita, M.; Rahmi, V.A. Advancement of Plant Extract/Ionic Liquid-Based Green Corrosion Inhibitor. Chem. Afr. 2023, 7, 505–538. [Google Scholar] [CrossRef]
- Rached, S.; Mzioud, K.; Habsaoui, A.; Galai, K.; Dahmani, K.; Ouakki, M.; Fartah, S.E.; Dkhireche , N.; Touhami, M.E. Inhibition of Copper Corrosion in Sulfuric Acid by Mentha pulegium L. Port. Electrochim. Acta 2024, 42, 137–153. [Google Scholar] [CrossRef]
- Rached, S.; Habsaoui, A.; Mzioud, K.; Lachhab, R.; Haida, S.; Errahmany, N.; Galai, M.; Touhami, M. Valorization of the green corrosion inhibitor Marrubium vulgare L.: Electrochemical, thermodynamic, theoretical & surface studies. Chem. Data Collect. 2023, 48, 101099. [Google Scholar] [CrossRef]
- Daoudi, W.; Dagdag, O.; Verma, C.; Berdimurodov, E.; Oussaid, A.; Berisha, A.; Oussaid, A.; Abboud, M.; El Aatiaoui, A. Rosmarinus officinalis L. Oil as an Eco-Friendly corrosion inhibitor for mild steel in acidic Solution: Experimental and computational studies. Inorg. Chem. Commun. 2024, 161, 112030. [Google Scholar] [CrossRef]
- Santana, C.; Nogueira da Cunha, J.; Rodrigues, J.G.; Duarte, J.; Freire, D.; D’Elia, E. Aqueous Extracts of the Castor Beans as a Corrosion Inhibitor of Mild Steel in HCl Media. J. Braz. Chem. Soc. 2020, 31, 1225–1238. [Google Scholar] [CrossRef]
- Ansari, A.; Youssefi, Y.; Tanghourte, M.; Ouassou, N.; Asoufar, N.; Znini, M.; Lgaz, H.; Mabrouk, E.H.; Azrour, M.; Lee, H.-S.; et al. Assessment of Warionia saharea Essential Oil as a Green Corrosion Inhibitor for Mild Steel in HCl: Experimental and Computational Studies. Coatings 2024, 14, 1164. [Google Scholar] [CrossRef]
- Mortadi, K.; El Amri, A.; Ouakki, M.; Hsissou, R.; Jebli, A.; Lebkiri, A.; Safi, Z.; Wazzan, N.; Berisha, A.; Cherkaoui, M.; et al. Electrochemical and theoretical studies on a bioactive Juniperus oxycedrus essential oil as a potential and ecofriendly corrosion inhibitor for mild steel in 1.0 M HCl environment. Inorg. Chem. Commun. 2024, 162, 112196. [Google Scholar] [CrossRef]
- Abdullah, H.; Anaee, R.; Khadom, A.; Ali, A.; Malik, A.; Kadhim, M. Experimental and theoretical assessments of the chamomile flower extract as a green corrosion inhibitor for aluminum in artificial seawater. Results Chem. 2023, 6, 101035. [Google Scholar] [CrossRef]
- Zhou, Z.; Min, X.; Wan, S.; Liu, J.; Liao, B.; Guo, X. A novel green corrosion inhibitor extracted from waste feverfew root for carbon steel in H2SO4 solution. Results Eng. 2023, 17, 100971. [Google Scholar] [CrossRef]
- Chowdhury, M.; Ahmed, M.M.; Hossain, N.; Islam, M.; Islam, S.; Rana, M. Tulsi and green tea extracts as efficient green corrosion inhibitor for the corrosion of aluminum alloy in acidic medium. Results Eng. 2023, 17, 100996. [Google Scholar] [CrossRef]
- Barboza, G.; Oliveira, M.; Neves, M.; Echevarria, A. Justicia brandegeeana as a green corrosion inhibitor for carbon steel in sulfuric acid. Green Chem. Lett. Rev. 2024, 17, 2320254. [Google Scholar] [CrossRef]
- Ezugha, S.; Chiedozie, A.; Eze, V. Inhibition Potentials of Solanum macrocarpon Leave Extract as a Green Corrosion Inhibitor of Mild Steel in an Acidic Solution. J. Bio- Tribo-Corros. 2024, 10, 81. [Google Scholar] [CrossRef]
- Malik, B.; Kaur, M.; Singh, K.; Kaur, J.; Saxena, A.; Sohal, H.S.; Husain, F.M.; Arshad, M.; Adil, M. An environmentally sustainable approach for the utilization of Erigeron bonariensis as a green inhibitor against weathering steel corrosion in acidic media. RSC Adv. 2024, 14, 16432–16444. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Saxena, A. Synergistic mixture of the Dactyloctenium aegyptium extract and KI as an environment friendly and highly efficient corrosion inhibitor for steel in 0.5 M HCl. J. Indian Chem. Soc. 2024, 101, 101317. [Google Scholar] [CrossRef]
- Hussein, S.A.; Khadom, A.A. Okra leaves extract as green corrosion inhibitor for steel in sulfuric acid: Gravimetric, electrochemical, and surface morphological investigations. Results Chem. 2024, 8, 101566. [Google Scholar] [CrossRef]
- Sajadi, G.S.; Salmanian, F.; Naghizade, R.; Hosseini, S.M.A. The inhibitive action of lemon verbena plant extract as an economical and eco-friendly corrosion inhibitor for mild steel in acidic solutions. Int. J. Electrochem. Sci. 2024, 19, 100699. [Google Scholar] [CrossRef]
- Mamudu, U.; Santos, J.H.; Umoren, S.A.; Alnarabiji, M.S.; Lim, R.C. Investigations of corrosion inhibition of ethanolic extract of Dillenia suffruticosa leaves as a green corrosion inhibitor of mild steel in hydrochloric acid medium. Corros. Commun. 2024, 15, 52–62. [Google Scholar] [CrossRef]
- Tan, B.; Liu, Y.; Gong, Z.; Zhang, X.; Chen, J.; Guo, L.; Xiong, J.; Liu, J.; Marzouki, R.; Li, W. Pyracantha fortuneana alcohol extracts as biodegradable corrosion inhibitors for copper in H2SO4 media. J. Mol. Liq. 2024, 397, 124117. [Google Scholar] [CrossRef]
- Fouda, A.; Molouk, A.; Atia, M.; El-Hossiany, A.; Almahdy, M. Verbena officinalis (VO) leaf extract as an anti-corrosion inhibitor for carbon steel in acidic environment. Sci. Rep. 2024, 14, 16112. [Google Scholar] [CrossRef]
- Nour El Houda, S.; Amel, B.; Malika, F. Trifolium repens extracts as a green corrosion inhibitor for carbon steel in a 3.5% NaCl solution. J. Taiwan Inst. Chem. Eng. 2024, 165, 105771. [Google Scholar] [CrossRef]
- Abeng, F.E.; Ita, B.I.; Ikpi, M.E.; Chukwuike, V.I.; Ikeuba, A.I.; Edim, M.M.; Chidiebere, M.A.; Thakur, A.; Anadebe, V.C. Millettia aboensis leaves extract as eco-friendly corrosion inhibitor for mild steel in acidizing solution: From experimental to molecular level prediction. Results Eng. 2024, 24, 102950. [Google Scholar] [CrossRef]
- Zhu, J.-H.; Lin, B.-L.; Duan, T.-H.; Lin, H.-Q.; Zhang, G.-Y.; Zhou, X.-X.; Xu, Y.-Y. Zea mays bracts extract as an eco-friendly corrosion inhibitor for steel in HCl pickling solution: Experimental and simulation studies. Arab. J. Chem. 2024, 17, 105895. [Google Scholar] [CrossRef]
- Raja, B.; Ismail, M.; Ghoreishiamiri, S.; Mirza, J.; Ismail, M.; Kakooei, S.; Rahim, A. Reviews on Corrosion Inhibitors—A Short View. Chem. Eng. Commun. 2016, 203, 1145–1156. [Google Scholar] [CrossRef]
- Shang, Z.; Zhu, J. Overview on plant extracts as green corrosion inhibitors in the oil and gas fields. J. Mater. Res. Technol. 2021, 15, 5078–5094. [Google Scholar] [CrossRef]
- Zehra, S.; Mobin, M.; Aslam, J. Chapter 13—Chromates as corrosion inhibitors. In Inorganic Anticorrosive Materials; Verma, C., Aslam, J., Hussain, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 251–268. [Google Scholar]
- Murmu, M.; Saha, S.K.; Murmu, N.C.; Banerjee, P. Chapter 14—Nitrate as corrosion inhibitor. In Inorganic Anticorrosive Materials; Verma, C., Aslam, J., Hussain, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 269–296. [Google Scholar]
- Refaey, S.; El-Rehim, S.; Taha, F.; Saleh, M.; Ahmed, R. Inhibition of chloride localized corrosion of mild steel by PO43−, CrO42−, MoO42−, and NO2− anions. Appl. Surf. Sci. 2000, 158, 190–196. [Google Scholar] [CrossRef]
- Eltmimi, A.; Al-Amiery, A.; Allami, A.J.; Yusop, R.M.; Kadhum, A.; Allami, T. Inhibitive effects of a novel efficient Schiff base on mild steel in hydrochloric acid environment. Int. J. Corros. Scale Inhib. 2021, 10, 634–648. [Google Scholar] [CrossRef]
- Bernal, S.; Botana, F.J.; Calvino, J.J.; Marcos, M.; Pérez-Omil, J.A.; Vidal, H. Lanthanide salts as alternative corrosion inhibitors. J. Alloys Compd. 1995, 225, 638–641. [Google Scholar] [CrossRef]
- Arenas, M.A.; Bethencourt, M.; Botana, F.J.; de Damborenea, J.; Marcos, M. Inhibition of 5083 aluminium alloy and galvanised steel by lanthanide salts. Corros. Sci. 2001, 43, 157–170. [Google Scholar] [CrossRef]
- Aljibori, H.; Al-Amiery, A.; Kadhum, A. Advances in corrosion protection coatings: A comprehensive review. Int. J. Corros. Scale Inhib. 2023, 12, 1476–1520. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, X.; Chen, Y.; Ji, J.; Zhang, F.; Guo, J.; Zhao, T.; Zhu, J.; Luo, H. Multifunctional organic-inorganic hybrid coating for enhanced bronze corrosion protection. J. Cult. Herit. 2024, 69, 113–125. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Zheng, H.; Deng, S.; Hou, Y.; Wu, Y.; Liu, M. A waterborne coating system for constructing inorganic-organic composite anti-corrosion and wear-resistant coating. Colloids Surf. A Physicochem. Eng. Asp. 2024, 694, 134120. [Google Scholar] [CrossRef]
- Kabeb, S.; Hassan, A.; Ahmad, F. Synergistic enhancement: Ammonium Polyphosphate’s impact on hybrid coating performance against corrosion and fire. Polym. Adv. Technol. 2024, 35, e6398. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, M.; Chen, H. Superhydrophobic anticorrosion coating with active protection effect: Graphene oxide-loaded inorganic/organic corrosion inhibitor for magnesium alloys. Surf. Coat. Technol. 2024, 480, 130586. [Google Scholar] [CrossRef]
- Sun, J.; Sun, W.; Wang, L.; Xu, K.; Yang, Z.; Ma, Y.; Zhao, L.; Ma, S.; Xing, W.; Liu, G. Boosting the acid penetration barrier of epoxy-silica organic-inorganic hybrid coating via adjusting its molecular structures: Experimental and molecular dynamics simulation study. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133634. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Govindaraj, S.; Gopala Krishna, N.; Thirumalaisamy, N.; Anne, R.; Sublime, N.; Philip, J. Enhancing microbiologically influenced corrosion protection of carbon steels with silanized epoxy-biocide hybrid coatings. Environ. Sci. Pollut. Res. 2024, 31, 13302–13326. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, X.; Zhou, J.; Wang, J.; Yang, C.; Hou, Z.; Zhu, Y.; Huang, L. Surface corrosion inhibition mechanism of sarcosine as a green novel inhibitor on a novel barrier layer material of cobalt in copper film CMP for GLSI. Mater. Sci. Semicond. Process. 2022, 140, 106402. [Google Scholar] [CrossRef]
- Belarbi, Z.; Singer, M.; Farelas, F.; Vu, N.; Young, D.; Nesic, S. Thiols as Volatile Corrosion Inhibitors for Top of the Line Corrosion. In Proceedings of the NACE CORROSION 2017, New Orleans, LA, USA, 26–30 March 2017. [Google Scholar]
- Ansari, F.A.; Verma, C.; Siddiqui, Y.S.; Ebenso, E.; Abdellaziz, B. Volatile corrosion inhibitors for ferrous and non-ferrous metals and alloys: A review. Int. J. Corros. Scale Inhib. 2018, 7, 126–150. [Google Scholar] [CrossRef]
- Furman, A.; Kharshan, M.; Chandler, C. Performance and Testing of Vapor Phase Corrosion Inhibitors. In Proceedings of the NACE CORROSION 2004, New Orleans, LA, USA, 28 March–1 April 2004. [Google Scholar]
- Shen, Y.; Zhang, D.; Zhang, Z.; Li, C.; Wu, W. High corrosion-resistant octanoic acid vapor-phase modified Ce-LDH surface film of aluminum alloy. Colloids Surf. A Physicochem. Eng. Asp. 2024, 686, 133370. [Google Scholar] [CrossRef]
- Zhao, J.; van Ommen, J.R.; Garcia, S.J. Gas-phase deposited nanolayers guard organic microparticles in polymer matrices for active corrosion protection at damages. Prog. Org. Coat. 2024, 192, 108522. [Google Scholar] [CrossRef]
- Wang, X.; Ren, J.; Li, Z.; Li, Y. Research progress of vapor phase corrosion inhibitors in marine environment. Environ. Sci. Pollut. Res. 2022, 29, 88432–88439. [Google Scholar] [CrossRef]
- Vorobyova, V.; Skiba, M.I.; Chygyrynets, O.J.P. A novel eco-friendly vapor phase corrosion inhibitor of mild steel. Pigment. Resin Technol. 2019, 48, 137–147. [Google Scholar] [CrossRef]
- Mahvidi, S.; Gharagozlou, M.; Mahdavian, M.; Naghibi, S. Potency of ZnFe2O4 Nanoparticles as Corrosion Inhibitor for Stainless Steel; the Pigment Extract Study. Mater. Res. 2017, 20, 1492–1502. [Google Scholar] [CrossRef]
- Shi, H.; Bi, H.; Yao, B.; Zhang, L. Dissolution of Au nanoparticles in hydrochloric acid solution as studied by optical absorption. Appl. Surf. Sci. 2000, 161, 276–278. [Google Scholar] [CrossRef]
- Roy, P.; Karfa, P.; Adhikari, U.; Sukul, D.D. Corrosion inhibition of mild steel in acidic medium by polyacrylamide grafted Guar gum with various grafting percentage: Effect of intramolecular synergism. Corros. Sci. 2014, 88, 246–253. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Tang, Y.; Zhang, X.; Qiu, Z. Synthesis and characterization of nano-SiO2@octadecylbisimidazoline quaternary ammonium salt used as acidizing corrosion inhibitor. Rev. Adv. Mater. Sci. 2022, 61, 186–194. [Google Scholar] [CrossRef]
- Liu, T.; Ma, L.; Wang, X.; Wang, J.; Qian, H.; Zhang, D.; Li, X. Self-healing corrosion protective coatings based on micro/nanocarriers: A review. Corros. Commun. 2021, 1, 18–25. [Google Scholar] [CrossRef]
- Stankiewicz, A.; Szczygieł, I.; Szczygieł, B. Self-healing coatings in anti-corrosion applications. J. Mater. Sci. 2013, 48, 8041–8051. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, X.; Schenderlein, M.; Borisova, D.; Cao, R.; Moehwald, H.; Shchukin, D. Self-Healing and Antifouling Multifunctional Coatings Based on pH and Sulfide Ion Sensitive Nanocontainers. Adv. Funct. Mater. 2013, 23, 3307–3314. [Google Scholar] [CrossRef]
- Montemor, M. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Zhang, C.; Qu, X.; Liu, J.; Yang, Z. Regenerative superhydrophobic coating from microcapsules. J. Mater. Chem. 2010, 20, 3211–3215. [Google Scholar] [CrossRef]
- García, S.; Fischer, H.; White, P.A.; Mardel, J.; Gonzalez-Garcia, Y.; Mol, J.M.C.; Hughes, A.E. Self-healing anticorrosive organic coating based on an encapsulated water reactive silyl ester: Synthesis and proof of concept. Prog. Org. Coat. 2011, 70, 142–149. [Google Scholar] [CrossRef]
- Vaszilcsin, C.; Putz, M.; Kellenberger, A.; Dan, M. On the evaluation of metal-corrosion inhibitor interactions by adsorption isotherms. J. Mol. Struct. 2023, 1286, 135643. [Google Scholar] [CrossRef]
- Kouakou, V.; Niamien, P.M.; Yapo, A.; Diaby, S.; Trokourey, A. Experimental and DFT Studies on the Behavior of Caffeine as Effective Corrosion Inhibitor of Copper in 1M HNO3. Orbital Electron. J. Chem. 2016, 8, 66–79. [Google Scholar] [CrossRef]
- He, Y.; Ren, S.; Wang, X.; Young, D.; Mohamed-Saïd, M.; Santos, B.A.; Serenario, M.; Singer, M. Temperature Dependence of Adsorption and Effectiveness for a Pyrimidinium-Type Corrosion Inhibitor on Mild Steel. Corrosion 2023, 80, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Ech-Chihbi, E.; Es-Sounni, B.; Kerdoune, C.; Mouhib, A.; Bakhouch, M.; Salim, R.; Salghi, R.; Hammouti, B.; Mazoir, N.; Chafiq, M.; et al. Corrosion inhibition performance and adsorption mechanism of new synthesized symmetrical diarylidenacetone-based inhibitor on C38 steel in 15 % HCl medium: Theoretical insight and experimental validation. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 135073. [Google Scholar] [CrossRef]
- Kokalj, A. On the use of the Langmuir and other adsorption isotherms in corrosion inhibition. Corros. Sci. 2023, 217, 111112. [Google Scholar] [CrossRef]
- Al-Amiery, A.; Shaker, L.; Betti, N. Corrosion inhibition effect and adsorption behaviour of nicotinic acid derivative on mild steel in HCl media. Mater. Today Proc. 2021, 56, 2204–2208. [Google Scholar] [CrossRef]
- Feng, C.; E, J.; Han, W.; Deng, Y.; Zhang, B.; Zhao, X.; Han, D. Key technology and application analysis of zeolite adsorption for energy storage and heat-mass transfer process: A review. Renew. Sustain. Energy Rev. 2021, 144, 110954. [Google Scholar] [CrossRef]
- Beda, R.H.B.; Niamien, P.M.; Avo Bilé, E.B.; Trokourey, A. Inhibition of Aluminium Corrosion in 1.0 M HCl by Caffeine: Experimental and DFT Studies. Adv. Chem. 2017, 2017, 6975248. [Google Scholar] [CrossRef]
- Gobara, M.; Baraka, A.; Zaghloul, B. Inhibition of mild steel corrosion in sulfuric acid solution using collagen. Res. Chem. Intermed. 2014, 41, 7245–7261. [Google Scholar] [CrossRef]
- Noor, E.; Al-Moubaraki, A.; Alghamdi, A. Continuous Studies on Using Camel’s Urine as Nontoxic Corrosion Inhibitor–Corrosion Inhibition of Al–Cu Alloy in Alkaline Solutions. Arab. J. Sci. Eng. 2018, 44, 237–250. [Google Scholar] [CrossRef]
- Vigdorowitsch, M.; Pchelintsev, A.; Tsygankova, L.; Tanygina, E. Freundlich Isotherm: An Adsorption Model Complete Framework. Appl. Sci. 2021, 11, 8078. [Google Scholar] [CrossRef]
- Vigdorowitsch, M.; Tsygankova, L.Y.; Shel, N.V. Polylogarithmic Adsorption Isotherm at Surface Linear Energetic Heterogeneity. Bull. Natl. Res. Nucl. Univ. MEPhI 2020, 9, 389–395. [Google Scholar] [CrossRef]
- Kovačević, N.; Milošev, I.; Kokalj, A. How relevant is the adsorption bonding of imidazoles and triazoles for their corrosion inhibition of copper? Corros. Sci. 2017, 124, 25–34. [Google Scholar] [CrossRef]
- Quraishi, M.; Chauhan, D. Heterocyclic Organic Corrosion Inhibitors: Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Goyal, M.; Kumar, S.; Bahadur, I.; Verma, C.; Ebenso, E.E. Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: A review. J. Mol. Liq. 2018, 256, 565–573. [Google Scholar] [CrossRef]
- Neupane, S.; Losada-Pérez, P.; Tiringer, U.; Taheri, P.; Desta, D.; Xie, C.; Crespo, D.; Mol, J.M.C.; Milošev, I.; Kokalj, A.; et al. Study of Mercaptobenzimidazoles as Inhibitors for Copper Corrosion: Down to the Molecular Scale. J. Electrochem. Soc. 2021, 168, 051504. [Google Scholar] [CrossRef]
- Kokalj, A.; Lozinšek, M.; Kapun, B.; Taheri, P.; Neupane, S.; Losada-Pérez, P.; Xie, C.; Stavber, S.; Crespo, D.; Renner, F.U.; et al. Simplistic correlations between molecular electronic properties and inhibition efficiencies: Do they really exist? Corros. Sci. 2021, 179, 108856. [Google Scholar] [CrossRef]
- Winkler, D.A.; Breedon, M.; Hughes, A.E.; Burden, F.R.; Barnard, A.S.; Harvey, T.G.; Cole, I. Towards chromate-free corrosion inhibitors: Structure–property models for organic alternatives. Green Chem. 2014, 16, 3349–3357. [Google Scholar] [CrossRef]
- Kokalj, A. Molecular modeling of organic corrosion inhibitors: Calculations, pitfalls, and conceptualization of molecule–surface bonding. Corros. Sci. 2021, 193, 109650. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Verma, C.; Olasunkanmi, L.O.; Akpan, E.D.; Verma, D.K.; Lgaz, H.; Guo, L.; Kaya, S.; Quraishi, M.A. Molecular modelling of compounds used for corrosion inhibition studies: A review. Phys. Chem. Chem. Phys. 2021, 23, 19987–20027. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Cruz-Borbolla, J.; Arizpe-Carreon, P.A.; Gutierrez, E. Mathematical Models Generated for the Prediction of Corrosion Inhibition Using Different Theoretical Chemistry Simulations. Materials 2020, 13, 5656. [Google Scholar] [CrossRef]
- Ikeuba, A.I.; Ita, B.I.; Okonkwo, C.P.; Ekuri, P.E.; Edet, H.O.; Amajama, J.; Iwuji, P.C. Review of computational methods used in the evaluation corrosion inhibition of metallic materials. Discov. Chem. Eng. 2024, 4, 28. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; El Basiony, N.M.; Sadeek, S.A.; Migahed, M.A. Scale and corrosion inhibition performance of the newly synthesized anionic surfactant in desalination plants: Experimental, and theoretical investigations. Desalination 2018, 437, 45–58. [Google Scholar] [CrossRef]
- Abdulazeez, M.; Oyebamiji, A.; Semire, B. DFT-QSAR studies on corrosion inhibition efficiency of derivatives of thiadiazole, oxadiazole and triazole. Int. J. Corros. Scale Inhib. 2016, 5, 248–262. [Google Scholar] [CrossRef]
- Radovanović, M.B.; Tasić, Ž.Z.; Mihajlović, M.B.P.; Simonović, A.T.; Antonijević, M.M. Electrochemical and DFT studies of brass corrosion inhibition in 3% NaCl in the presence of environmentally friendly compounds. Sci. Rep. 2019, 9, 16081. [Google Scholar] [CrossRef] [PubMed]
- Chiter, F.; Bulteau, Y.; Bonin, P.; Pébère, N.; Lacaze-Dufaure, C. On the identification of favourable factors for corrosion inhibition of aluminium by 8-hydroxyquinoline and its derivatives: DFT and electrochemical studies. Corros. Sci. 2024, 233, 112104. [Google Scholar] [CrossRef]
- Cen, H.; Zhu, Z.; Chen, M.; Guo, X.; Chen, Z. Effect of Magnetic Field on the Inhibition Performance of Corrosion Inhibitors with Different Dipole Moment Gradients. Met. Mater. Int. 2021, 27, 5046–5058. [Google Scholar] [CrossRef]
- Kuruvilla, M.; Prasad, A.R.; John, S.; Joseph, A. Enhanced Inhibition of the Corrosion of Metallic Copper Exposed in Sulphuric Acid Through the Synergistic Interaction of Cysteine and Alanine: Electrochemical and Computational Studies. J. Bio- Tribo-Corros. 2016, 3, 5. [Google Scholar] [CrossRef]
- Challouf, H.; Souissi, N.; Ben Messaouda, M.; Rym, A.; Madani, A. Origanum majorana Extracts as Mild Steel Corrosion Green Inhibitors in Aqueous Chloride Medium. J. Environ. Prot. 2016, 07, 532–544. [Google Scholar] [CrossRef]
- Khaled, K.; Abdel-Rehim, S.S.; Saker, G. On the corrosion inhibition of iron in hydrochloric acid solutions, Part I: Electrochemical DC and AC studies. Arab. J. Chem. 2010, 5, 213–218. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, B.; Wang, X.; Lu, Y.; Li, F.; Li, C. Improved corrosion resistance of carbon steel in soft water with dendritic-polymer corrosion inhibitors. Chem. Eng. J. 2023, 452, 139043. [Google Scholar] [CrossRef]
- Lukovits, I.; Kálmán, E.; Zucchi, F. Corrosion Inhibitors—Correlation between Electronic Structure and Efficiency. Corrosion 2001, 57, 3–8. [Google Scholar] [CrossRef]
- Pérez, P.; Domingo, L.; Aizman, A.; Contreras, R. The Electrophilicity Index in Organic Chemistry. Theor. Comput. Chem. 2007, 19, 139–201. [Google Scholar] [CrossRef]
- Lakbaibi, Z.; Damej, M.; Molhi, A.; Benmessaoud, M.; Tighadouini, S.; Jaafar, A.; Tariq, B.; Ansari, A.; Driouich, A.; Tabyaoui, M. Evaluation of inhibitive corrosion potential of symmetrical hydrazine derivatives containing nitrophenyl moiety in 1M HCl for C38 steel: Experimental and Theoretical studies. Heliyon 2022, 8, e09087. [Google Scholar] [CrossRef]
- Prakashaiah, B.G.; Jayaprakash, G.K.; Rani, B.E.A. A Study of Corrosion Behavior of (E)-2-(3,4-dihydroxybenzylidene)hydrazinecarbothioamide and Bis [[3,4-dihydroxyphenylmethylene] Carbonothioicdihydrazide]-Sealed Anodized AA2024-T3. J. Bio- Tribo-Corros. 2022, 8, 75. [Google Scholar] [CrossRef]
- Mahdi, B.S.; Abbass, M.K.; Mohsin, M.K.; Al-azzawi, W.K.; Hanoon, M.M.; Al-kaabi, M.H.; Shaker, L.M.; Al-amiery, A.A.; Isahak, W.N.; Kadhum, A.A.H.; et al. Corrosion Inhibition of Mild Steel in Hydrochloric Acid Environment Using Terephthaldehyde Based on Schiff Base: Gravimetric, Thermodynamic, and Computational Studies. Molecules 2022, 27, 4857. [Google Scholar] [CrossRef]
- Razali, N.Z.K.; Wan Hassan, W.N.S.; Sheikh Mohd Ghazali, S.A.I.; Mohd Shotor, S.N.; Dzulkifli, N.N. DFT, Fukui indices, and molecular dynamic simulation studies on corrosion inhibition characteristics: A review. Chem. Pap. 2024, 78, 715–731. [Google Scholar] [CrossRef]
- Huerta-Servin, R.; Ahuatzi-Chacón, D.; Salmerón-Alcocer, A.; García-Medina, S.; Mena-Cervantes, V.Y.; Hernández-Altamirano, R. Effect of solubility and partition coefficient on the toxicity of corrosion inhibitors used in the oil industry. Chem. Pap. 2024, 78, 3063–3081. [Google Scholar] [CrossRef]



| Plant and Part of the Plant Used as GIC | Extraction Medium | Metal | Active Phyto-Components | Corrosive Medium | MIE % | Temp °C | Max Conc | Analysis Techniques | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Camellia chrysantha- (Matricaria recutita L.) flower | Ethanolic. | Al. | Bisabolol, camphen, cineole. | Artificial seawater (3.5 wt.% NaCl solution). | 75.66 | 19. 85 | 20 mL L−1 | OCP, Tafel, kinetic thermodynamic adsorption isotherm (Langmuir, Freundlich, Temkin), FTIR, AFM, SEM. | [167] |
| Feverfew root | Deionized water. | Q235 carbon steel. | Parthenolide. | 0.5 mol/L H2SO4. | 97.2 | 25 | 400 mg L−1 | Weight loss, EIS, PDP, OCP, FTIR, SEM, XPS. | [168] |
| Tea leaves | 5 mL of 10% H2SO4 and 100 mL of water. | Al alloy 1100. | - | 10% H2SO4. | 71.43 85.7 | - | - | Weight loss, OCP, EIS, SEM, EDS, XRD, FTIR. | [169] |
| Tulsi plants | H2SO4 and 100 mL of water. | Al alloy 1100. | - | 10% H2SO4. | 85.7 | - | - | Weight loss, OCP, EIS, SEM, EDS, XRD, FTIR. | [169] |
| Castor beans | Double-distilled water. | Mid steel. | Ricinoleic acid. | 1 M HCl. | 94.2 | 45 | 200 mg L−1 | Weight loss, OCP, EIS, LP, PPC, adsorption isotherm (Langmuir, Temkin, Flory–Huggins, El-Awady), EDS, SDS-PAGE and FTIR. | [164] |
| Justicia brandegeean Aerial parts (leaves, flowers, and stems) autumn and spring | Ethanol. | Carbon steel AISI 1020. | Alcohols (68.42%), aldehydes and ketones (9.92%), esters (6.59%), and alkanes (1.10%) palmitic acid; 86.49% unsaturated fatty acids, the main component being linoleic acid with 37.4%. | H2SO4 1 mol L−1 Solution. | 93.54 | 30 | 1500 ppm | FTIR, weight loss, NMR, ATR, OCP, EIS, LPR, PP, SEM, Adsorption isotherm (Frumkin, Langmuir, Temkin, Flory–Huggins, El-Awady). | [170] |
| Solanum macrocarpon leaves | Methyl alcohol extract. | Mild steel. | Alkaloids, flavonoids, tannins, saponins, steroids. | 2 M H2SO4. | 91.4 | 29.85 | 0.5% w/v | FTIR, gasometrical techniques, hydrogen evolution data, kinetic and thermodynamic data, CR. | [171] |
| Erigeron bonariensis leaves, flowers, and stems | Ethanol. | Weathering steel–mild steel. | Quercetin, rutin, naringenin, luteolin, caffeic acid, apigenin. | 1 M H2SO4. | 99.5 (leaf) 94.35 (flower) 85.22 (steam) | 27 ± 1 | 2000 mg L−1 | Weight loss, absorption studies, UV spectroscopy, phytochemical analysis, PDP, OCP, EIS, SEM. | [172] |
| Dactylocte-nium aegyptium entire plant | Ethanolic extract. | Steel. | Tricin, Vanillic acid, p-hydroxybenzaldehyde, p-hydroxybenzoic acid. | 0.5 M HCl solution. | 95.7 | - | 800 ppm | UV–Vis, IR, SEM, electrochemical measurements, adsorption isotherm models (Langmuir, Temkin and Freundlich). | [173] |
| Okra leaves | Distilled water. | N80 steel. | Tannin, lectin, saponin, many phenolic compounds. | 1 M H2SO4. | 96 | 30 | 100 mL L−1 | Gravimetrical and electrochemical techniques, FTIR, UV, optical microscopy, AFM, SEM, TGA. | [174] |
| Lemon verbena leaves | Water. | Mild steel (st37). | - | 0.5 M H2SO4. 1 M HCl. | 90 (H2SO4) 94 (HCl) | 25 | 2000 ppm (H2SO4) 2500 ppm (HCl) | ECN, EIS, PP, SEM. Quantum chemical computation. MD simulation. | [175] |
| Dillenia suffruticosa leaves | Ethanolic extract. | Mild steel. | Flavonoids and glycosides, anthraquinone glycosides, phenolic derivatives and tannins, saponins, steroids, and triterpenoids. | HCl. | 81.4 | 22.35 | 1000 mg L−1 | Gravimetric and electrochemical methods, surface analysis of the corroded steel samples: SEM, FTIR. | [176] |
| Pyracantha fortuneana fruit | Absolute ethanol. | Copper. | HTP, DTT, AGA, APA, ACA, DTP. | H2SO4. | 95 | - | 600 mg L−1 | Electrochemical test. Morphology analysis, FTIR, SEM, AFM. | [177] |
| Verbena officinalis leaves | Ethanol. | Carbon steel. | Luteolin, diosmosing -7-neohesperidoside. | 0.5 M H2SO4. | 91.1 | 25 | 1000 ppm | Weight loss, PDP, EIS, AFM, XPS. | [178] |
| Trifolium repens entire plant | Dichloro-methane. | Carbon steel API5LX60. | Flavones: acacetin, luteolin, and several others with hydroxy and methoxy groups. | 3.5% NaCl. | 98 | 20–25 | 20 ppm | FTIR, PP, EIS, SEM, AFM. | [179] |
| Millettia aboensis leaves | Methanolic extract. | Mild steel. | Alkaloids, tannins, glycosides, phenolic compounds and flavonoids. | 0.3 M HCl. | 88.6 | - | 3 g L−1 | Electrochemical measurements, XPS, SEM, GC–MS. | [180] |
| Zea mays bracts | Ethanol aqueous solution 4:1 v/v%. | Mild steel. | Flavonoids, carbohydrates phenolic compounds phenyl-propanoids. | 1 M HCl. | 96.2 | 45 | 5.0 g L−1 | FT-IR, UV–Vis, XPS, PDP, EIS. | [181] |
| Parameter | Symbol | Signification | Reference |
|---|---|---|---|
| Energy of the highest occupied molecular orbital | EHOMO | Electron-donating ability of a molecule. | [237] |
| Energy of the lowest unoccupied molecular orbital | ELUMO | Ability of the molecule to accept electrons. | [238] |
| Ionization potential | IP | Capacity of a chemical compound to eliminate an electron. | [239] |
| Electron affinity | A | Capacity of a molecule to interact with nucleophile. | [240] |
| Dipole moment | µD | Related to asymmetry of charge in a molecule and a good indicator of the stability of complex on a metal surface. | [241] |
| Energy gap | ΔE ΔE = EHOMO − ELUMO | Related to a good adsorption on surface of inhibitor molecule. Lower ΔE results in higher stability of the metal–inhibitor interaction. | [242] |
| Fraction of electron transferred | ΔN | If ΔN > 3.6, the inhibition efficiency increases. | [243] |
| Global hardness | η | Resistance of an atom to transfer its charge. | [244] |
| Softness | σ | How easily an inhibitor performs a charge transfer. | [245] |
| Electronegativity | χ | Attraction of electrons by inhibitor molecules. | [246] |
| Electrophilicity index | ω | Assessment of electrophilic properties of a molecule. | [247] |
| Electro-donating power | Ω− | Assessment of the ability of a species to donate electrons. | [248] |
| Electro-accepting power | ω+ | Assessment of the ability of a species to accept electrons. | [249] |
| Dipole polarizability | α | α is a measure of polarizability. Large values of α results in a strong adsorption process. | [250] |
| Fukui functions | fk+, fk− | This is an indicator for the zones of a molecule with nucleophilic, electrophilic, or potential radical properties. | [251] |
| Partition coefficient | Log P | Log P, or octanol–water partition coefficient, is a measure of how hydrophilic or hydrophobic a molecule is. | [252] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Răuță, D.-I.; Matei, E.; Avramescu, S.-M. Recent Development of Corrosion Inhibitors: Types, Mechanisms, Electrochemical Behavior, Efficiency, and Environmental Impact. Technologies 2025, 13, 103. https://doi.org/10.3390/technologies13030103
Răuță D-I, Matei E, Avramescu S-M. Recent Development of Corrosion Inhibitors: Types, Mechanisms, Electrochemical Behavior, Efficiency, and Environmental Impact. Technologies. 2025; 13(3):103. https://doi.org/10.3390/technologies13030103
Chicago/Turabian StyleRăuță, Denisa-Ioana (Gheorghe), Ecaterina Matei, and Sorin-Marius Avramescu. 2025. "Recent Development of Corrosion Inhibitors: Types, Mechanisms, Electrochemical Behavior, Efficiency, and Environmental Impact" Technologies 13, no. 3: 103. https://doi.org/10.3390/technologies13030103
APA StyleRăuță, D.-I., Matei, E., & Avramescu, S.-M. (2025). Recent Development of Corrosion Inhibitors: Types, Mechanisms, Electrochemical Behavior, Efficiency, and Environmental Impact. Technologies, 13(3), 103. https://doi.org/10.3390/technologies13030103








