Resonance-Induced Capacitively Coupled Contactless Conductivity Detection (ReC4D) Unit for Nucleic Acid Amplification Testing
Abstract
:1. Introduction
2. Materials and Methods
2.1. RT-LAMP Reaction Preparation
- (i)
- The RNA can be extracted from any sample or matrix, preventing measurement interference, as all samples are prepared using the same RT-LAMP reaction mix.
- (ii)
- The RT-LAMP reaction mix contains primers specifically designed for the SARS-CoV-2 N and ORF1ab genes. These primers ensure high selectivity in the RT-LAMP assay, as genetic amplification occurs only when these genes are present.
2.2. Electrical Conductivity of Nucleic Acid Amplification Products
2.3. Operation Principle
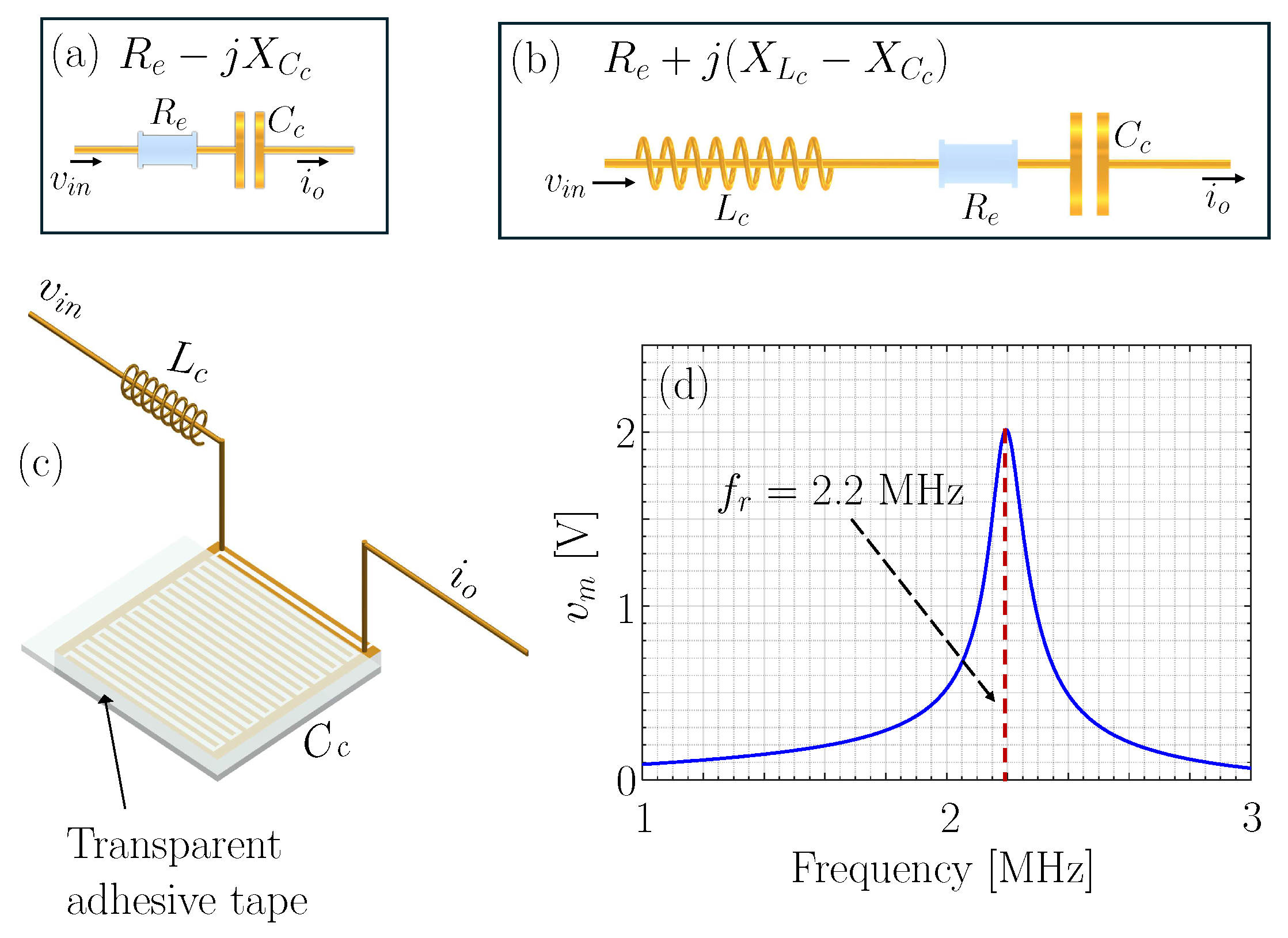
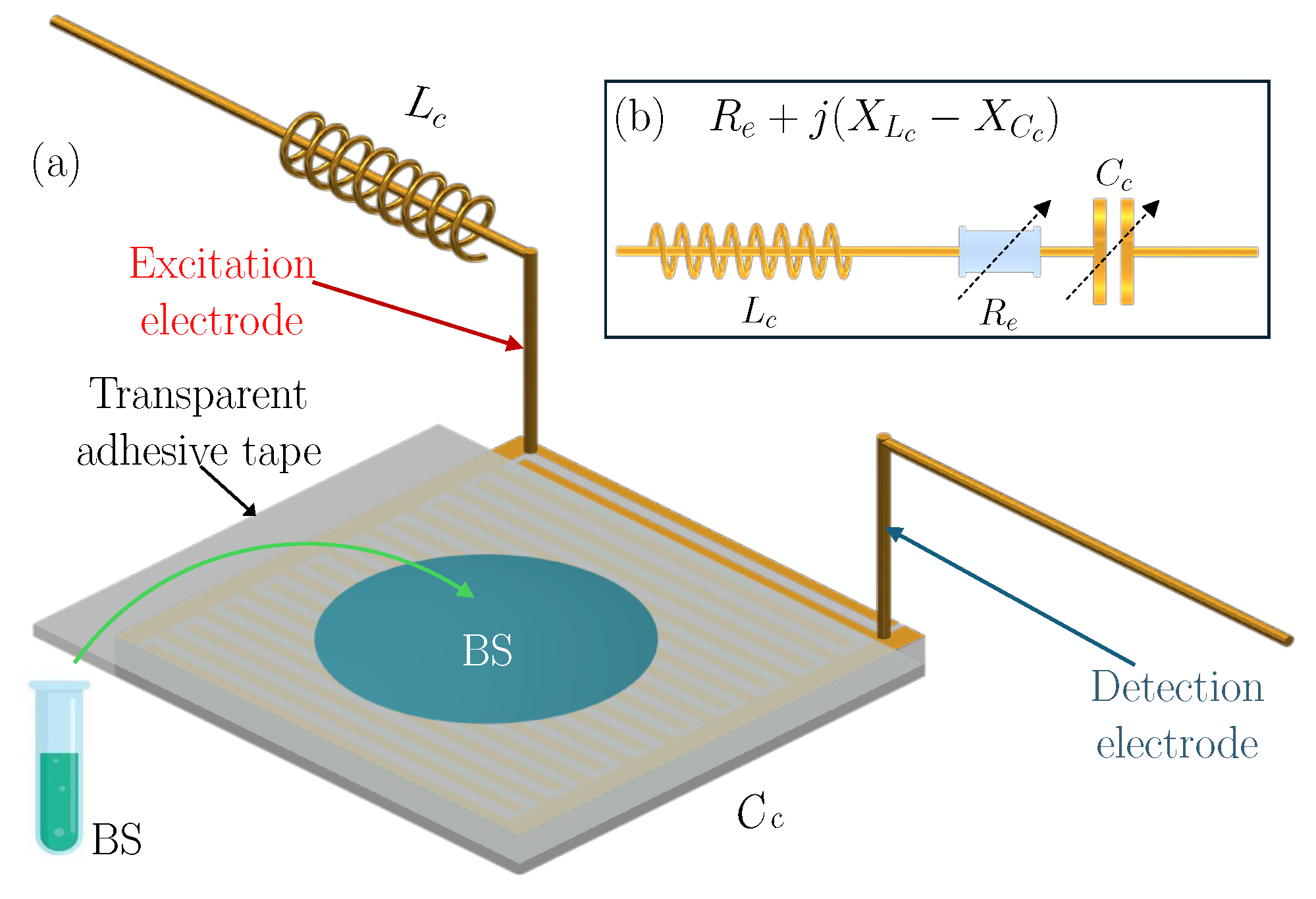
2.4. Resonance-Induced Capacitively Coupled Contactless Conductivity Detection Unit
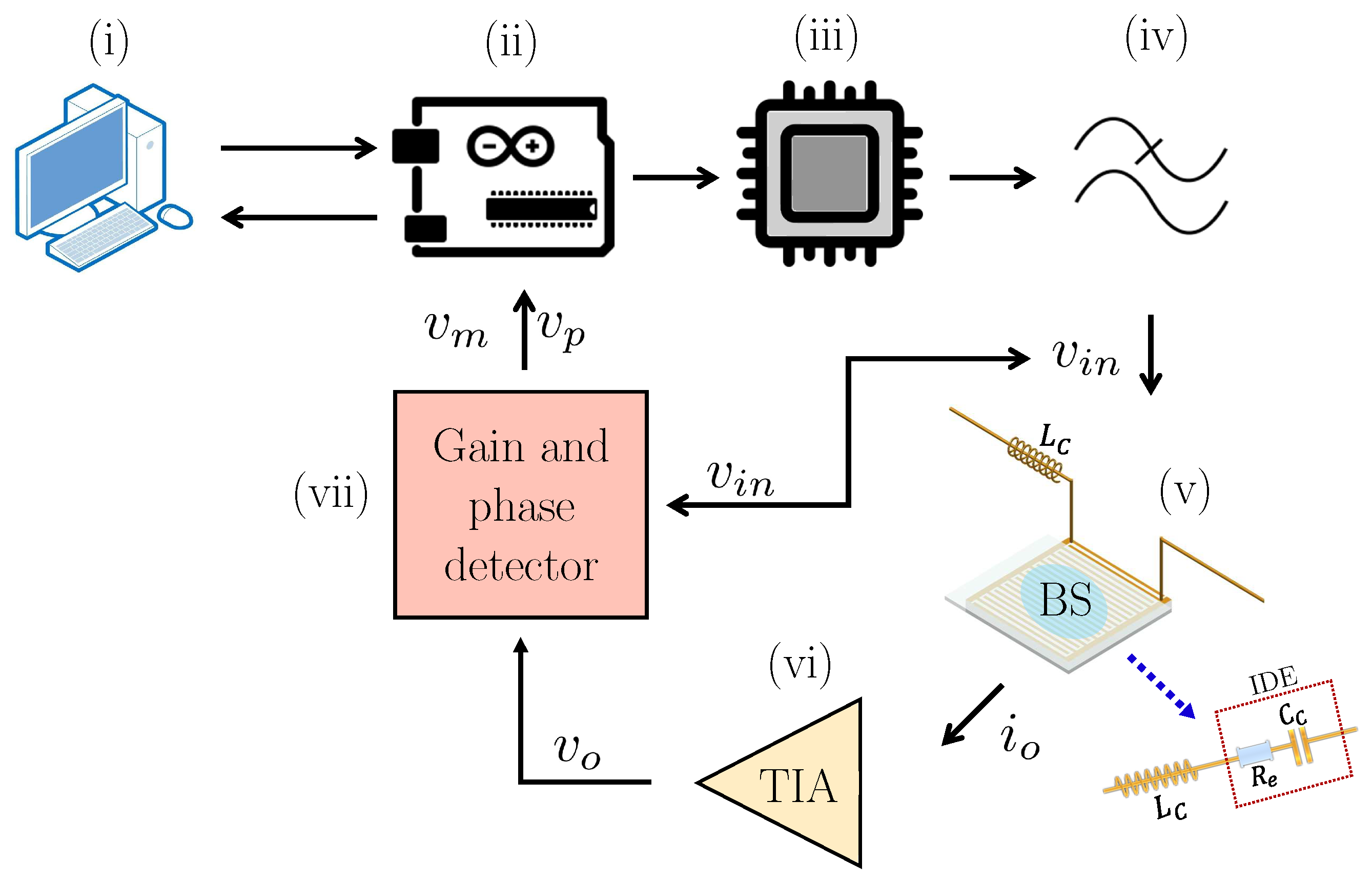
2.5. Measurement Setup
3. Results
3.1. Measurement Platform Validation
3.2. Measuring the Conductivity of KCl Concentrations
3.3. Quantifying Nucleic Acid Amplification
3.4. Performance Comparison with State-of-the-Art Detection Platforms for NAA Products to Detect SARS-CoV-2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, C.H. (Ed.) Chapter 9—Amplification of Nucleic Acids. In Diagnostic Molecular Biology; Academic Press: New York, NY, USA, 2019; pp. 215–247. [Google Scholar] [CrossRef]
- Fakruddin, M.; Mannan, K.S.B.; Chowdhury, A.; Mazumdar, R.M.; Hossain, M.N.; Islam, S.; Chowdhury, M.A. Nucleic Acid Amplification: Alternative Methods of Polymerase Chain Reaction. J. Pharm. Bioallied Sci. 2013, 5, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, X.; Feng, H.; Ji, F.; Bai, D.; Dong, X.; Huang, W. Isothermal Nucleic Acid Amplification Technology for Rapid Detection of Virus. Crit. Rev. Biotechnol. 2023, 43, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Kubista, M.; Andrade, J.M.; Bengtsson, M.; Forootan, A.; Jonák, J.; Lind, K.; Sindelka, R.; Sjöback, R.; Sjögreen, B.; Strömbom, L.; et al. The real-time polymerase chain reaction. Mol. Asp. Med. 2006, 27, 95–125. [Google Scholar] [CrossRef]
- Moon, Y.J.; Lee, S.Y.; Oh, S.W. A Review of Isothermal Amplification Methods and Food-Origin Inhibitors against Detecting Food-Borne Pathogens. Foods 2022, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.E.; Lim, B.; Hsu, C.C.; Xiong, D.; Wu, W.; Yu, Y.; Jia, H.; Wang, Y.; Zeng, Y.; Ji, M.; et al. RT-LAMP for Rapid Diagnosis of Coronavirus SARS-CoV-2. Microb. Biotechnol. 2020, 13, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H. Nucleic Acid Amplification Strategies for Biosensing, Bioimaging, and Biomedicine. In Nucleic Acid Amplification Strategies for Biosensing, Bioimaging and Biomedicine; Zhang, S., Bi, S., Song, X., Eds.; Springer: New York, NY, USA, 2019; pp. 3–14. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Li, P.; Cui, L.; Qiu, J.G.; Jiang, B.; Zhang, C.Y. Integration of Nanomaterials with Nucleic Acid Amplification Approaches for Biosensing. TrAC Trends Anal. Chem. 2020, 129, 115959. [Google Scholar] [CrossRef]
- Amoah, I.D.; Mthethwa, N.P.; Pillay, L.; Deepnarain, N.; Pillay, K.; Awolusi, O.O.; Kumari, S.; Bux, F. RT-LAMP: A Cheaper, Simpler and Faster Alternative for the Detection of SARS-CoV-2 in Wastewater. Food Environ. Virol. 2021, 13, 447–456. [Google Scholar] [CrossRef]
- Mujawar, M.A.; Gohel, H.; Bhardwaj, S.K.; Srinivasan, S.; Hickman, N.; Kaushik, A. Nano-Enabled Biosensing Systems for Intelligent Healthcare: Towards COVID-19 Management. Mater. Today Chem. 2020, 17, 100306. [Google Scholar] [CrossRef]
- Pilevar, M.; Kim, K.T.; Lee, W.H. Recent Advances in Biosensors for Detecting Viruses in Water and Wastewater. J. Hazard. Mater. 2021, 410, 124656. [Google Scholar] [CrossRef]
- Moon, J.; Kwon, H.J.; Yong, D.; Lee, I.C.; Kim, H.; Kang, H.; Lim, E.K.; Lee, K.S.; Jung, J.; Park, H.G.; et al. Colorimetric Detection of SARS-CoV-2 and Drug-Resistant pH1N1 Using CRISPR/dCas9. ACS Sens. 2020, 5, 4017–4026. [Google Scholar] [CrossRef]
- Fan, Z.; Yao, B.; Ding, Y.; Zhao, J.; Xie, M.; Zhang, K. Entropy-Driven Amplified Electrochemiluminescence Biosensor for RdRp Gene of SARS-CoV-2 Detection with Self-Assembled DNA Tetrahedron Scaffolds. Biosens. Bioelectron. 2021, 178, 113015. [Google Scholar] [CrossRef]
- Kirimli, C.; Lin, S.; Su, Y.H.; Shih, W.H.; Shih, W.Y. In Situ, Amplification-Free Double-Stranded Mutation Detection at 60 Copies/Ml with Thousand-Fold Wild Type in Urine. Biosens. Bioelectron. 2018, 119, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Wu, T.H.; Chang, C.C.; Lo, H.Y.; Liu, H.W.; Huang, N.T.; Lin, C.W. Biosensing Amplification by Hybridization Chain Reaction on Phase-Sensitive Surface Plasmon Resonance. Biosensors 2021, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Pan, Y.; Sun, Z.; Li, J.; Yi, Y.; Yang, J.; Li, G. An Electrochemical Biosensor for Sensitive Analysis of the SARS-CoV-2 RNA. Biosens. Bioelectron. 2021, 186, 113309. [Google Scholar] [CrossRef]
- Ahmad, M.; Sharma, P.; Kamai, A.; Agrawal, A.; Faruq, M.; Kulshreshtha, A. HRPZyme Assisted Recognition of SARS-CoV-2 Infection by Optical Measurement (HARIOM). Biosens. Bioelectron. 2021, 187, 113280. [Google Scholar] [CrossRef]
- Ochmann, S.E.; Vietz, C.; Trofymchuk, K.; Acuna, G.P.; Lalkens, B.; Tinnefeld, P. Optical Nanoantenna for Single Molecule-Based Detection of Zika Virus Nucleic Acids without Molecular Multiplication. Anal. Chem. 2017, 89, 13000–13007. [Google Scholar] [CrossRef]
- Han, S.; Soylu, M.C.; Kirimli, C.E.; Wu, W.; Sen, B.; Joshi, S.G.; Emery, C.L.; Au, G.; Niu, X.; Hamilton, R.; et al. Rapid, Label-Free Genetic Detection of Enteropathogens in Stool without Genetic Isolation or Amplification. Biosens. Bioelectron. 2019, 130, 73–80. [Google Scholar] [CrossRef]
- Aura, A.M.; D’Agata, R.; Spoto, G. Ultrasensitive Detection of Staphylococcus Aureus and Listeria Monocytogenes Genomic DNA by Nanoparticle-Enhanced Surface Plasmon Resonance Imaging. ChemistrySelect 2017, 2, 7024–7030. [Google Scholar] [CrossRef]
- Hajian, R.; Balderston, S.; Tran, T.; deBoer, T.; Etienne, J.; Sandhu, M.; Wauford, N.A.; Chung, J.Y.; Nokes, J.; Athaiya, M.; et al. Detection of Unamplified Target Genes via CRISPR–Cas9 Immobilized on a Graphene Field-Effect Transistor. Nat. Biomed. Eng. 2019, 3, 427–437. [Google Scholar] [CrossRef]
- Xie, S.; Chai, Y.; Yuan, Y.; Bai, L.; Yuan, R. Development of an Electrochemical Method for Ochratoxin A Detection Based on Aptamer and Loop-Mediated Isothermal Amplification. Biosens. Bioelectron. 2014, 55, 324–329. [Google Scholar] [CrossRef]
- Salm, E.; Zhong, Y.; Reddy, B.J.; Duarte-Guevara, C.; Swaminathan, V.; Liu, Y.S.; Bashir, R. Electrical Detection of Nucleic Acid Amplification Using an On-Chip Quasi-Reference Electrode and a PVC REFET. Anal. Chem. 2014, 86, 6968–6975. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Hill, A.; Tait, S.; Baillie, C.; Virdis, B.; McCabe, B. Microbial Electrochemical Sensors for Volatile Fatty Acid Measurement in High Strength Wastewaters: A Review. Biosens. Bioelectron. 2020, 165, 112409. [Google Scholar] [CrossRef]
- Huang, Z.; Long, J.; Xu, W.; Ji, H.; Wang, B.; Li, H. Design of capacitively coupled contactless conductivity detection sensor. Flow Meas. Instrum. 2012, 27, 67–70. [Google Scholar] [CrossRef]
- Li, L.; Ren, D.D.; Zhang, P.Y.; Song, Y.P.; Li, T.X.; Gao, M.H.; Xu, J.N.; Zhou, L.; Zeng, Z.C.; Pu, Q. Pushing the Limits of Capacitively Coupled Contactless Conductivity Detection for Capillary Electrophoresis. Anal. Chem. 2024, 96, 10356–10364. [Google Scholar] [CrossRef]
- Tůma, P.; Sommerová, B.; Šiklová, M. Monitoring of adipose tissue metabolism using microdialysis and capillary electrophoresis with contactless conductivity detection. Talanta 2019, 192, 380–386. [Google Scholar] [CrossRef]
- Duong, H.A.; Vu, M.T.; Nguyen, T.D.; Nguyen, M.H.; Mai, T.D. Determination of 10-hydroxy-2-decenoic acid and free amino acids in royal jelly supplements with purpose-made capillary electrophoresis coupled with contactless conductivity detection. J. Food Compos. Anal. 2020, 87, 103422. [Google Scholar] [CrossRef]
- Do Quang, L.; Bui, T.T.; Hoang, A.B.; Van, T.P.; Jen, C.P.; Chu Duc, T. Development of a Passive Capacitively Coupled Contactless Conductivity Detection (PC4D) Sensor System for Fluidic Channel Analysis Toward Point-of-Care Applications. IEEE Sens. J. 2019, 19, 6371–6380. [Google Scholar] [CrossRef]
- Hu, C.; Xie, B.; Li, H.; Xiao, D. A five-electrode capacitively coupled contactless conductivity detector with a low limit of detection. Anal. Methods 2023, 15, 2253–2261. [Google Scholar] [CrossRef]
- Chantipmanee, N.; Sonsa-ard, T.; Fukana, N.; Kotakanok, K.; Mantim, T.; Wilairat, P.; Hauser, P.C.; Nacapricha, D. Contactless conductivity detector from printed circuit board for paper-based analytical systems. Talanta 2020, 206, 120227. [Google Scholar] [CrossRef]
- Sonsa-ard, T.; Chantipmanee, N.; Fukana, N.; Hauser, P.C.; Wilairat, P.; Nacapricha, D. Contactless conductivity sensor employing moist paper as absorbent for in-situ detection of generated carbon dioxide gas. Anal. Chim. Acta 2020, 1118, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Brito-Neto, J.G.A.; Fracassi da Silva, J.A.; Blanes, L.; do Lago, C.L. Understanding capacitively coupled contactless conductivity detection in capillary and microchip electrophoresis. Part 1. Fundamentals. Electroanalysis 2005, 17, 1198–1206. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; He, W.; Chen, Y.; You, H. A novel planar grounded capacitively coupled contactless conductivity detector for microchip electrophoresis. Micromachines 2022, 13, 394. [Google Scholar] [CrossRef]
- Kang, Q.; Shen, D.; Li, Q.; Hu, Q.; Dong, J.; Du, J.; Tang, B. Reduction of the impedance of a contactless conductivity detector for microchip capillary electrophoresis: Compensation of the electrode impedance by addition of a series inductance from a piezoelectric quartz crystal. Anal. Chem. 2008, 80, 7826–7832. [Google Scholar] [CrossRef]
- Alvarez-Serna, B.E.; Ramírez-Chavarría, R.G.; Castillo-Villanueva, E.; Carrillo-Reyes, J.; Ramírez-Zamora, R.M.; Buitrón, G.; Alvarez-Icaza, L. Label-Free and Portable Field-Effect Sensor for Monitoring RT-LAMP Products to Detect SARS-CoV-2 in Wastewater. Talanta 2023, 253, 124060. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Lu, X.; Justin Gooding, J.; Li, Q.; Qu, K. Monitoring the Progression of Loop-Mediated Isothermal Amplification Using Conductivity. Anal. Biochem. 2014, 466, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Q.; Jin, X.; Jiang, C.; Lu, Y.; Tavallaie, R.; Gooding, J.J. Quantitative determination of target gene with electrical sensor. Sci. Rep. 2015, 5, 12539. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.; Abdou, J.; Delzeit, L. Capacitively-Coupled Contactless Conductivity Detection (C4D) for In-Line Ionic Silver Monitoring. In Proceedings of the 2023 International Conference on Environmental Systems, Rome, Italy, 21–23 September 2023. [Google Scholar]
- Coltro, W.K.T.; Lima, R.S.; Segato, T.P.; Carrilho, E.; de Jesus, D.P.; do Lago, C.L.; da Silva, J.A.F. Capacitively Coupled Contactless Conductivity Detection on Microfluidic Systems—Ten Years of Development. Anal. Methods 2012, 4, 25–33. [Google Scholar] [CrossRef]
- Hauser, P.C.; Kubáň, P. Capacitively Coupled Contactless Conductivity Detection for Analytical Techniques—Developments from 2018 to 2020. J. Chromatogr. A 2020, 1632, 461616. [Google Scholar] [CrossRef]
- Ramírez-Chavarría, R.G.; Sánchez-Pérez, C.; Matatagui, D.; Qureshi, N.; Pérez-García, A.; Hernández-Ruíz, J. Ex-Vivo Biological Tissue Differentiation by the Distribution of Relaxation Times Method Applied to Electrical Impedance Spectroscopy. Electrochim. Acta 2018, 276, 214–222. [Google Scholar] [CrossRef]
- Chahbi, M.; Mortadi, A.; Zaim, S.; El Ghyati, N.; Monkade, M.; El Moznine, R. A new approach to investigate the ionic conductivity of NaCl and KCl solutions via impedance spectroscopy. Mater. Today Proc. 2022, 66, 205–211. [Google Scholar] [CrossRef]
- Ramírez-Chavarría, R.G.; Castillo-Villanueva, E.; Alvarez-Serna, B.E.; Carrillo-Reyes, J.; Ramírez-Zamora, R.M.; Buitrón, G.; Alvarez-Icaza, L. Loop-mediated isothermal amplification-based electrochemical sensor for detecting SARS-CoV-2 in wastewater samples. J. Environ. Chem. Eng. 2022, 10, 107488. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Shin, S.; Hwang, J.; Kim, Y.J.; Choi, S. Open-Source Fluorescence Spectrometer for Noncontact Scientific Research and Education. J. Chem. Educ. 2021, 98, 3493–3501. [Google Scholar] [CrossRef]
- Cancelliere, R.; Paialunga, E.; Grattagliano, A.; Micheli, L. Label-free electrochemical immunosensors: A practical guide. TrAC Trends Anal. Chem. 2024, 180, 117949. [Google Scholar] [CrossRef]
- Ménard-Moyon, C.; Bianco, A.; Kalantar-Zadeh, K. Two-dimensional material-based biosensors for virus detection. ACS Sens. 2020, 5, 3739–3769. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Zhou, Z.; Si, J.; Li, S.; Zeng, Y.; Deng, Y.; Chen, Z. Advances in simple, rapid, and contamination-free instantaneous nucleic acid devices for pathogen detection. Biosensors 2023, 13, 732. [Google Scholar] [CrossRef]
- Ye, H.; Nowak, C.; Liu, Y.; Li, Y.; Zhang, T.; Bleris, L.; Qin, Z. Plasmonic LAMP: Improving the detection specificity and sensitivity for SARS-CoV-2 by plasmonic sensing of isothermally amplified nucleic acids. Small 2022, 18, 2107832. [Google Scholar] [CrossRef]
- Liu, D.; Shen, H.; Zhang, Y.; Shen, D.; Zhu, M.; Song, Y.; Zhu, Z.; Yang, C. A microfluidic-integrated lateral flow recombinase polymerase amplification (MI-IF-RPA) assay for rapid COVID-19 detection. Lab Chip 2021, 21, 2019–2026. [Google Scholar] [CrossRef]
- Wu, Y.; Dang, H.; Park, S.G.; Chen, L.; Choo, J. SERS-PCR assays of SARS-CoV-2 target genes using Au nanoparticles-internalized Au nanodimple substrates. Biosens Bioelectron. 2022, 197, 113736. [Google Scholar] [CrossRef]
- Bel Hadj Ali, I.; Souguir, H.; Melliti, M.; Mohamed, M.V.T.; Ardhaoui, M.; Ayouni, K.; Haddad-Boubaker, S.; Saadi Ben Aoun, Y.; Triki, H.; Guizani, I. Rapid detection of SARS-CoV-2 RNA using a one-step fast multiplex RT-PCR coupled to lateral flow immunoassay. BMC Infec. Dis. 2024, 24, 1417. [Google Scholar] [CrossRef]
- Lynch, C.R.; Drummond, R.S.; Jelley, L.; Baker, L.; Smit, E.; Fleming, R.; Billington, C. Optimization and Benchmarking of RT-LAMP-CRISPR-Cas12a for the Detection of SARS-CoV-2 in Saliva. Int. J. Mol. Sci. 2025, 26, 1806. [Google Scholar] [CrossRef] [PubMed]
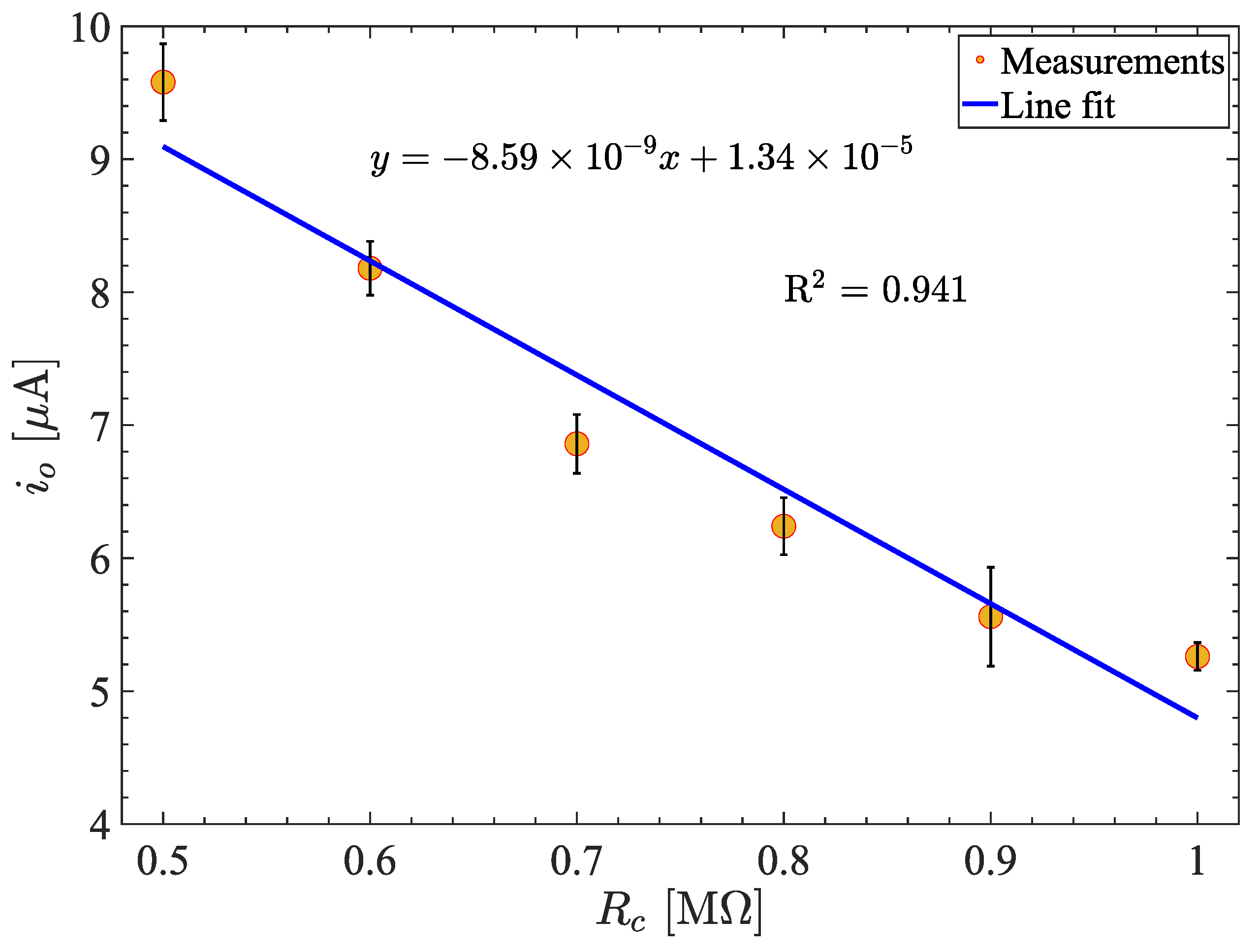


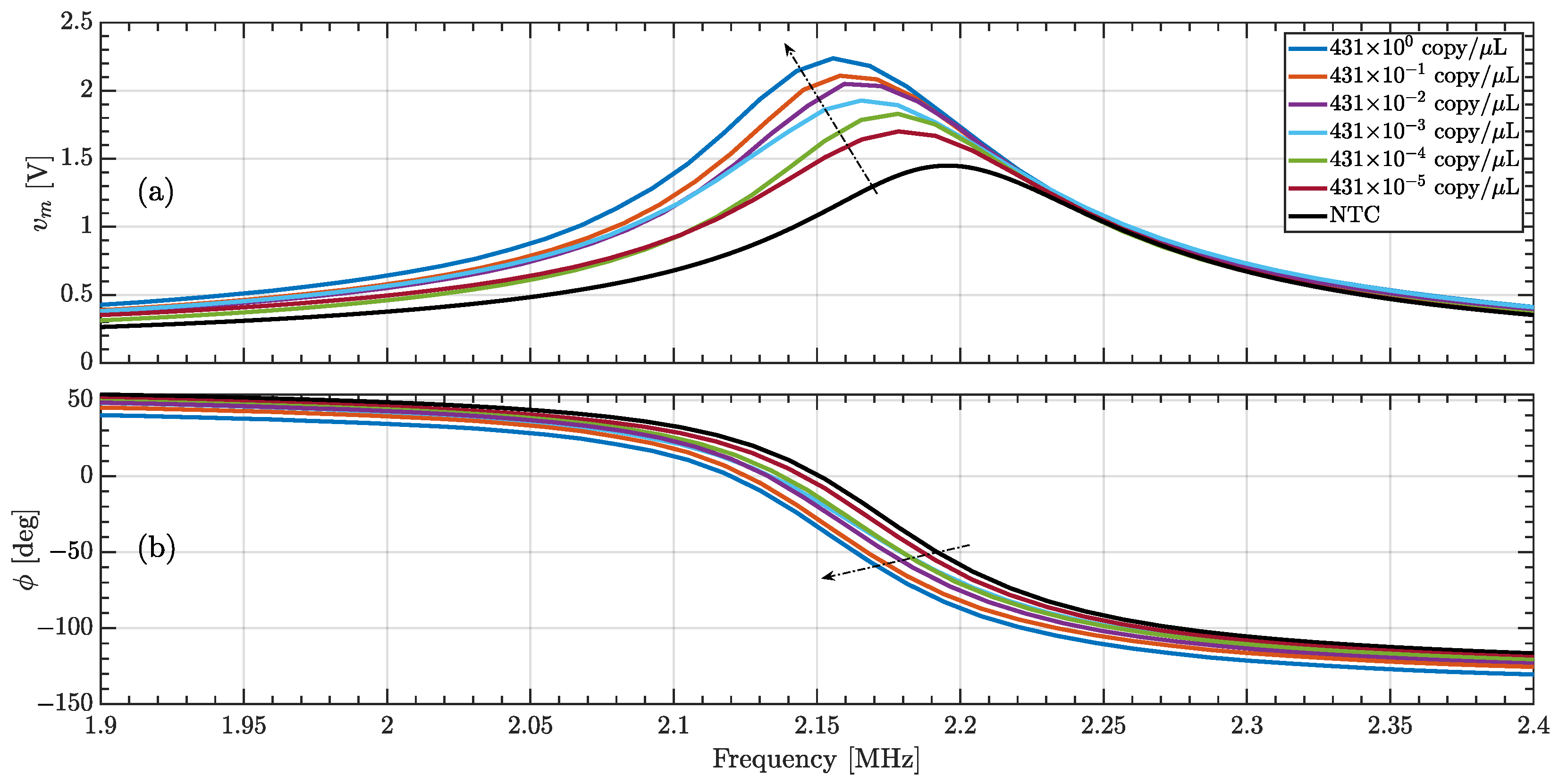
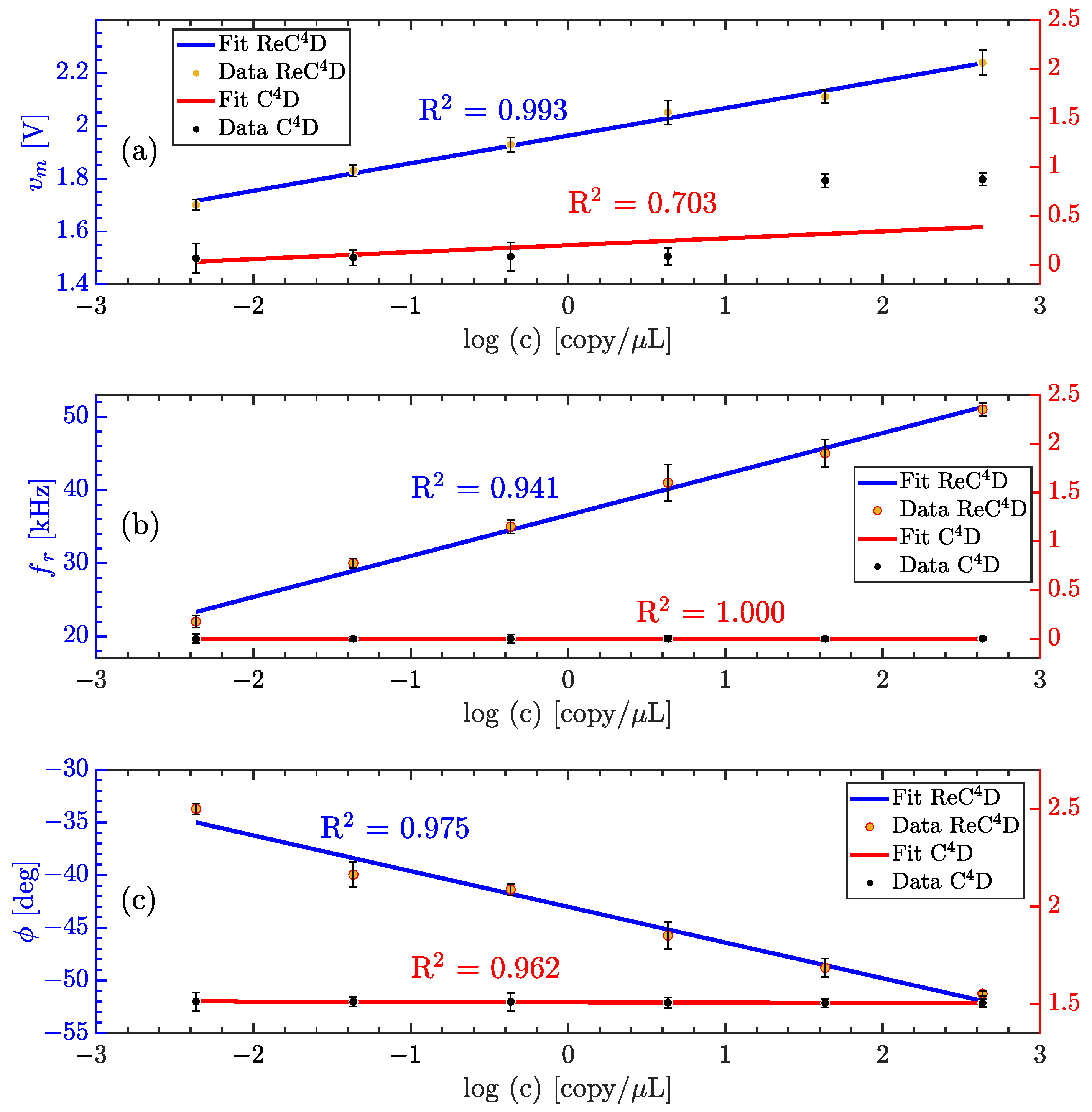
| [M] | [V] | [A] | [A] | %E |
|---|---|---|---|---|
| 0.5 | 4.79 | 9.85 | 10.00 | 4.22 |
| 0.6 | 4.09 | 8.18 | 8.33 | 1.79 |
| 0.7 | 3.43 | 6.87 | 7.17 | 3.85 |
| 0.8 | 3.12 | 6.24 | 6.25 | 0.09 |
| 0.9 | 2.78 | 5.57 | 5.56 | 0.23 |
| 1.0 | 2.63 | 5.26 | 5.00 | 5.21 |
| Detection Parameter | Sensitivity |
|---|---|
| 0.371 | |
| 88.060 | |
| 80.260 |
| Parameter | Sensitivity ReD | LoD ReD | Sensitivity D | LoD D |
|---|---|---|---|---|
| 0.071 | 0.61 | 0.183 | 1.66 | |
| 5.618 | 0.24 | N/A | N/A | |
| 3.381 | 0.41 | 0.017 | 0.83 |
| Sensing Technology | Sample | NAA Method | Target | LOD | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|
| Electrochemical sensor | Wastewater | RT-LAMP | ORF1ab and N genes | 0.038 pg/L | A simple and cost-effective sensor for resource-limited environments. The device is capable of monitoring the reaction time course of RT-LAMP at low concentrations. | The electrodes are disposable after each measurement. The RT-LAMP mix requires methylene blue as a redox probe to obtain a diffusion-controlled electrochemical response. | [45] |
| Plasmonic sensor based on gold and silver alloy nanoshells | Synthetic | LAMP | N gene | 10 copies/reaction | The plasmonic sensor-based detection technique shows better sensitivity and specificity than the LAMP kits commercially available. The NAA method eliminates non-template amplification contamination. | The sensors are not cost-effective as they require gold and silver alloy nanoshells. LAMP method requires 75 min of analysis time. | [50] |
| Microfluidic-integrated lateral flow assay | Clinical | RT-RPA | N gene | 1 copy/L | The assay is simple and accessible, as the genetic material is loaded, then incubated and mixed on the microfluidic platform. The microfluidic platform protects the sample from cross-contamination and allows for sensitive detection. | It requires approximately 30 minutes of incubation. The assay can be used as a complement to RT-PCR. | [51] |
| Surface-enhanced Raman scattering assay using Au nanodimple substrate | Synthetic | RT-PCR | E and RdRP genes | N.R. | The platform reduces the number of cycles needed to amplify genetic material compared to conventional RT-PCR amplification. By reducing the amplification time, the analysis and diagnostic time is also drastically shortened. | It requires the preparation of a gold substrate internalized with nanoparticles to shorten the amplification time, which is not cost-effective and requires additional processes. The measurements require Raman spectroscopy equipment, which is expensive and not easily accessible in resource-limited environments. | [52] |
| Lateral flow immunoassay based on fluorescein | Clinical | PCR | N and E genes | N.R. | The detection method provides an alternative for rapid PCR amplicon detection for qualitative testing. The amplification method, coupled with the immunoassay platform, is more cost-effective than conventional assays. | The primers used in the amplification require double labeling to enable detection. It is a qualitative test that requires 1 h and 20 min to deliver results. | [53] |
| CRISPR-Cas12a fluorescence detection | Saliva | RT-LAMP and RT-RPA | N and E genes | N.R. | The amplification and detection method provide results with performance similar to RT-qPCR in saliva samples. The optimization of measurements allows for a relationship between genetic amplification and fluorescence levels, which can be measured with minimal hardware. | The assay can only provide qualitative results at different stages of amplification. The preparation of the mixtures for CRISPR-Cas12a detection requires specialized preparation to control stability and make it replicable in resource-limited environments. | [54] |
| Resonance-induced capacitively coupled contactless conductivity detection | Synthetic | RT-LAMP | ORF1ab and N genes | 0.24 copy/L | The RT-LAMP amplification mixture and method do not require modifications or additional reagents. The detection method offers a reusable device, as it does not come into direct contact with the sample, and the sensor element does not require a bioreceptor or functionalization. | The sample is at risk of contamination from the air when placed on the sensor surface without any protection. The preparation of the RT-LAMP mixture solution and the experimental conditions must be controlled by the experimenter to ensure reproducibility. | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Chavarría, R.G.; Uc-Martín, J.A.; Alvarez-Serna, B.E.; Padilla-Morán, R.F. Resonance-Induced Capacitively Coupled Contactless Conductivity Detection (ReC4D) Unit for Nucleic Acid Amplification Testing. Technologies 2025, 13, 138. https://doi.org/10.3390/technologies13040138
Ramírez-Chavarría RG, Uc-Martín JA, Alvarez-Serna BE, Padilla-Morán RF. Resonance-Induced Capacitively Coupled Contactless Conductivity Detection (ReC4D) Unit for Nucleic Acid Amplification Testing. Technologies. 2025; 13(4):138. https://doi.org/10.3390/technologies13040138
Chicago/Turabian StyleRamírez-Chavarría, Roberto G., Jorge A. Uc-Martín, Bryan E. Alvarez-Serna, and Ramón F. Padilla-Morán. 2025. "Resonance-Induced Capacitively Coupled Contactless Conductivity Detection (ReC4D) Unit for Nucleic Acid Amplification Testing" Technologies 13, no. 4: 138. https://doi.org/10.3390/technologies13040138
APA StyleRamírez-Chavarría, R. G., Uc-Martín, J. A., Alvarez-Serna, B. E., & Padilla-Morán, R. F. (2025). Resonance-Induced Capacitively Coupled Contactless Conductivity Detection (ReC4D) Unit for Nucleic Acid Amplification Testing. Technologies, 13(4), 138. https://doi.org/10.3390/technologies13040138









