Evaluation of Operational Efficiency in China’s Pharmaceutical Industry and Analysis of Environmental Impacts
Abstract
:1. Introduction
2. Literature Review
2.1. The Basic Theory and General Measurement Methods of Efficiency
2.2. Efficiency Measurement Methods: A Comparison of SFA and DEA
2.3. The Method of Three-Stage DEA and Advantage
2.4. The Current Research on Efficiency in the PI
2.4.1. Innovation Efficiency Research
2.4.2. Pharmaceutical Companies’ Financial Efficiency Evaluation
2.5. Research Gap
- (1)
- Current evaluations of the operational efficiency of the PI are based solely on financial or innovation dimensions, lacking comprehensive research.In the innovation dimension, scholars typically use indicators such as sales of new products and the number of patent applications (Hao & Ruan, 2022; Lai et al., 2020; Qiu et al., 2023). Other scholars also evaluate the operational efficiency use the new molecular entity (NME) and impact factors of the publication (Gascón et al., 2017; Schuhmacher et al., 2023, 2021). The financial evaluation dimension includes a broader range of indicators, with operation revenue and operation profit being the most widely used (Gascón et al., 2017; Lin et al., 2021; Yang, 2024). Other financial indicators, such as asset turnover, returns on equity (ROE), and earnings per share (EPS), are also employed (Hamad & Tarnoczi, 2021; Riaz et al., 2023; Xia et al., 2022). To date, the only study that has combined both financial and innovation dimensions in evaluating the operational efficiency of pharmaceutical companies is Gascón et al. (2017). No other research has conducted a combination evaluation of both dimensions. However, the financial indicators primarily reflect a company’s short-term profitability; innovation serves as a measure of its long-term growth potential. Therefore, a separate evaluation of the financial and innovation performance overlooks the balance between short-term and long-term interest.
- (2)
- The current research on the impact of environmental factors on efficiency is limited.Although previous studies have employed three-stage DEA to analyze the impact of the environment on efficiency, the use of a single indicator to represent a dimension lacks a comprehensive understanding of the environment. Qiu et al. (2023) used the number of employees to represent company size, the number of employees with a bachelor’s degree or higher to represent employee quality, and returns on equity (ROE) and the ratio of total liabilities to total assets (LEV) as environmental indicators. Yang (2024) used government subsidies, per capita GDP, and the years that a company has been established to measure and explain the environmental influence on efficiency. Sun et al. (2024), construct environmental indicators using per capita disposable income to represent wealth levels, the working-age population to represent the labor supply, and local GDP to represent the local economic level. Although these studies explore the impact of environmental factors on efficiency from different perspectives, they remain inadequate. Environmental factors are complex, and using a single indicator for regression analysis cannot fully capture the overall environmental impact. Moreover, when too many environmental indicators are included in regression, potential multicollinearity issues may arise, which could compromise the accuracy of regression results (Baird & Bieber, 2016; Haitovsky, 1969; Shrestha, 2020).
2.6. The Conceptual Framework of This Study
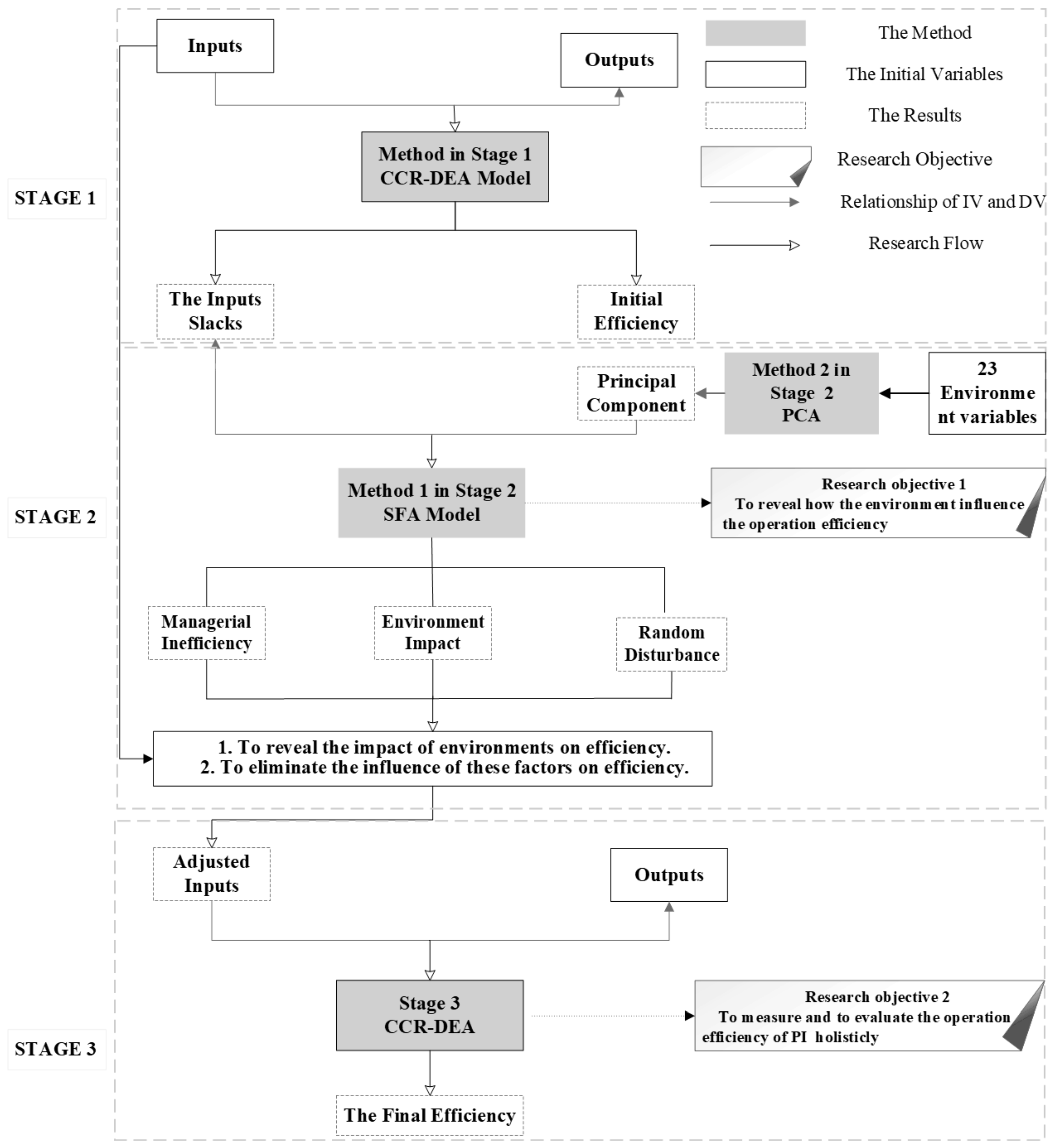
- (1)
- Stage 1: initial efficiency measurement.In this stage, DEA was used to calculate the efficiency values of 31 provincial-level regions in China. The main objective was to measure the current efficiency distribution and calculate the sales of input variables.
- (2)
- Stage 2: environmental factor analysis and adjustment.At the beginning of this stage, PCA was employed to extract principle components from multiple potential environmental variables. The environmental variable values were calculated based on the variance contribution of each component. The extracted environmental variables were used as independent variables (IV), and the slacks of the inputs from Stage 1 were used as the dependent variable (DV) in the SFA regression. This stage aimed to reveal how the comprehensive environment influences efficiency and then eliminate the impact of environmental factors and random disturbances on the input variables by adjusting the input indicators.
- (3)
- Stage 3: efficiency adjustment and recalculation.Adjusted inputs were obtained in Stage 2, and the original output was employed in this stage to recalculate the final and accurate efficiency using the DEA model. This stage aimed to measure the accurate efficiency of PI in China while removing the effects of environmental factors.
3. Materials and Methods
3.1. Data Source and Software
3.2. Theratical Model
3.2.1. BCC-DEA Model
3.2.2. SFA Model
3.2.3. PCA Method
- (1)
- Variable standardization: Standardize the original data so that all variables have the same scale. Due to significant scale differences between the pharmaceutical industries across provinces and large variations in the data, this study applied the min–max normalization method for standardization).
- (2)
- Calculation of the correlation or covariance matrix: compute the correlation matrix or covariance matrix based on the standardized data.
- (3)
- Eigenvalue and eigenvector decomposition: perform eigenvalue decomposition of the correlation or covariance matrix to obtain the eigenvalues and their corresponding eigenvectors.
- (4)
- Selection of principal components: select the principal components that explain most of the variance, usually choosing components that cumulatively explain more than 60% of the variance.
- (5)
- Calculation of principal component scores: Use the eigenvectors as weights to calculate the projections of the original data along the principal component axes, resulting in principal component scores. Through these steps, PCA reduces the number of initial variables, retaining principal components that carry the main information and form the underlying structure of the data.
3.3. The Design of the Indicators
4. Empirical Result
4.1. Positive Verification of Input-Output Variables
4.2. Efficiency Measurement Result in the First Stage
4.3. The Results of the SFA Regression in Stage 2
4.3.1. Extraction of Environmental Variables
- (1)
- Step 1: Method suitability verification.The suitability of the data for principal component analysis (PCA) was verified using the Kaiser–Meyer–Olkin (KMO) measure, and cumulative variance was explained. When the KMO value is greater than 0.6, it indicates strong correlations between the variables, making the data suitable for PCA. If the KMO value is below 0.6, it suggests weak correlations between the variables, making the data unsuitable for PCA. Additionally, when the cumulative variance explained is 70% or higher, it indicates that the extracted principal components effectively explain the majority of the variation in the original data, making the data suitable for further analysis. Conversely, if the cumulative variance explained is below 70%, it suggests that the extracted components do not adequately capture the main information in the data (Hair et al., 2010). Table 4 shows the KMO measure and Bartlett’s test results, while Table 5 presents the analysis of cumulative variance before and after rotation.As shown in Table 4, the KMO value is 0.902, which exceeds the acceptable threshold of 0.60, indicating that these environmental factors are well suited for the principal PCA method. Furthermore, Bartlett’s test of sphericity results (, ) demonstrate significant correlations between the variables, further validating the appropriateness of conducting PCA.As shown in Table 5, after PCA was applied, only four main principle components were extracted from the 23 potential environmental factors. These principal components collectively already explain 89.35% of the total variance, indicating a very high level of explanatory power in the dataset.
- (2)
- Step 2: Rotated component.In PCA, the initially extracted components may be complex, with variable loadings that are difficult to clearly distinguish, which hinders the interpretation of the results. Therefore, rotating the components helps simplify the structure of variable loadings, allowing each variable to concentrate on a few main components. This increases the interpretability of the principal components, clarifies their actual meaning, and enhances the explanatory power regarding the study subject. Table 6 presents the results of the rotated component loading matrix.Based on the results of the rotated component matrix in Table 6, four principal components were extracted. To better interpret the environmental factors reflected by each principal component, only variables with loadings greater than 0.5 were retained. For the purpose of the subsequent efficiency analysis, the extracted factors were classified and named according to the characteristics of the principal components.
- (3)
- Step 3: Name component.Principal Component 1: economic and technological foundation level ().The first principal component includes variables such as regional economic levels, government fiscal revenue, and government investments in technology, education, innovation, and technological outputs. Therefore, this component reflects the region’s economic development and innovation capacity, and it is named “Economic and technological foundation level”.Principal Component 2: residents’ living standards ().The second principal component comprises variables like per capita GDP, resident income, and consumption levels, which reflect the living quality and economic status of the region’s residents. Thus, this component is named “Residents’ Living Standards”.Principal Component 3: local pollution levels ().The third principal component primarily consists of waste emissions and pollution levels in the natural environment. The higher the pollutant emissions, the higher the local pollution level, which is why this component is named “Local Pollution Level”.Principal Component 4: openness to the foreign market ().The fourth principal component is mainly composed of variables related to foreign investment, reflecting the region’s level of openness and its ability to attract foreign capital. Hence, this component is named “Openness to the foreign market”.
4.3.2. The Result of the SFA Regression
4.4. The Result of Stage 3
5. Discussion
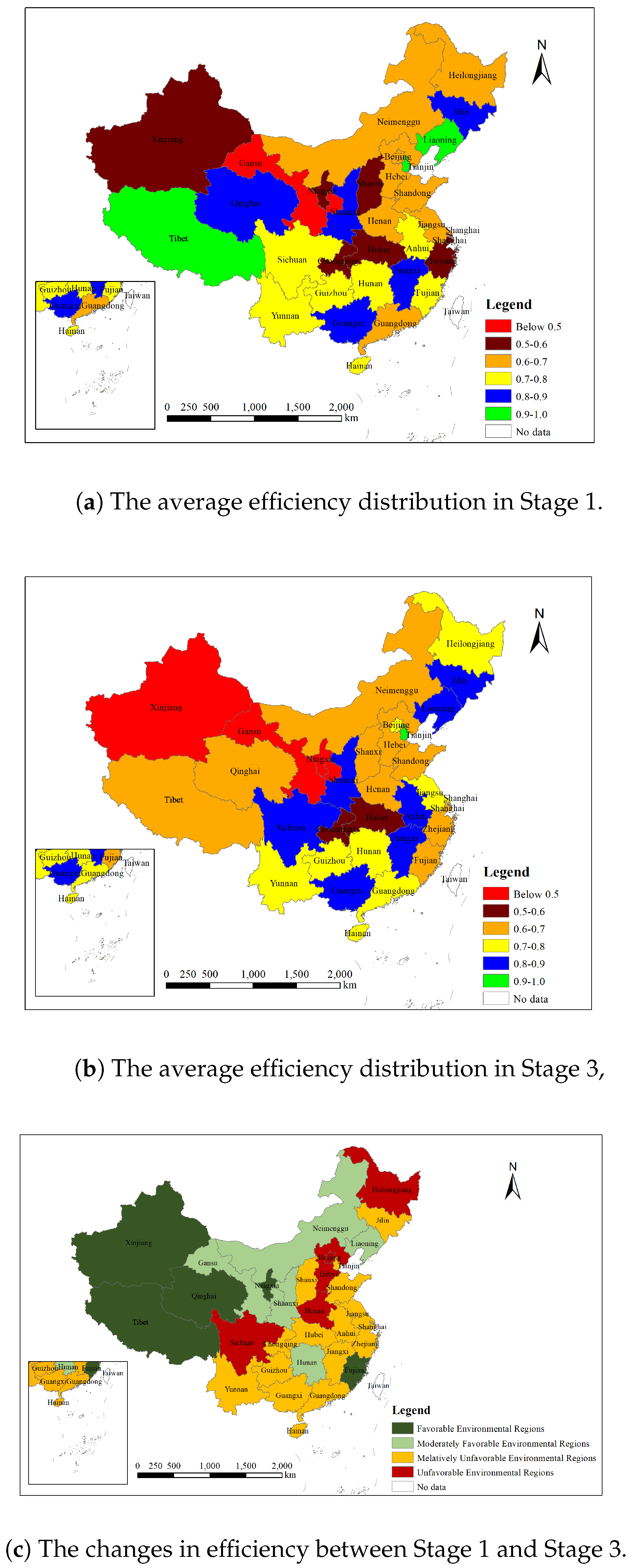
5.1. Discussion on the Efficiency Distribution of the Pharmaceutical Industry Across China’s Regions
- (1)
- Efficiency analysis of pharmaceutical enterprises in first-tier regions.Pharmaceutical enterprises in first-tier regions, including Tianjin, Liaoning, and Jiangxi, exhibit high PTE and SE. These regions demonstrate strong resource allocation capabilities and significant economies of scale, leading to higher overall efficiency. While the economic strength of these regions is not the highest, they effectively leverage abundant human resources, low labor costs, and strong government support to optimize resource utilization. Through the synergistic effects of technology, resources, and policies, these regions have developed their unique high-efficiency models. Additionally, the well-established industrial support foundation in these regions promotes inter-industry coordination, which further enhances both technical and scale efficiencies.
- (2)
- Efficiency analysis of pharmaceutical enterprises in second-tier regions.Pharmaceutical enterprises in second-tier regions also show high PTE but relatively low SE. These regions include Jiangsu, Shandong, Guangdong, and Ningxia. Except for Ningxia, the other provinces (Jiangsu, Shandong, Guangdong) are among the economically strongest in China. According to the SFA analysis, the high efficiency in Ningxia is primarily due to favorable environmental factors such as strong national support, a large environmental capacity, and the relatively low income and living standards of the local population, which together create a high level of resource allocation.However, enterprises in Jiangsu, Shandong, and Guangdong, despite their strong performance in technical innovation and resource allocation, efficiently utilize technology and management methods to achieve high technical efficiency. These regions’ enterprises possess advanced production technologies and robust R&D capabilities. However, despite their strengths in technological efficiency, they have struggled to achieve economies of scale during expansion, resulting in a persistent situation of decreasing returns to scale (DRS) over the past decade. This phenomenon can be attributed to factors such as market saturation, with enterprises finding it difficult to match increased production capacity with sufficient market demand, inefficient resource allocation, despite strong technological performance, with issues in the distribution of resources such as funds, talent, and equipment, and management bottlenecks, through which the original management system is unable to adapt to the complexity brought about via scale expansion, thereby limiting the improvement of scale efficiency.
- (3)
- Efficiency analysis of pharmaceutical enterprises in third-tier regions.Pharmaceutical enterprises in third-tier regions show high SE but relatively low PTE. These regions include Beijing, Shanghai, Chongqing, Hunan, Henan, and Yunnan. The potential causes for the low PTE in these regions vary. For instance, in Chongqing, Beijing, and Shanghai, although these regions possess strong economic and technological foundations, they are hindered by strict environmental and safety policies. High environmental protection requirements in places like Beijing and Shanghai have restricted investment in technological upgrades and innovation, thereby limiting improvements in technical efficiency. Additionally, high production factor costs, such as labor, land, and energy prices, have become significant obstacles to enhancing technological performance. Despite these constraints, enterprises in these regions can achieve economies of scale through market size advantages and industrial agglomeration effects, which allow them to lower unit costs through increased production.In contrast, enterprises in regions like Hunan and Yunnan, despite facing challenges in technical efficiency, have performed well in scale efficiency. These regions’ enterprises have relatively limited investments in technological innovation and R&D, which results in stagnation in technological progress and affects their production efficiency. However, due to lower production factor costs and government policy support, such as tax reductions and industrial subsidies, enterprises in these regions can achieve economies of scale. The local government’s policies further enhance the efficiency of enterprises when expanding production to meet the growing market demand, thereby boosting scale efficiency.
- (4)
- Efficiency analysis of pharmaceutical enterprises in fourth-tier regions.Pharmaceutical enterprises in fourth-tier regions, such as Gansu and Shanxi, generally exhibit low TE and PTE. These regions’ enterprises mainly rely on traditional technologies and relatively extensive management models, lacking advanced technical support and process optimization. As a result of insufficient technological innovation, the production efficiency in these regions is low. Moreover, the geographical locations and economic foundations of Gansu and Shanxi make them less attractive for highly skilled talent and technological capital, preventing enterprises from absorbing external advanced technologies and management experience. The severe shortage and outflow of local talent have further constrained technological improvements. Additionally, the weak economic foundation and limited government support for technological innovation and research restrict the ability of enterprises to invest in necessary upgrades, leading to a lack of improvement in technical and pure technical efficiency.
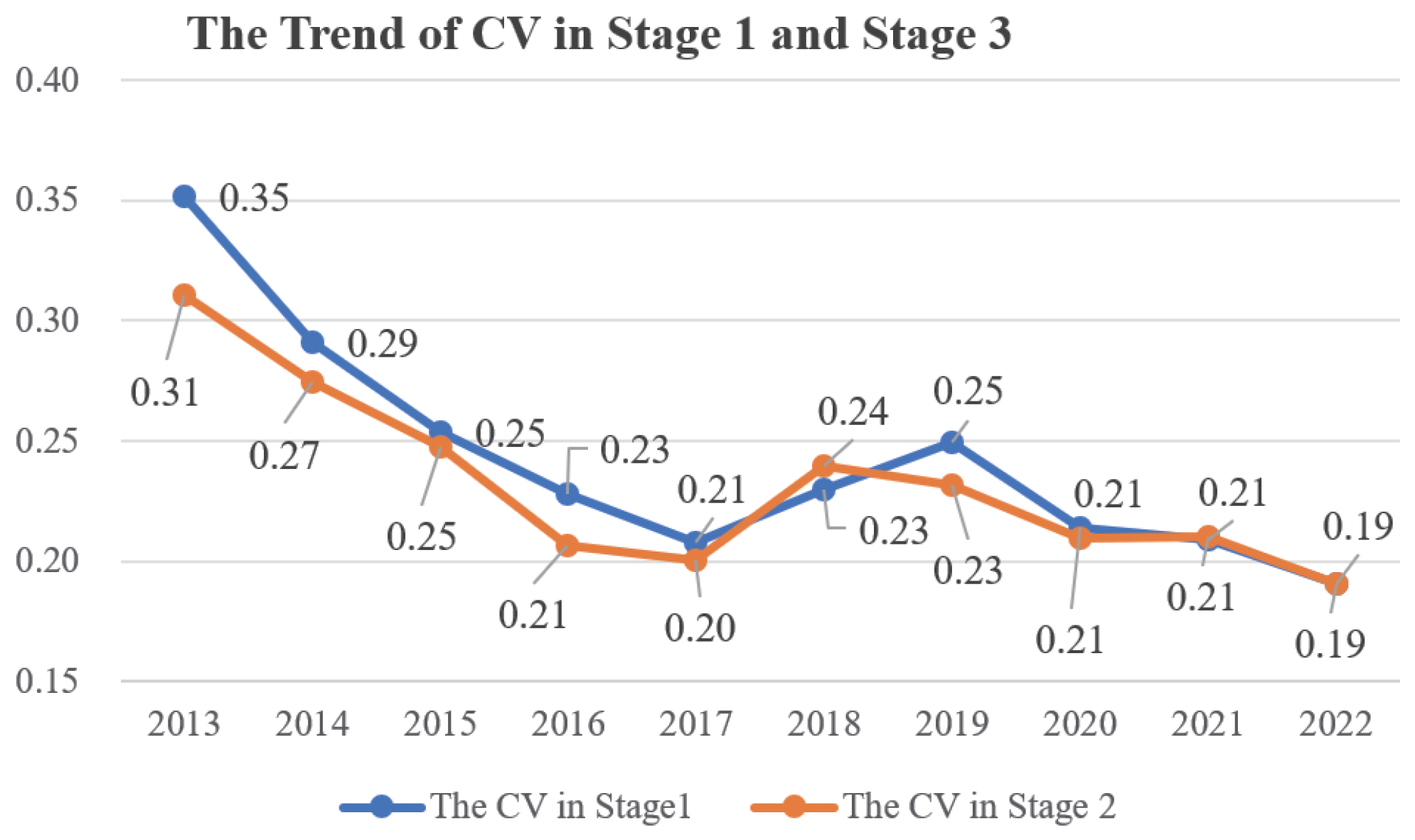
5.2. Discussion on the Changes in Regional Efficiency and Their Causes
5.3. The Discussion of Regional Disparities in China’s Pharmaceutical Industry
5.4. Discussion of the Impact of the Environment in Different Regions
5.5. The Relationship with Existing Research
6. Conclusions
6.1. Main Findings of This Study
6.2. Implication of This Study for Policy-Makers
6.3. Limitations of This Study
6.4. Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
| 1 | Note: This study’s data sources do not include Hong Kong, Macau, or Taiwan, as their statistical data are processed separately in official Chinese reports. |
References
- Ahmed, M. H., & Melesse, K. A. (2018). Impact of off-farm activities on technical efficiency: Evidence from maize producers of eastern Ethiopia. Agricultural and Food Economics, 6(1), 1–15. [Google Scholar] [CrossRef]
- Aigner, D., Lovell, C. K., & Schmidt, P. (1977). Formulation and estimation of stochastic frontier production function models. Journal of Econometrics, 6(1), 21–37. [Google Scholar] [CrossRef]
- André, F. J., Buendía, A., & Santos-Arteaga, F. J. (2024). Efficient water use and reusing processes across Spanish regions: A circular data envelopment analysis with undesirable inputs. Journal of Cleaner Production, 434, 139929. [Google Scholar] [CrossRef]
- Avkiran, N. K., & Rowlands, T. (2008). How to better identify the true managerial performance: State of the art using DEA. Omega, 36(2), 317–324. [Google Scholar] [CrossRef]
- Bai, H., Irfan, M., & Hao, Y. (2022). How does industrial transfer affect environmental quality? Evidence from China. Journal of Asian Economics, 82, 101530. [Google Scholar] [CrossRef]
- Baird, G. L., & Bieber, S. L. (2016). The Goldilocks dilemma: Impacts of multicollinearity—A comparison of simple linear regression, multiple regression, and ordered variable regression models. Journal of Modern Applied Statistical Methods, 15(1), 18. [Google Scholar]
- Banker, R. D., Charnes, A., & Cooper, W. W. (1984). Some models for estimating technical and scale inefficiencies in data envelopment analysis. Management Science, 30(9), 1078–1092. [Google Scholar] [CrossRef]
- Barney, J. (1991). Firm resources and sustained competitive advantage. Journal of Management, 17(1), 99–120. [Google Scholar] [CrossRef]
- Barney, J. B., & Arikan, A. M. (2005). The resource-based view: Origins and implications. In The Blackwell handbook of strategic management (pp. 123–182). Blackwell Publishers Ltd. [Google Scholar]
- Bhardwaj, R. (2024). Economics of the pharmaceutical and medical device industry: Supply chain, trade and innovation. Taylor & Francis. [Google Scholar] [CrossRef]
- Borja Reis, C. F. d., & Pinto, J. P. G. (2022). Center–periphery relationships of pharmaceutical value chains: A critical analysis based on goods and knowledge trade flows. Review of Political Economy, 34(1), 124–145. [Google Scholar] [CrossRef]
- Cai, L., & Sun, Y. (2013). Research on the efficiency of biopharmaceutical enterprises based on DEA and SFA. Science Technology and Management Research, 33(2), 24. [Google Scholar]
- Camanho, A. S., Silva, M. C., Piran, F. S., & Lacerda, D. P. (2024). A literature review of economic efficiency assessments using Data Envelopment Analysis. European Journal of Operational Research, 315(1), 1–18. [Google Scholar] [CrossRef]
- Charnes, A., Cooper, W. W., & Rhodes, E. (1978). Measuring the efficiency of decision making units. European Journal of Operational Research, 2(6), 429–444. [Google Scholar] [CrossRef]
- Chaturvedi, P., Shukla, P., Giri, B. S., Chowdhary, P., Chandra, R., Gupta, P., & Pandey, A. (2021). Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environmental Research, 194, 110664. [Google Scholar] [CrossRef] [PubMed]
- Chen, G., & Chen, F. (2024). Changes in technological innovation efficiency and influencing factors of listed textile and apparel companies research—Based on three-stage DEA with Tobit modeling. PLoS ONE, 19(8), e0307820. [Google Scholar] [CrossRef]
- Chen, P., Ding, X., Chen, M., Song, H., & Imran, M. (2025). The impact of resource spatial mismatch on the configuration analysis of agricultural green total factor productivity. Agriculture, 15(1), 23. [Google Scholar] [CrossRef]
- Coelli, T. J., Rao, D. S. P., O’donnell, C. J., & Battese, G. E. (2005). An introduction to efficiency and productivity analysis. Springer Science & Business Media. [Google Scholar] [CrossRef]
- Colm, G. (1960). The theory of public finance: A study in public economy. JSTOR. [Google Scholar]
- Cooper, W. W., Seiford, L. M., & Tone, K. (2007). Data envelopment analysis: A comprehensive text with models, applications, references and dea-solver software (Vol. 2). Springer. [Google Scholar] [CrossRef]
- Deore, A. B., Dhumane, J. R., Wagh, R., & Sonawane, R. (2019). The stages of drug discovery and development process. Asian Journal of Pharmaceutical Research and Development, 7(6), 62–67. [Google Scholar] [CrossRef]
- Dou, J., & Han, X. (2019). How does the industry mobility affect pollution industry transfer in China: Empirical test on Pollution Haven Hypothesis and Porter Hypothesis. Journal of Cleaner Production, 217, 105–115. [Google Scholar] [CrossRef]
- Dyson, R. G., Allen, R., Camanho, A. S., Podinovski, V. V., Sarrico, C. S., & Shale, E. A. (2001). Pitfalls and protocols in DEA. European Journal of Operational Research, 132(2), 245–259. [Google Scholar] [CrossRef]
- Farrell, M. J. (1957). The measurement of productive efficiency. Journal of the Royal Statistical Society Series A: Statistics in Society, 120(3), 253–281. [Google Scholar] [CrossRef]
- Feng, M., & Li, X. (2020). Evaluating the efficiency of industrial environmental regulation in China: A three-stage data envelopment analysis approach. Journal of Cleaner Production, 242, 118535. [Google Scholar] [CrossRef]
- Fried, H. O., Lovell, C. K., Schmidt, S. S., & Yaisawarng, S. (2002). Accounting for environmental effects and statistical noise in data envelopment analysis. Journal of Productivity Analysis, 17, 157–174. [Google Scholar] [CrossRef]
- Friedman, M. (2007). The social responsibility of business is to increase its profits. In Corporate ethics and corporate governance (pp. 173–178). Springer. [Google Scholar]
- Gascón, F., Lozano, J., Ponte, B., & de la Fuente, D. (2017). Measuring the efficiency of large pharmaceutical companies: An industry analysis. The European Journal of Health Economics, 18, 587–608. [Google Scholar] [CrossRef] [PubMed]
- Ge, X., Imran, M., & Ali, K. (2024). Natural resource-driven prosperity: Unveiling the catalysts of sustainable economic development in the United States. In Natural resources forum. Blackwell Publishing Ltd. [Google Scholar]
- Greenacre, M., Groenen, P. J., Hastie, T., d’Enza, A. I., Markos, A., & Tuzhilina, E. (2022). Principal component analysis. Nature Reviews Methods Primers, 2(1), 100. [Google Scholar] [CrossRef]
- Greene, W. H. (2008). The econometric approach to efficiency analysis. The Measurement of Productive Efficiency and Productivity Growth, 1(1), 92–250. [Google Scholar]
- Grossman, S. J., & Stiglitz, J. E. (1977). On value maximization and alternative objectives of the firm. The Journal of Finance, 32(2), 389–402. [Google Scholar]
- Guanglan, Z., & Zhening, Z. (2024). Evaluation and influencing factors of regional environmental efficiency in China based on three-stage DEA model. SAGE Open, 14(4), 21582440241280825. [Google Scholar] [CrossRef]
- Guo, C., Song, Q., Yu, M.-M., & Zhang, J. (2024). A digital economy development index based on an improved hierarchical data envelopment analysis approach. European Journal of Operational Research, 316(3), 1146–1157. [Google Scholar] [CrossRef]
- Guo, H., & Shi, K. (2021). Evaluation of the international competitiveness of China’s pharmaceutical industry and high-quality development strategies during the 14th Five-Year Plan. Journal of Beijing University of Technology (Social Sciences Edition), 21(3), 65–79. [Google Scholar] [CrossRef]
- Guo, Y., Yu, M.-M., & See, K. F. (2024). Developing a sustainable development goals index for OECD countries: An effectiveness-based hierarchical data envelopment analysis. Environmental Science & Policy, 160, 103836. [Google Scholar] [CrossRef]
- Hair, J., Black, W., Babin, B., & Anderson, R. (2010). Multivariate data analysis (7th ed.). Prentice Hall. [Google Scholar]
- Haitovsky, Y. (1969). Multicollinearity in regression analysis: Comment. The Review of Economics and Statistics, 51, 486–489. [Google Scholar] [CrossRef]
- Hamad, M., & Tarnoczi, T. (2021). Efficiency analysis of companies operating in the pharmaceutical industry in the Visegrad countries. Intellectual Economics, 15(2), 131–155. [Google Scholar] [CrossRef]
- Hao, B., & Ruan, X. (2022). Research on the technological innovation efficiency of Chinese listed biopharmaceutical enterprises based on the two-stage DEA model. China Pharmacy, 12, 7–12. [Google Scholar] [CrossRef]
- Hjalmarsson, L., Kumbhakar, S. C., & Heshmati, A. (1996). DEA, DFA and SFA: A comparison. Journal of Productivity Analysis, 7, 303–327. [Google Scholar] [CrossRef]
- Imran, M., Alam, M. S., Jijian, Z., Ozturk, I., Wahab, S., & Doğan, M. (2024a). From resource curse to green growth: Exploring the role of energy utilization and natural resource abundance in economic development. In Natural resources forum. Blackwell Publishing Ltd. [Google Scholar] [CrossRef]
- Imran, M., & Jijian, Z. (2023). Exploring the relationship between financial inclusion and natural resource utilization in QUAD economies. Environmental Science and Pollution Research, 30(58), 122958–122971. [Google Scholar] [CrossRef]
- Imran, M., Khan, M. K., Alam, S., Wahab, S., Tufail, M., & Jijian, Z. (2024b). The implications of the ecological footprint and renewable energy usage on the financial stability of South Asian countries. Financial Innovation, 10(1), 102. [Google Scholar] [CrossRef]
- Imran, M., Khan, M. K., Wahab, S., Ahmed, B., & Jijian, Z. (2025a). The paradox of resource-richness: Unraveling the effects on financial markets in natural resource abundant economies. Financial Innovation, 11(1), 63. [Google Scholar] [CrossRef]
- Imran, M., Sattar, A., & Alam, M. S. (2024c). Heterogeneous analysis of free trade agreement between Pakistan and China: A policy guideline for CPEC. Journal of Economic and Administrative Sciences, 40(1), 76–94. [Google Scholar] [CrossRef]
- Imran, M., Tufail, M., Mo, C., Wahab, S., Khan, M. K., Hoo, W. C., & Ling, Z. (2025b). From resources to resilience: Understanding the impact of standard of living and energy consumption on natural resource rent in Asia. Energy Strategy Reviews, 57, 101590. [Google Scholar] [CrossRef]
- Imran, M., Zhang, G., & An, H. (2017). Impact of market access and comparative advantage on regional distribution of manufacturing sector. China Finance and Economic Review, 5, 1–14. [Google Scholar] [CrossRef]
- Jia, F. (2022). Research on the international competitiveness of China’s pharmaceutical industry from the perspective of the global value chain [Unpublished Master’s thesis, Kunming University of Science and Technology]. [Google Scholar]
- Joe, Z., & Wu, G. (1996). Dea theory, methods and applications. Science Press. [Google Scholar]
- Kalirajan, K. P., & Shand, R. (1994). Stochastic frontier production functions and technical efficiency measurements: A review. Palgrave Macmillan. [Google Scholar] [CrossRef]
- Kaur, N., & Singh, B. (2024). Dynamics of resource allocation–firm performance relationship: Panel autoregressive distributed lag approach. Managerial and Decision Economics, 45(6), 3663–3676. [Google Scholar]
- Krugman, P. (1991). Increasing returns and economic geography. Journal of Political Economy, 99(3), 483–499. [Google Scholar] [CrossRef]
- Kumbhakar, S. C., & Tsionas, E. G. (2011). Some recent developments in efficiency measurement in stochastic frontier models. Journal of Probability and Statistics, 2011(1), 603512. [Google Scholar] [CrossRef]
- Lai, H., Shi, H., & Zhou, Y. (2020). Regional technology gap and innovation efficiency trap in Chinese pharmaceutical manufacturing industry. PLoS ONE, 15(5), e0233093. [Google Scholar] [CrossRef]
- Lampe, H. W., & Hilgers, D. (2015). Trajectories of efficiency measurement: A bibliometric analysis of DEA and SFA. European Journal of Operational Research, 240(1), 1–21. [Google Scholar] [CrossRef]
- Lee, G. H., & Jeon, H. W. (2023). A stochastic frontier analysis (SFA)-based method for detecting changes in manufacturing energy efficiency by sector and time. In IFIP international conference on advances in production management systems (pp. 29–42). Springer. [Google Scholar]
- Li, S., & Fan, C. (2009). A review and comparison of SFA and DEA methods. Statistics and Decision, 7(283), 25–28. [Google Scholar]
- Lin, T.-X., Wu, Z.-h., Ji, X.-x., & Yang, J.-j. (2021). Research on the operating efficiency of Chinese listed pharmaceutical companies based on two-stage network DEA and Malmquist. Mathematical Problems in Engineering, 2021(1), 1475781. [Google Scholar] [CrossRef]
- Liu, T., Pan, S., Hou, H., & Xu, H. (2020). Analyzing the environmental and economic impact of industrial transfer based on an improved CGE model: Taking the Beijing–Tianjin–Hebei region as an example. Environmental Impact Assessment Review, 83, 106386. [Google Scholar] [CrossRef]
- Liu, Y., Zhang, N., Xie, C., Jiang, Y., Qin, Y., Zhou, L., Fan, Y., Ren, L., Yin, C., Yang, H., Xie, W., Zhai, Q., Li, G., Chen, H., & Chen, X. (2022). Evolution of drug regulations and regulatory innovation for anticancer drugs in China. Acta Pharmaceutica Sinica B, 12(12), 4365–4377. [Google Scholar] [CrossRef]
- Luo, D. (2012). A note on estimating managerial inefficiency of three-stage DEA model. Statistical Research, 29(4), 104–107. [Google Scholar]
- Ma, Z. X. (2010). Data envelopment analysis models and methods. Science Press. [Google Scholar]
- Madabushi, R., Seo, P., Zhao, L., Tegenge, M., & Zhu, H. (2022). Role of model-informed drug development approaches in the lifecycle of drug development and regulatory decision-making. Pharmaceutical Research, 39(8), 1669–1680. [Google Scholar] [CrossRef]
- Madaleno, M., & Moutinho, V. (2023). Stochastic frontier analysis: A review and synthesis. Advanced Mathematical Methods for Economic Efficiency Analysis, 692, 55–78. [Google Scholar] [CrossRef]
- Martin, R., & Sunley, P. (2003). Deconstructing clusters: Chaotic concept or policy panacea? Journal of Economic Geography, 3(1), 5–35. [Google Scholar] [CrossRef]
- Mergoni, A., Emrouznejad, A., & De Witte, K. (2024). Fifty years of data envelopment analysis. European Journal of Operational Research. [Google Scholar] [CrossRef]
- Mohs, R. C., & Greig, N. H. (2017). Drug discovery and development: Role of basic biological research. Alzh Dement-Trci, 3(4), 651–657. [Google Scholar] [CrossRef] [PubMed]
- Moulay Ali, H., Guellil, M. S., Mokhtari, F., & Tsabet, A. (2024). The effect of subsidies on tecnical efficiency of Algerian agricultural sector: Using stochastic frontier model (SFA). Discover Sustainability, 5(1), 98. [Google Scholar] [CrossRef]
- Na, H., Du, T., Sun, W., He, J., Sun, J., Yuan, Y., & Qiu, Z. (2019). Review of evaluation methodologies and influencing factors for energy efficiency of the iron and steel industry. International Journal of Energy Research, 43(11), 5659–5677. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China. (2023). China statistical yearbook 2023. China Statistics Press. Available online: https://www.stats.gov.cn (accessed on 27 October 2024).
- Okeke, E. S., Ezeorba, T. P. C., Okoye, C. O., Chen, Y., Mao, G., Feng, W., & Wu, X. (2022). Environmental and health impact of unrecovered API from pharmaceutical manufacturing wastes: A review of contemporary treatment, recycling and management strategies. Sustainable Chemistry and Pharmacy, 30, 100865. [Google Scholar] [CrossRef]
- Đokić, D., Novaković, T., Tekić, D., Matkovski, B., Zekić, S., & Milić, D. (2022). Technical efficiency of agriculture in the European Union and Western Balkans: SFA method. Agriculture, 12(12), 1992. [Google Scholar] [CrossRef]
- Porter, M. E. (1990a). New global strategies for competitive advantage. Planning Review, 18(3), 4–14. [Google Scholar] [CrossRef]
- Porter, M. E. (1990b). The competitive advantage of nations. Macmillan Press LTD. [Google Scholar]
- Porter, M. E. (1998). Clusters and the new economics of competition. Harvard Business Review, 76(6), 77–90. [Google Scholar]
- Qin, Y., Zhang, P., Deng, X., & Liao, G. (2023). Innovation efficiency evaluation of industrial technology research institute based on three-stage DEA. Expert Systems with Applications, 224, 120004. [Google Scholar] [CrossRef]
- Qiu, L., Yu, R., Hu, F., Zhou, H., & Hu, H. (2023). How can China’s medical manufacturing listed firms improve their technological innovation efficiency? An analysis based on a three-stage DEA model and corporate governance configurations. Technological Forecasting and Social Change, 194, 122684. [Google Scholar] [CrossRef]
- Rashid, A. A., See, K. F., & Yu, M.-M. (2024). Measuring airline efficiency using a dynamic network data envelopment analysis in the presence of innovation capital. Technological Forecasting and Social Change, 206, 123457. [Google Scholar] [CrossRef]
- Resti, A. (2000). Efficiency measurement for multi-product industries: A comparison of classic and recent techniques based on simulated data. European Journal of Operational Research, 121(3), 559–578. [Google Scholar] [CrossRef]
- Riaz, M., Kazmi, S. M. A., Iqbal, M. S., & Hussain, A. (2023). Efficiency analysis of pharmaceutical companies in Pakistan: A case study of ten famous companies. Pakistan Journal of Humanities and Social Sciences, 11(3), 3823–3830. [Google Scholar] [CrossRef]
- Schuhmacher, A., Hinder, M., von Stegmann und Stein, A., Hartl, D., & Gassmann, O. (2023). Analysis of pharma R&D productivity—A new perspective needed. Drug Discovery Today, 28(10), 103726. [Google Scholar] [CrossRef]
- Schuhmacher, A., Wilisch, L., Kuss, M., Kandelbauer, A., Hinder, M., & Gassmann, O. (2021). R&D efficiency of leading pharmaceutical companies—A 20-year analysis. Drug Discovery Today, 26(8), 1784–1789. [Google Scholar] [CrossRef]
- Shi, Y., Charles, V., & Zhu, J. (2025). Bank financial sustainability evaluation: Data envelopment analysis with random forest and Shapley additive explanations. European Journal of Operational Research, 321(2), 614–630. [Google Scholar] [CrossRef]
- Shin, K., Lee, D., Shin, K., & Kim, E. (2018). Measuring the efficiency of US pharmaceutical companies based on open innovation types. Journal of Open Innovation: Technology, Market, and Complexity, 4(3), 34. [Google Scholar] [CrossRef]
- Shrestha, N. (2020). Detecting multicollinearity in regression analysis. American Journal of Applied Mathematics and Statistics, 8(2), 39–42. [Google Scholar]
- Singh, P. K., Bhatt, R. K., & Kiran, R. (2020). Investigating allocative efficiency, cost efficiency, technical efficiency using stochastic frontier analysis (SFA) of groundnut farm households: Evidence from a case in India. Custos e Agronegocio on Line, 16(4), 105–129. [Google Scholar]
- Solanki, N., Verma, J., Kumar, S., Seema, Mundlia, J., Saini, R., & Saini, S. (2024). Impact of contract research organizations on pharmaceutical industries: A review. Applied Drug Research, Clinical Trials and Regulatory Affairs, e26673371332371. [Google Scholar] [CrossRef]
- Song, H., & Ma, Y. (2024). Measurement and spatial and temporal characterization of logistics efficiency–Based on a three-stage DEA model. Heliyon, 10(19), e38455. [Google Scholar] [CrossRef] [PubMed]
- Stević, Ž., Miškić, S., Vojinović, D., Huskanović, E., Stanković, M., & Pamučar, D. (2022). Development of a model for evaluating the efficiency of transport companies: PCA–DEA–MCDM model. Axioms, 11(3), 140. [Google Scholar] [CrossRef]
- Sun, J., Rosli, A. B., & Daud, A. (2024). Operational efficiency of pharmaceutical companies in China: Based on three-stage DEA with undesirable outputs. Sustainability, 17(1), 207. [Google Scholar] [CrossRef]
- Tang, Y., & Imran, M. (2024). Trade-offs and synergies: Examining the impact of natural resource rents and energy efficiency on financial development in the RCEP context. In Natural resources forum. Blackwell Publishing Ltd. [Google Scholar] [CrossRef]
- Tang, Y., Yin, M., Yang, W., Li, H., Zhong, Y., Mo, L., Liang, Y., Ma, X., & Sun, X. (2019). Emerging pollutants in water environment: Occurrence, monitoring, fate, and risk assessment. Water Environment Research, 91(10), 984–991. [Google Scholar] [CrossRef]
- Teece, D. J., Pisano, G., & Shuen, A. (1997). Dynamic capabilities and strategic management. Strategic Management Journal, 18(7), 509–533. [Google Scholar] [CrossRef]
- Tone, K. (2001). A slacks-based measure of efficiency in data envelopment analysis. European Journal of Operational Research, 130(3), 498–509. [Google Scholar] [CrossRef]
- Wang, G., & Zhang, S. (2023). Evaluation of the international competitiveness and country dependence of China’s pharmaceutical manufacturing industry based on the global value chain. Science Technology and Industry, 23(13), 16–22. [Google Scholar]
- Wang, J., Han, D., & Wang, Y. (2020). Empirical research on innovation efficiency in China based on SFA model. In Iop conference series: Earth and environmental science (Vol. 474, p. 072055). IOP. [Google Scholar]
- Wang, R., & Duan, Y. (2023). Dynamic comparison on the technical efficiency between China’s EPEs and PEs: Two-dimensional measurement based on SFA. Journal of Cleaner Production, 406, 136986. [Google Scholar] [CrossRef]
- Wang, W., Imran, M., Ali, K., & Sattar, A. (2024). Green policies and financial development in G7 economies: An in-depth analysis of environmental regulations and green economic growth. In Natural resources forum. Blackwell Publishing Ltd. [Google Scholar] [CrossRef]
- Wang, Z., Li, Y., Wang, K., & Huang, Z. (2017). Environment-adjusted operational performance evaluation of solar photovoltaic power plants: A three stage efficiency analysis. Renewable and Sustainable Energy Reviews, 76, 1153–1162. [Google Scholar] [CrossRef]
- Wang, Z., Zhou, Y., Wang, T., & Zhao, N. (2024). Efficiency of construction waste and carbon reduction in the construction industry: Based on improved three stage SBM-DEA model in China. Engineering, Construction and Architectural Management. [Google Scholar] [CrossRef]
- Wei, X., & Zhao, R. (2024). Evaluation and spatial convergence of carbon emission reduction efficiency in China’s power industry: Based on a three-stage DEA model with game cross-efficiency. Science of the Total Environment, 906, 167851. [Google Scholar] [CrossRef]
- Wu, B., Li, J., Yao, Z., Li, X., Wang, W., Wu, Z., & Zhou, Q. (2023). Characteristics and reduction assessment of GHG emissions from crop residue open burning in China under the targets of carbon peak and carbon neutrality. Science of the Total Environment, 905, 167235. [Google Scholar] [CrossRef] [PubMed]
- Xia, F., Cui, Y. Y., Liu, J. P., & He, Y. F. (2022). Analysis of operational efficiency of listed pharmaceutical companies in China from 2013 to 2019. Chinese Pharmaceutical Journal, 31(11), 1–5. [Google Scholar]
- Xiong, A., & Meng, G. (2019). Research on the technological innovation efficiency of pharmaceutical enterprises based on DEA method: A case study of the top 15 listed companies in Shenzhen and Shanghai. Chinese Journal of New Drugs, 6. [Google Scholar] [CrossRef]
- Xu, T., Kang, C., & Zhang, H. (2022). China’s efforts towards carbon neutrality: Does energy-saving and emission-reduction policy mitigate carbon emissions? Journal of Environmental Management, 316, 115286. [Google Scholar] [CrossRef] [PubMed]
- Yan, L. (2012). Empirical study on the regional innovation efficiency evaluation index system in China. Management World, 174–175. [Google Scholar] [CrossRef]
- Yang, R. (2024). Operational efficiency analysis of listed biomedical companies in China: Three-stage DEA and Malmquist index. Advances in Economics and Management Research, 10(1), 264–273. [Google Scholar] [CrossRef]
- Zhang, L., & Cui, J. (2024). Research on total factor energy efficiency in western China based on the three-stage DEA-Tobit model. PLoS ONE, 19(4), e0294329. [Google Scholar] [CrossRef]
- Zhang, M., Imran, M., & Juanatas, R. A. (2024). Innovate, conserve, grow: A comprehensive analysis of technological innovation, energy utilization, and carbon emission in BRICS. In Natural resources forum. Blackwell Publishing Ltd. [Google Scholar]
- Zhang, Y., Wang, Y., Zhang, J., Liu, J., Ruan, J., Jin, X., Liu, D., Lu, Z., & Xu, Z. (2024). Research on waste gas treatment technology and comprehensive environmental performance evaluation for collaborative management of pollution and carbon in China’s pharmaceutical industry based on life cycle assessment (LCA). Science of The Total Environment, 919, 170555. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H., Guo, S., & Zhao, H. (2019). Provincial energy efficiency of China quantified by three-stage data envelopment analysis. Energy, 166, 96–107. [Google Scholar] [CrossRef]
- Zhong, S., Liang, S., Zhong, Y., Zheng, Y., & Wang, F. (2022). Measure on innovation efficiency of China’s pharmaceutical manufacturing industry. Frontiers in Public Health, 10, 1024997. [Google Scholar] [CrossRef]
- Zhu, J. (2009). Quantitative models for performance evaluation and benchmarking: Data envelopment analysis with spreadsheets (Vol. 2). Springer. [Google Scholar]



| Variable Category | Indicator | Indicator Definition | Unit |
|---|---|---|---|
| Input Indicators | Assets () | Local total assets of PI | Millon CNY |
| Personnel () | Local total number of employees in PI | Person | |
| R&D investment () | Local total R&D investment of enterprises | Millon CNY | |
| Output Indicators | Operation revenue () | Main business revenue of the PI | Millon CNY |
| Operation profit () | Total operating profit of the PI | Millon CNY | |
| New product sales () | Total sales revenue from new products in the PI | Million CNY | |
| Number of patents () | Total number of patents owned by the PI | Patents | |
| Environmental Indicators 1 | Economic and technological foundation () | ||
| Residents’ living standards () | - | ||
| Local pollution levels () | - | ||
| Openness to the foreign market () | - | ||
| Variables | Operation Revenue () | Operation Profit () | New Product Sales () | Number of Patents () |
|---|---|---|---|---|
| Assets () | 0.736 *** | 0.609 *** | 0.783 *** | 0.879 *** |
| Personnel () | 0.939 *** | 0.642 *** | 0.749 *** | 0.759 *** |
| R&D Investment () | 0.829 *** | 0.701 *** | 0.899 *** | 0.925 *** |
| Region | The Efficiency in Stage 1 | The Efficiency in Stage 3 | |||||
|---|---|---|---|---|---|---|---|
| TE | PTE | SE | TE | PTE | SE | ||
| National Wide | Average | 0.708 | 0.772 | 0.923 | 0.705 | 0.793 | 0.891 |
| SD | 0.165 | 0.178 | 0.086 | 0.173 | 0.148 | 0.144 | |
| North China | Beijing | 0.683 | 0.737 | 0.924 | 0.745 | 0.758 | 0.987 |
| Tianjin | 0.925 | 0.940 | 0.985 | 0.935 | 0.939 | 0.995 | |
| Hebei | 0.641 | 0.695 | 0.927 | 0.691 | 0.703 | 0.985 | |
| Shanxi | 0.588 | 0.598 | 0.985 | 0.601 | 0.682 | 0.871 | |
| Inner Mongolia | 0.689 | 0.698 | 0.986 | 0.677 | 0.741 | 0.912 | |
| Northeast China | Liaoning | 0.925 | 0.946 | 0.977 | 0.881 | 0.903 | 0.976 |
| Jilin | 0.824 | 0.874 | 0.938 | 0.854 | 0.882 | 0.967 | |
| Heilongjiang | 0.656 | 0.760 | 0.886 | 0.744 | 0.774 | 0.955 | |
| East China | Shanghai | 0.597 | 0.613 | 0.973 | 0.618 | 0.630 | 0.981 |
| Jiangsu | 0.695 | 0.922 | 0.755 | 0.720 | 0.920 | 0.785 | |
| Zhejiang | 0.552 | 0.690 | 0.819 | 0.601 | 0.694 | 0.889 | |
| Anhui | 0.779 | 0.842 | 0.925 | 0.809 | 0.817 | 0.990 | |
| Jiangxi | 0.860 | 0.876 | 0.980 | 0.864 | 0.876 | 0.986 | |
| Fujian | 0.728 | 0.750 | 0.971 | 0.647 | 0.717 | 0.894 | |
| Shandong | 0.628 | 0.897 | 0.698 | 0.655 | 0.897 | 0.729 | |
| Central China | Henan | 0.629 | 0.707 | 0.904 | 0.678 | 0.715 | 0.951 |
| Hubei | 0.555 | 0.583 | 0.952 | 0.579 | 0.595 | 0.975 | |
| Hunan | 0.782 | 0.810 | 0.963 | 0.745 | 0.764 | 0.976 | |
| South China | Guangdong | 0.662 | 0.862 | 0.782 | 0.706 | 0.864 | 0.835 |
| Guangxi | 0.824 | 0.866 | 0.956 | 0.827 | 0.883 | 0.929 | |
| Hainan | 0.782 | 0.844 | 0.927 | 0.800 | 0.865 | 0.916 | |
| Southwest China | Chongqing | 0.577 | 0.599 | 0.962 | 0.584 | 0.613 | 0.952 |
| Sichuan | 0.773 | 0.904 | 0.862 | 0.833 | 0.906 | 0.924 | |
| Guizhou | 0.766 | 0.817 | 0.931 | 0.796 | 0.824 | 0.965 | |
| Yunnan | 0.704 | 0.785 | 0.902 | 0.745 | 0.779 | 0.956 | |
| Tibet | 0.961 | 0.996 | 0.965 | 0.605 | 0.984 | 0.612 | |
| Northwest China | Shaanxi | 0.820 | 0.833 | 0.984 | 0.806 | 0.846 | 0.947 |
| Gansu | 0.494 | 0.511 | 0.968 | 0.475 | 0.620 | 0.766 | |
| Ningxia | 0.500 | 0.518 | 0.960 | 0.448 | 0.739 | 0.613 | |
| Qinghai | 0.806 | 0.869 | 0.929 | 0.695 | 0.889 | 0.768 | |
| Xinjiang | 0.544 | 0.580 | 0.942 | 0.476 | 0.748 | 0.643 | |
| KMO measure of sampling adequacy | 0.902 |
| Bartlett’s test of sphericity approximate Chi-Square | 15,737 |
| Degrees of freedom | 253 |
| Significance | 0.000 |
| Component | Initial Eigenvalues | Extraction Sums of Squared Loadings | Rotation Sums of Squared Loadings | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | % Variance | Cum. % | Total | % of Variance | Cum. % | Total | % of Variance | Cum. % | |
| 1 | 15.18 | 66.00 | 66.00 | 15.18 | 66.00 | 66.00 | 11.82 | 51.37 | 51.37 |
| 2 | 2.95 | 12.83 | 78.83 | 2.95 | 12.83 | 78.83 | 4.19 | 18.21 | 69.58 |
| 3 | 1.40 | 6.07 | 84.90 | 1.40 | 6.07 | 84.90 | 2.37 | 10.29 | 79.87 |
| 4 | 1.02 | 4.43 | 89.33 | 1.02 | 4.43 | 89.33 | 2.18 | 9.46 | 89.33 |
| 5 | 0.58 | 2.56 | 90.81 | - | - | - | - | - | - |
| ... | - | - | - | - | - | - | - | - | - |
| 23 | 0.03 | 0.02 | 100.00 | - | - | - | - | - | - |
| Component | Principle Component 1 | Principle Component 2 | Principle Component 3 | Principle Component 4 |
|---|---|---|---|---|
| Water Pollution Equivalent | - | - | 0.769 | - |
| Air Pollution Equivalent | - | - | 0.807 | - |
| Full-time R&D Hours | 0.927 | - | - | - |
| Annual Number of R&D Projects | 0.916 | - | - | - |
| Annual R&D Investment | 0.899 | - | - | - |
| New Product Projects | 0.95 | - | - | - |
| New Product Investment | 0.933 | - | - | - |
| Annual New Product Sales | 0.922 | - | - | - |
| Authorized Inventions | 0.76 | 0.516 | - | |
| Authorized Utility Models | 0.898 | - | - | - |
| Authorized Designs | 0.885 | - | - | - |
| Per Capita Disposable Income | - | 0.905 | - | - |
| Per Capita Consumption Level | - | 0.908 | - | - |
| Number of Foreign-Funded Enterprises Registered | 0.723 | - | - | - |
| Foreign Investment Amount | - | - | - | 0.895 |
| Registered Capital of Foreign Investment | - | - | 0.964 | |
| Higher Education Enrollment | 0.727 | - | - | - |
| Local General Budget Revenue | 0.777 | - | - | - |
| Government Support for Education | 0.809 | - | - | - |
| Government Support for Science and Technology | 0.809 | - | - | - |
| Government Support for Environmental Protection | 0.563 | - | - | - |
| Regional GDP | 0.832 | - | - | - |
| Per Capita GDP | - | 0.880 | - | - |
| Independent Variable | Dependent Variable | |||
|---|---|---|---|---|
| Slack of Total Asset | Slack of Employees Number | Slack of R&D Investment | ||
| Constant Term | −17,973.69 *** | −2638.33 *** | −32.16 *** | |
| t-ratio | −14.03 | −23.19 | −5.09 | |
| Economic and technological foundation () | −6568.12 *** | 218.76 | 4.51 * | |
| t-ratio | −2.68 | 0.70 | 1.77 | |
| Residents’ living standards () | 16,586.74 *** | 2117.79 *** | 40.48 *** | |
| t-ratio | 25.28 | 5.75 | 10.56 | |
| Local pollution levels () | 7163.00 *** | 2942.94 ** | −42.76 ** | |
| t-ratio | 39.44 | 2.01 | −2.41 | |
| Openness to the foreign market () | 45,187.43 *** | −4467.38 *** | 4.66 | |
| t-ratio | 9.31 | −4.63 | 0.10 | |
| 3,560,829,700 | 57,626,093.00 | 33,054.70 | ||
| 0.999 | 0.999 | 0.999 | ||
| LR test of the one-sided error | 204.38 | 204.72 | 310.85 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Rosli, A.B.; Daud, A.; Yan, X. Evaluation of Operational Efficiency in China’s Pharmaceutical Industry and Analysis of Environmental Impacts. Economies 2025, 13, 90. https://doi.org/10.3390/economies13040090
Sun J, Rosli AB, Daud A, Yan X. Evaluation of Operational Efficiency in China’s Pharmaceutical Industry and Analysis of Environmental Impacts. Economies. 2025; 13(4):90. https://doi.org/10.3390/economies13040090
Chicago/Turabian StyleSun, Jiaqiang, Anita Binti Rosli, Adrian Daud, and Xia Yan. 2025. "Evaluation of Operational Efficiency in China’s Pharmaceutical Industry and Analysis of Environmental Impacts" Economies 13, no. 4: 90. https://doi.org/10.3390/economies13040090
APA StyleSun, J., Rosli, A. B., Daud, A., & Yan, X. (2025). Evaluation of Operational Efficiency in China’s Pharmaceutical Industry and Analysis of Environmental Impacts. Economies, 13(4), 90. https://doi.org/10.3390/economies13040090






