Abstract
The aim of the study was to explore students’ own translation of the symbolic level of a chemical reaction, including the information provided with the use of coefficients, indices, and signs, as well as the preservation of atoms. Students were asked to translate the symbolic level of the combustion of methane with the use of clay modelling. The students had to make active choices regarding the size, shape, two- or three-dimensional structure, and the number of atoms in the molecules included in the reaction using modelling clay. The analysis followed the three levels of analysis as presented by Hedegaard. The results highlight the variations in students’ answers and show the importance of investigating unrestricted translations of the symbolic level of chemistry. Including clay modelling in the educational process is helpful for both educators and students, as it fosters comprehension of underlying processes and enhances awareness of substance structure and atom redistribution across various substances.
1. Introduction
Chemistry as a school subject is important for learners of all ages as it provides the basis for understanding many of today’s societal challenges [1,2,3,4,5], especially the goals related to economic growth and active participation in issues in such as sustainability in the techno-scientific society [6]. Unfortunately, chemistry is not always an apparent and easily experienced science. Learning chemistry as a school subject can be viewed as learning multilevel thinking, as many of the levels are abstract because they are on a scale of one femtometre or smaller. This level is difficult to provide experience of, as we can only describe it by relating the size of something familiar. Few, if any, familiar things exist at such a small size to use them as a point of reference. It also becomes difficult to probe students’ understanding of the submicroscopic world, as the opposite is also an issue: How can a student express their understanding of a level so small that we have no familiar point of reference?
Chemists have circumvented the problem by using chemical symbols that exist on a macroscopic level, although they are intended to represent the submicroscopic level. The symbols can be viewed as the language of chemistry because, in the same way as a letter in the alphabet represents sounds, the chemical letters represent subatomic particles, atoms, charges, molecules, and chemical transformations. With the use of stoichiometry, the symbols change from being seen on a submicroscopic level to being seen on a macroscopic visual level. This study was designed to explore students’ own interpretations of the symbolic level of chemical transformations, including the submicroscopic–macro-level shift using stoichiometry. Clay modelling was used as it provides artistic freedom in terms of shape, size, two- or three-dimensionality, composition and distances between particles, and particle arrangement.
2. Background
2.1. The Knowledge Space of Chemistry as a School Subject
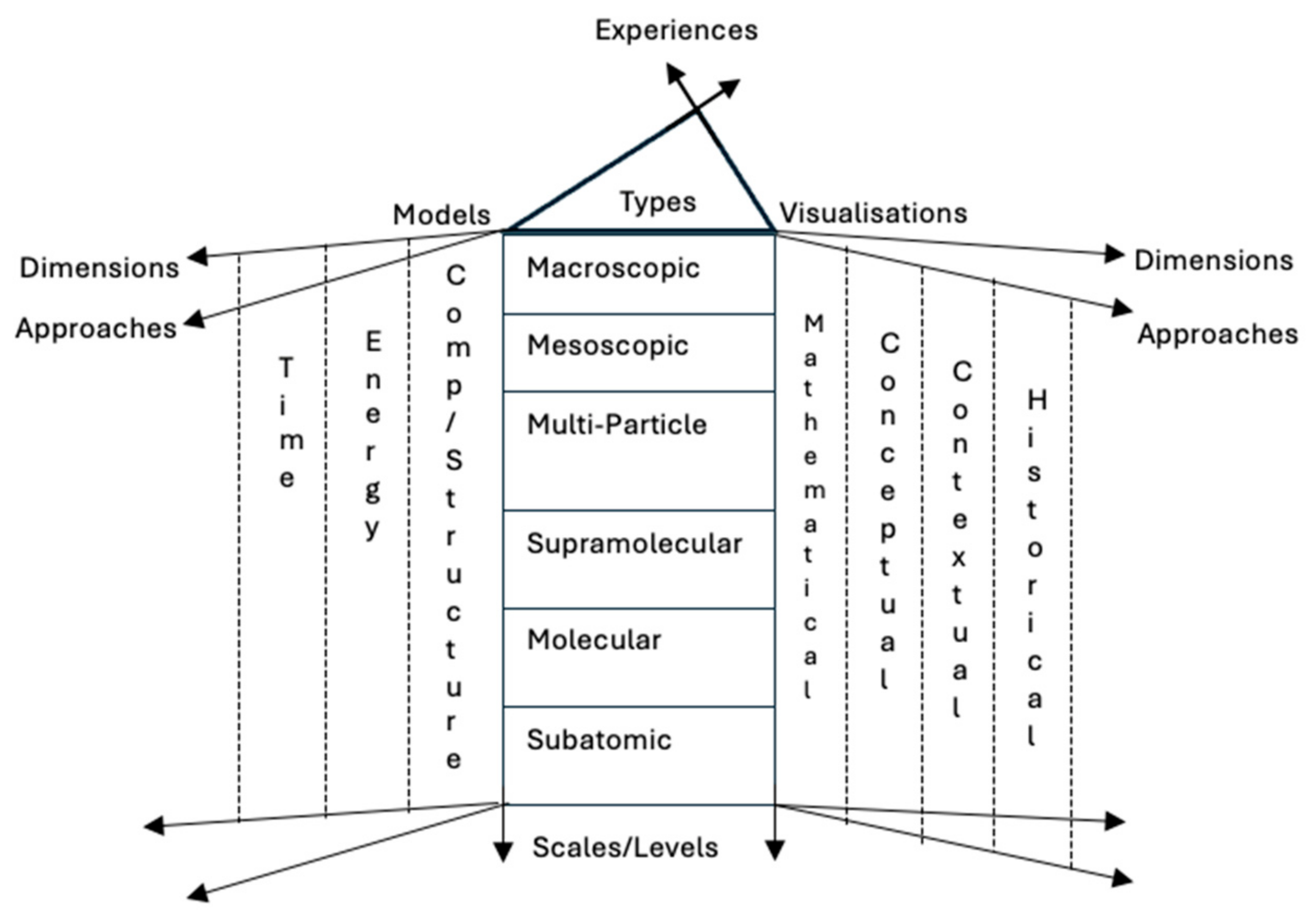
Learning chemistry is a complex educational journey that spans several years of compulsory and secondary school. Describing its complexity has been attempted for decades by educational researchers. The general point of agreement is that learning chemistry is a time-consuming process [7] that involves many abstract concepts, phenomena, and explanatory concepts on fundamentally different levels. The complexity of the subject has been described as a case of multilevel thinking [8]. Talanquer [9] attempted to describe the many aspects of the knowledge space of chemistry as a school subject (see Figure 1). The figure shows the many different levels of chemistry, ranging from the macroscopic experienced world to the subatomic level. Content is also described using different approaches such as visualisations, mathematics, concepts, and can be seen from both different contextual and historical perspectives. It includes knowledge of composition and structure, energy, and the speed of chemical processes—something that makes time an important factor for understanding natural chemical processes.
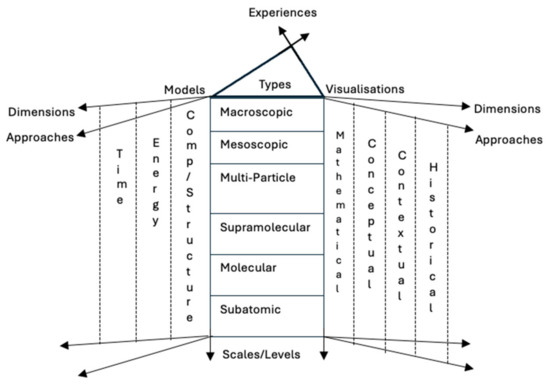
Figure 1.
A summary of the knowledge space of chemistry based on Talanquier [9].
Another more general way to describe chemistry as a school subject was suggested by Johnstone [10], who described the three levels of chemistry as: descriptive, symbolic, and explanatory. The descriptive level represents the macroscopic level of observed phenomena, such as the melting of ice or boiling of water. The symbolic level is our translation of chemistry into symbols such as chemical formulas, symbols that represent atoms, atomic charges, stoichiometry, aggregation states, and chemical names [11]. The explanatory level is the submicroscopic level of chemistry, a level that cannot easily be visualised.
2.2. Models
The abstract level of chemistry as a school subject involves numerous forms of models and visual representations such as graphs, illustrations, games, animations, and models, among others. These are models/tools for communication are made to support learners in making sense of abstract content and solving problems. The word “model” is here used to describe a simplified model, which is a representation of a physical reality, used to explain, predict, or communicate natural science [12]. It can be an object, phenomena, process, idea, or system. Research suggests that one of the most important factors for scientific understanding is learning to reason with models [13]. Indeed, research shows that the translation between the learner and the representation is important. It is especially important for learners to translate the same content through many forms of representations [14], such as, for example, chemical formulae, animations, or drawings. Working with multiple representations [15] can invoke different stages of insights, e.g., what does each representation mean? Which part of the knowledge space is it related to? [16] How are the representations related to each other? What similarities and differences are there between representations? [17] This processes of connecting and integrating the different levels of the representation are important for developing deep and nuanced understanding [17,18].
2.3. Learning Chemistry
To learn chemistry, students need to move between the levels of chemistry [9], master the language of chemistry, i.e., the symbolic level and be able to imagine that the symbolic level represents the invisible world of atoms and molecules on a scale so small, it is not easily imagined. Research indicates that students often believe that atoms are on a scale big enough to be observed using regular microscopes or that textbook illustrations depict the actual atomic size [19]. So far, the scale of the subatomic level can only be accessed by relating size to something familiar or through animations. Unfortunately, the different possibilities for relating the size of an atom to something meaningful that can be experienced are few (if there are in fact any). Animations can provide a sense of transition between the macroscopic and submicroscopic world and give a bodily experience of just how small the submicroscopic level is through the time elapsed between the different levels [20]. Additionally, symbolic-level illustrations are often used without clarification, something that may obstruct students from making the connection between submicro- and symbolic representations. Other difficulties occur as the representations themselves are not consistent, and the same round circle may for example symbolise single atoms, a particle, or a group of particles [21,22,23,24].
2.4. Challenges When Learning about Chemical Reactions
The above-described challenges are valid for most of the areas of chemistry, including chemical transformations, which are the focus of this study. Only some chemical transformations can be experienced in real life, such as the denaturation of proteins while frying an egg; other examples are combustions or explosions [25]. Slower transformations such as the breakdown of matter in nature are so slow that most of us don’t even notice that the transformation is taking place. Also, many of the visual transformations are irreversible, such as exploding fireworks [25], something that may lead to assumptions that all chemical reactions are irreversible when, in fact, most chemical transformations are reversible.
In formal education, chemical transformations on the symbolic level are commonly described to students using either a basic particle theory or with the use of the atomic model. The particle theory uses particles generalized to the level of circles and shows the change in the form of straightforward reengagements of particles and sometimes collision theory. The second approach, the atomic model, is specific and makes use of electrons, protons, atoms, ions, and molecules, and shows, for example, the transition of electrons between different particles [26]. Research shows that both perspectives are useful for supporting learners and students that make use of the more generalized particle model can use it to support complex reasoning [26]. These two models can also be used as an intended student progressions in learning about chemical reactions; moving from rearrangement to describing the process based on specific particles.
When depicting a chemical reaction using the symbolic level, the level changes from submicroscopic to the macro level with the addition of stochiometric information [27]. With the addition of numbers, the chemical equation resembles a mathematical equation, and it is not uncommon for students to view a chemical reaction as math problem rather than a tool for explanation that is linked to phenomena or the submicroscopic level [28,29,30,31,32,33,34]. This is a reasonable assumption since mathematics and chemistry share signs, but the signs hold different meanings in different subjects. In a chemical reaction, the + sign between reactants does not refer to an addition; instead, it means “and” [25]. The arrow in the reaction formula signifies the direction of the conversion of reactants into products and can be translated to “forms”, but students commonly see it as an equal sign (=) leading to a view of a chemical reaction as being equal on both sides of the symbol; even though the total number of atoms of each type and the total mass are the same on both sides of the arrow, there are not an equal number of products and reactants during the reaction [25]. Considering the chemical reaction as a mathematical problem can also result in students memorising chemical reaction formulas symbolically or perceiving it as an implementation of a set of rules instead of as a transfer of atoms and the breaking and forming of chemical bonds [31,32,35,36,37,38,39,40].
Balancing chemical reaction formulas is also a challenge since a comprehensive understanding of stoichiometry also requires the students to separate coefficients from indices [41]. Examples of such challenges include understanding the two different meanings of the number 2 in the symbol 2Ag2O, as well as determining when a symbol represents a mole of atoms or a mole of molecules. One common error in students’ illustrations of the products of a chemical transformation is combining multiple molecules into one molecule [35,42], or suggesting that mass can transform into some form of energy during a chemical or physical change [38,43,44,45]. Even though students commonly encounter submicroscopic representations in texts and animations, and during instruction, their understanding of these submicroscopic representations and their ability to draw accurate depictions themselves is less satisfactory [35]. Studies have highlighted the importance of engaging students with multiple representations to support learning [23,35,42,46,47,48].
2.5. Models for Teaching the Symbolic Level of Chemistry
Teaching methods become important in the chemistry classroom where multilevel thinking and the abstract language and symbols of chemistry meet students’ everyday experiences and macro-level understanding. There are models for teaching that can be used for illustrating what occurs at the submicroscopic level, such as visual aids like drawings, diagrams, animations, illustrated narratives, gestures, bodily representations, and symbolic representations through formulas. Also, molecular models that visualise the 3D molecules that represent the current consensus models of molecules and chemical bonds are traditionally used in the chemistry classroom. Research has explored how teaching tools can enhance chemistry learning, and a combination of using symbols alongside the submicroscopic models has shown promise in enhancing students’ ability to assess their own understanding and for teachers to address student questions. For example, visualisations of the molecular level have been shown to be important as helps students combining the three levels of chemistry in a holistic manner [49,50,51,52]. However, only allowing students to interpret and understand presented models provides limited insight into their thoughts and comprehension of concepts and models.
2.6. Students Expressed Models
Student-generated models are another way to provide teachers with a deeper understanding of student thinking [21,35,53,54,55,56,57]. Allowing students to express their own ideas can be a way for teachers to make sense of student ideas [55,56], work with the multiple levels of chemistry [58] and support discussions of temporal, spatial, and casual relationships.
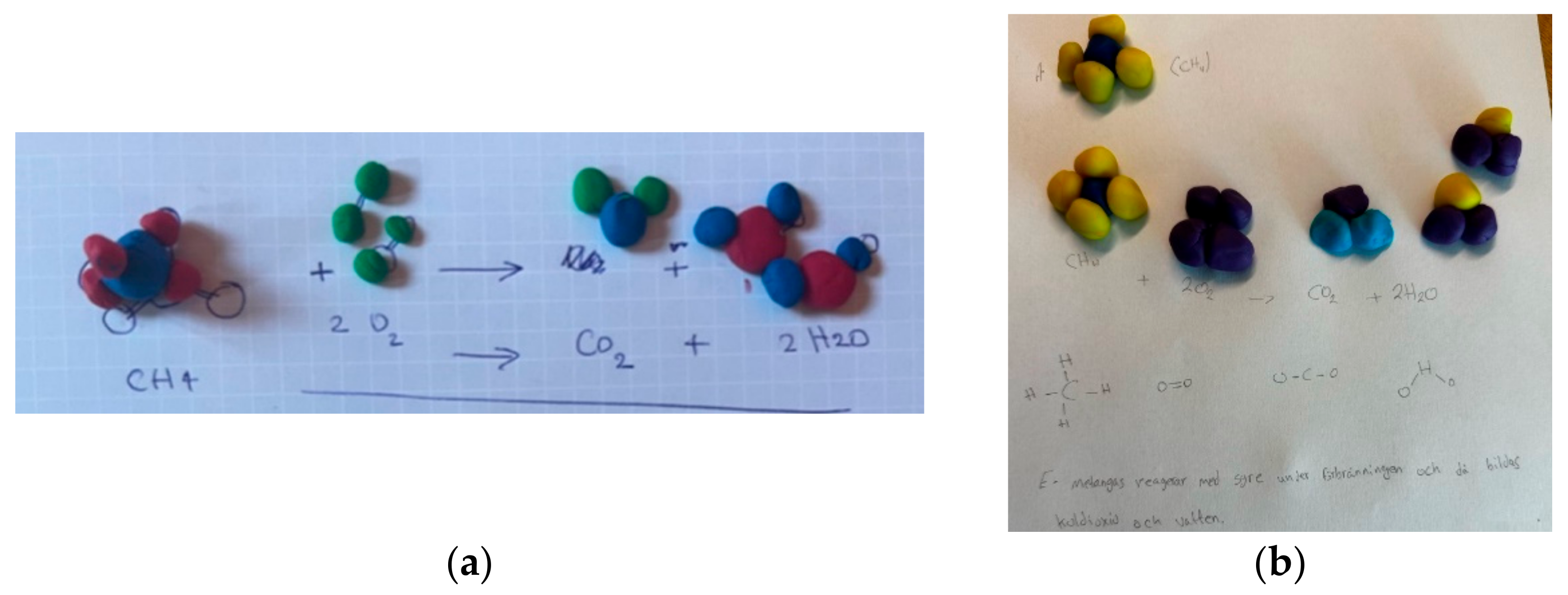
Different approaches for generating student models i.e., students’ own interpretation of a chemical content have been explored. The material utilized imposes limitations on the representation of models. For instance, drawings are primarily confined to a two-dimensional format, while the use of items like pasta [59] restricts the range of shapes that can be created. Here, the inclusion of gestures (embodied actions), and verbal support can be support an otherwise static expression [56]. A discrepancy arises between the expressed and intended forms, as students may face difficulty in visually expressing the intended goal. Drawing in three dimensions can, for example, be challenging for some students. The opposite is also true—not all students can express what they intend using words. One example of the latter was a student who gave a written explanation of the hydrogen nucleus by stating that “hydrogen has a proton in the nucleus”, a statement that most teachers would have accepted as the consensus model until the student made a ball and referred to as the nucleus, then added another ball firmly pushed into the nucleus, symbolising that the proton was in the nucleus (see Figure 2) [53].

Figure 2.
A student model of the hydrogen nuclei: “hydrogen has a proton in the nucleus”. The yellow ball represents the nucleus and the blue ball “the proton”, from Adbo and Akesson-Nilsson [53].
Studies indicate that many students struggle with the transition from the symbolic to the particulate nature of chemistry. The purpose of this study was to investigate the aforementioned transition, which encompasses both the conservation of atoms and the application of indices and coefficients using clay modelling. The study aims to:
- -
- Explore student translations between the symbolic and the particulate nature of chemistry.
- -
- Analyse how students creatively interpret chemical processes when given total artistic freedom.
2.7. Design of Study
The students included in this study were provided with the opportunity to illustrate molecules using clay models. An example of this includes allowing students to transition from molecular to structural formulas and switch between line formulas and drawings to show the tetrahedral structure of carbon compounds.
3. Method
3.1. Context
The case study involved 68 voluntary participants enrolled in the first of two chemistry courses during a foundational year within a university program. This year offers preparatory science education at a level equivalent to upper secondary education, for admission to higher education in science. This program includes students completing required courses for eligibility and others improving grades. The students included in this study were formed into groups and included students that had not previously studied chemistry (39 students), called Group 1, and students that had previously studied chemistry (29 students), called Group 2. The participants in this study spanned a wide age range, from 20 to 50 years old, with the majority being between 20 and 25 years old. Given that the course is being offered at the university level, the inclusion of students from the uptake area is predominantly from the southern regions of Sweden, which comprises both rural and urban localities. The data analysis was comprehensive but did not incorporate gender considerations.
All students had formally studied chemistry during lower secondary years, approximately ages 13 to 16. This curriculum covered matter’s structure, elements, molecular and ionic compounds, and chemical reactions, including atoms, electrons, and nuclear particles. Prior to undertaking the task in this study, the students were introduced to the following concepts: atomic model, chemical compounds, molecules, chemical symbols, the periodic table, mass number and atomic mass calculation, VSEPR (for diatomic molecules, methane, ammonia, water, and carbon dioxide), mass conservation, calculation of the amount of substance and molar mass, chemical reactions, and balance chemical equations. The students had previously practiced using the particle model by drawing atoms, protons, neutrons, electrons, and molecules, and calculated the quantities of substances, determined molar masses, and balanced chemical equations.
3.2. Data Collection
The study investigated students’ translation between the symbolic and particulate level of chemistry, where they were required to transfer the symbolic level into 3D models using clay modelling. This transfer has also been described as modelling ability, and have been defined as: “the understanding of spatial molecular structures and the ability to transfer between molecular representations and chemical understanding levels” [60].
Working on students’ skills to draw and transfer between a molecular formula, a structural formula, and a model are seen as sub-skills in a hierarchy developed by Dori and Kaberman [60]. In this hierarchy, molecular formulas are transformed to structural formulas then computer-generated models. In this study, students were familiarized with molecular formulas and then asked to create a 3D model using clay for modelling. The purpose of taking the reverse path in this hierarchy was to concretize the symbols used in molecular or structural formulas. In order to achieve the purpose, the students were asked to build a balanced chemical equation of methane reacting with oxygen forming carbon dioxide and water, using modelling clay. The task also included presenting a balanced reaction formula using modelling clay, providing drawn images of the built models, showing molecular formulas below the images of the models, and explaining the process using their own words. The student received the following instructions;
“First, you are required to illustrate the transformation by constructing the various molecules involved in the reaction. Then, you depict the chemical conversion by moving atoms, which is accomplished by disassembling the molecular models and then reassembling the atoms in a new configuration. Show a balanced chemical reaction”.
The time they had available was 60 min, and the study was conducted with approximately 34 students at a time, working individually. Additional information provided were as follows:
“When methane gas (CH4), is burned, carbon dioxide (CO2) and water (H2O) are formed if the combustion is complete. Combustion involves a reaction with oxygen (O2) in the form of oxygen gas.
3.3. Data Analysis
Data were analysed using mixed methods. Clay models, depictions of the symbolic level, and written explanations were analysed independently by two researchers using Hedegaard’s three levels of qualitative analysis [61] where the first level is called “commonsense interpretation”. At this level of interpretation, data from the 68 students were merged into individual illustrations of molecular models (clay models + symbolic level illustrations), and an explanation of the process. The second level of analysis, called “situated interpretation”, was then applied to the data set. At this level of analysis, focus was placed on students’ written explanations and common structural and functional patterns of the symbolic level representations as well as for their models of the particulate level. Specifically, differences between the formal content and the student-expressed model in literal appearance, structure, and images were analysed and then sorted into four different categories, depending on their closeness to the formally introduced content. Following that, the frequency of answers in each category was determined. Analysis from the perspective of the two different student groups was also performed. The third level of analysis, called thematic and conceptual interpretation, was performed here with an analytical focus on identifying general connections between the results and the existing research literature.
4. Results
The analysis of the difference between the instructional model and the student model focused on the conservation of atoms, use of the lowest coefficients of reactants and products, the symbols + for “and” together with the arrow showing the direction of the reaction process and the three-dimensional structure of the clay models according to the VSEPR model. The students’ answers were divided into four different categories:
- Both the symbolic level and the particulate models were in accordance with the formally introduced content. An example of a representation from category A can be found in Figure 3a.
- The symbolic level was correct, but the translation to particulate models was incorrect. An example can be found in Figure 3b.
- The symbolic level was incorrect, but the translation to particulate models was correct. Examples can be found in Figure 3c,d.
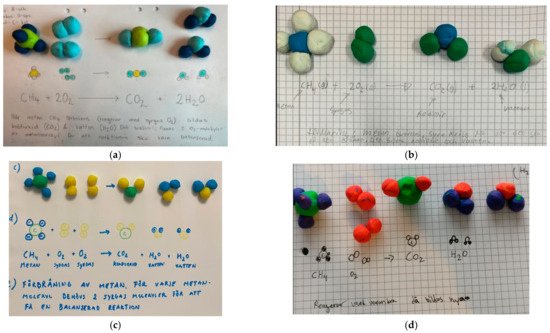
Figure 3.
The students’ clay modelling and answers of combustion of methane were divided into four different categories: listed as (a) Both the symbolic level and the particulate models were in accordance with the formally introduced content; (b) The symbolic level was correct, but the translation to particulate models was incorrect; (c) The symbolic level was incorrect, but the translation to particulate models was correct. Oxygen (O2) and water (H2O) are repeated twice in the reaction formula instead of using coefficients; (d) Coefficients are missing in the reaction formula.
Figure 3.
The students’ clay modelling and answers of combustion of methane were divided into four different categories: listed as (a) Both the symbolic level and the particulate models were in accordance with the formally introduced content; (b) The symbolic level was correct, but the translation to particulate models was incorrect; (c) The symbolic level was incorrect, but the translation to particulate models was correct. Oxygen (O2) and water (H2O) are repeated twice in the reaction formula instead of using coefficients; (d) Coefficients are missing in the reaction formula.
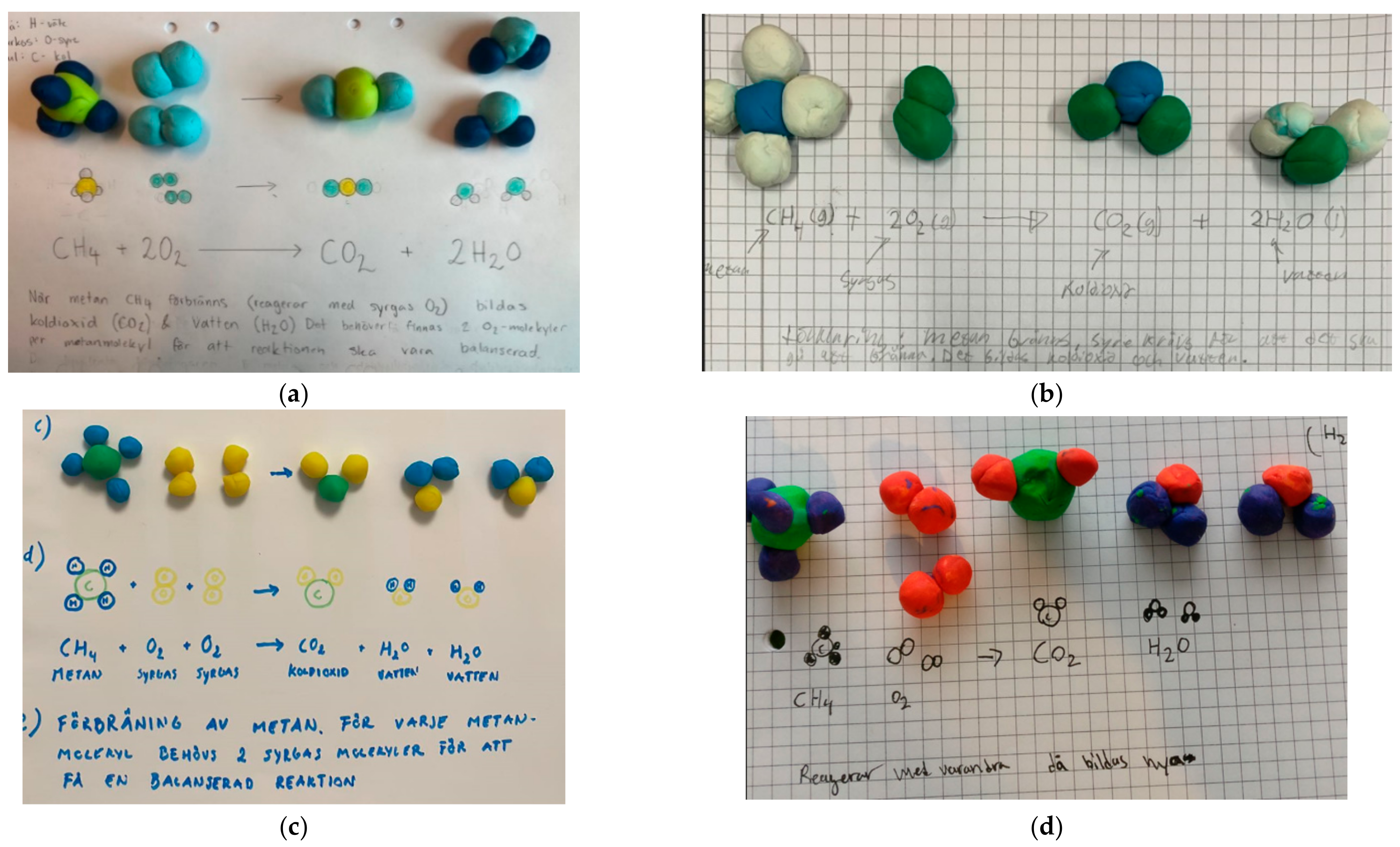

Figure 4.
Category D: Neither the symbolic level nor particulates were correct. (a) It is not possible to distinguish between reactants and products; (b) It is not possible to distinguish between reactants and products as the connection between molecular formula and clay models is inadequate.
Figure 4.
Category D: Neither the symbolic level nor particulates were correct. (a) It is not possible to distinguish between reactants and products; (b) It is not possible to distinguish between reactants and products as the connection between molecular formula and clay models is inadequate.



Figure 5.
Category D: Neither the symbolic level nor particulates were correct. (a) Models built for CO2 and H2O but does not use them in the reaction formula. Presents CH4O2 as a product; (b) Shows 2CH2O as a product in the reaction formula, but the connection between molecular formulas and the clay models is inadequate; (c) Neither the reaction formula nor the clay models are balanced; (d) Uses drawn symbols instead of molecular formulas in the reaction formula.
Figure 5.
Category D: Neither the symbolic level nor particulates were correct. (a) Models built for CO2 and H2O but does not use them in the reaction formula. Presents CH4O2 as a product; (b) Shows 2CH2O as a product in the reaction formula, but the connection between molecular formulas and the clay models is inadequate; (c) Neither the reaction formula nor the clay models are balanced; (d) Uses drawn symbols instead of molecular formulas in the reaction formula.

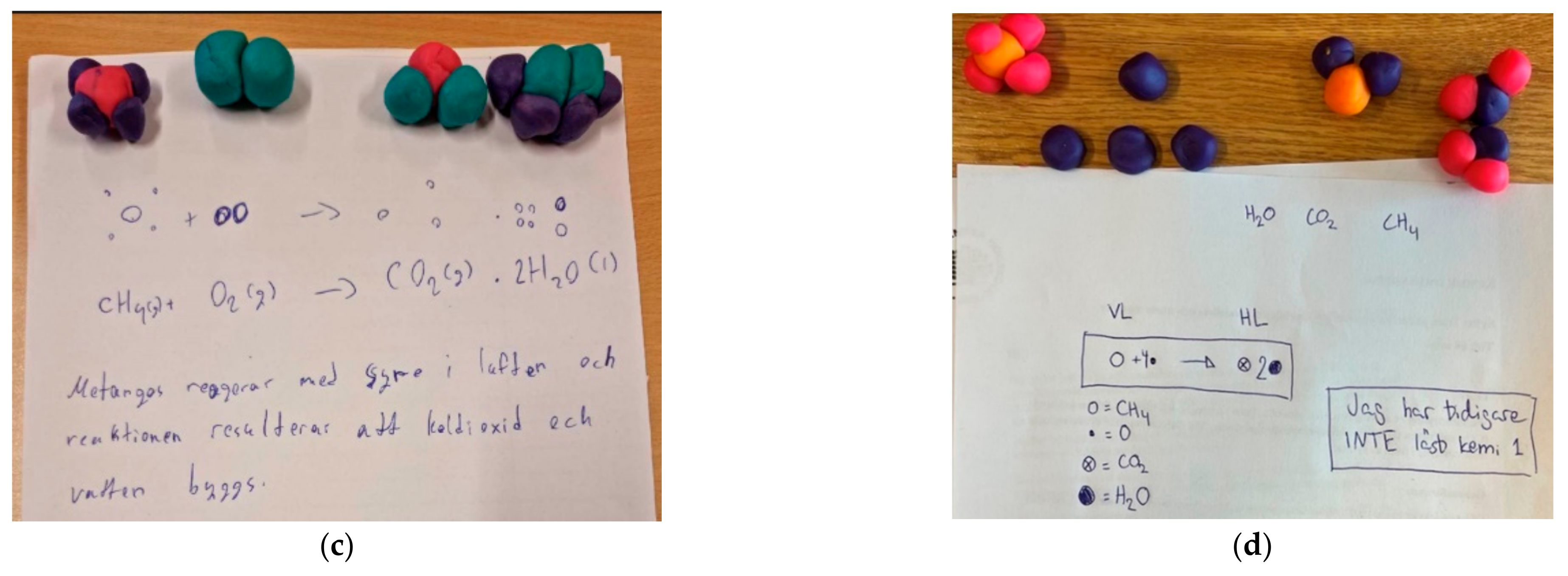
4.1. Preservation of Atoms in Chemical Reactions and Writing Chemical Equations
There was a significant difference between the two student groups for Category A and D (Table 1). For Category A, 72% in Group 2 (the students who had previously studied chemistry) correctly illustrated the conservation of atoms on both the symbolic and particulate levels, while only 41% of the students in Group 1 (the students who had not previously studied chemistry) could be categorized into this category. For Category D, only 3% of the students in Group 2 were classified as belonging to this category, while 36% of the students in Group 1 showed significant deviations from the formally introduced content in their expressions.

Table 1.
The four different categories A–D concern the students’ responses regarding the balanced reaction formula and its representation in clay. Data is provided for two different groups: 1. Students who have not previously studied chemistry (Group 1); 2. Students who have previously studied chemistry 1 (Group 2), and part of all. The responses are indicated in percentages. The number of participating students is indicated in parentheses. The analysis of the students’ translation difficulties between the two levels revealed specific challenges in symbols, molecular formula, and conservation of mass.
A closer comparison between the two groups was made by merging Category A and C. When these two categories were merged, Group 2 (89%) had a greater tendency to preserve atoms at the particulate level than Group 1 (51%). In an analysis of how many students presented a balanced reaction formula at the symbolic level, Category A and B were combined. The results showed that 79% of the students in Group 2, compared to only 51% of the students in Group 1, were able to write a balanced reaction formula.
The data suggest that the students who had previous experience in chemistry found it easier to comprehend the particulate level than the symbolic level. An analysis of individual translation reveals that difficulties revolve around the utilisation of symbols, molecular formulas, and the preservation of mass.
One of the most common issues concerned the direction of the reaction process were some students struggled with the use of the arrow symbol. Two of these examples are illustrated in Figure 4, where students seem to understand that the arrow symbol is indicative of a change but is sometimes used between each molecular formula, so it is not possible to separate reactants from products.
The molecular formulas themselves were also difficult for some, and alternative models such as CH4O2 and 2CH2O (see Figure 5a,b) were suggested. Some students used parentheses around each molecular formula in the reaction formula, and one student only used parentheses around the molecules where the coefficient 2 was indicated, such as 2(O2) and 2(H2O).
Several students also failed to consider the conservation of atoms in the chemical reaction. One typical example is shown in Figure 3d, where only the molecular formulas for the reactants and products are specified. Others illustrated the conservation of atoms by repeating the molecular formula for a substance instead of using coefficients (Figure 3c).
Most students (94% of 68 students) had the same number of atoms in the clay model for each substance as in their corresponding molecular formula. In Figure 3a, an example is presented where the number of atoms in each molecule in the clay model corresponds to the number of atoms in the respective molecular formula. However, in 4b, examples are presented where the number of clay balls in the models does not correspond for each molecular formula presented under the models.
The use of modelling clay also placed another difficulty on the chemical equations themselves. The student needed to be creative in their constructions in order to distinguish between different types of atoms (Table 2) allowing them to show the redistribution of atoms in the chemical reaction (Figure 3a). A large proportion of students (85%) used colours or other markers to distinguish the different types of atoms (Table 2). Among the remaining 15%, it was not possible to follow the redistribution of the different types of atoms. Either the same colour was used for two different types of atoms, or the same colour was not used for the same type of atom in the reactants and products. For examples, see Figure 6a,b.

Table 2.
Constructed clay models showing which products and reactants have been built, how they adhere to the teaching model regarding composition and choice of colour for different types of atoms. (A) Built correct reactants and products following the teaching model and choice of colour. (B) Did not build correct reactants and products following the teaching model and/or where the choice of colour for the types of atoms cannot distinguish the different types of atoms or where the same type of atom is illustrated with different colours. The responses are indicated in percentages. The number of participating students is indicated in parentheses.
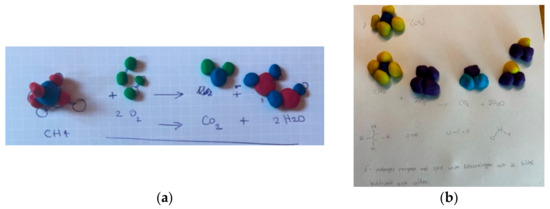
Figure 6.
Representation of methane combustion in clay models: (a) In the clay models, the colours for each type of atom in the reactants (carbon—blue, hydrogen—red, oxygen—green) are not the same in the products (carbon—blue, hydrogen—blue, and oxygen—green and red); (b) In the clay models, the colours for each type of atom in the reactants (carbon—dark blue, hydrogen—yellow, oxygen–dark blue) are not the same in the products, where the oxygen in the product CO2 is light blue. Water was built as HO2.
Upon closer analysis of those who did not present a correctly balanced reaction formula, there was a significant variation between the two different groups of students. In Group 2 the most common issue (14%) was that they did not use the coefficient 2 in front of O2 and H2O. Instead, they repeated the molecular formulas for these twice in their reaction formula, such as O2 + O2 and H2O + H2O (see Table 3). The most common errors in the Group 1 were either to only indicate each molecular formula once and without coefficients (18%) or to specify incorrect molecular formulas (18%).

Table 3.
Compilation of the diversity of incorrectly presented reaction formulas. The responses are indicated in percentages. The number of participating students is indicated in parentheses.
One student wrote C2O instead of CO2, and another student wrote OH2 instead of H2O. These students were in Group 2. Five students correctly presented the molecular formula for H2O but then presented an incorrect structural formula or clay model of the water molecule by including two oxygen atoms and one hydrogen atom (Figure 6b), missing the index. Three of these students were in Group 2.
4.2. Analysis of the VSEPR Model
The teaching models for VSEPR theory (3D structure of molecules) included tetrahedral methane, double-bonded, linear oxygen, linear carbon dioxide, and angled water molecules (see Figure 3a).
Only about one-third (32%) of students made clay models in accordance with the VSEPR theory. There was also very little difference between the two student groups (Table 4).

Table 4.
The proportion of models focusing on whether they adhere to or do not adhere to the VSEPR theory. The responses are indicated in percentages. The number of participating students is indicated in parentheses.
The most common deviating model, according to the VSEPR theory, was the model of CO2. 46% of the students built an angular, not linear CO2 (Figure 3b–d, Figure 5a,c,d, and Figure 6a,b). In Group 2 almost half of the students (48%) built an angular CO2 molecule (Table 5). Within this group almost half of the students did not construct CH4 in tetrahedral form (Figure 3b,c, Figure 4a and Figure 5c) they instead used the 2D structure (see Table 5). In Group 1, the proportion of angled CO2 and CH4 in 2D was lower, which can be explained by the fact that in this group, it was much more common for them to present molecular models that deviated regarding the composition of types of atoms in the molecules. Despite oxygen’s simplicity, some students imaged separate oxygen atoms (Figure 5d) and put four atoms close together. Another student placed two water molecules close to each other (Figure 5c).

Table 5.
Distribution of models that do not follow the VSEPR theory. Methane (CH4)—built in 2D, carbon dioxide (CO2)—built as angled. Other: oxygen (O2) built as split or four together, built together 2 H2O, built a hybrid model. The responses are indicated in percentages. The number of participating students is indicated in parentheses.
In a deeper analysis of the shapes and structures of the molecular models, the relative sizes of the atoms were also examined. When assessing the relative size, slightly over one-third (34%) of the built models closely resembled the teaching model, where carbon and oxygen are similar in size, while hydrogen is roughly half their size (Table 4). However, there was a division among student groups. Among Group 2, 38% of the students’ models closely aligned in relative sizes, compared to 31% in Group 1 (Table 6). Figure 3a shows a model closely aligned in relative sizes. A common deviation was using the same atom size for all atom types (31%), as exemplified in Figure 3b, Figure 5d and Figure 6b, and in Group 2, this was the most common deviation (38%). In Group 1, it was more common (44%) to use different sizes for their atoms, but they did not follow the rules for the relative sizes (see Figure 4a,b) where the carbon atom is twice the size of hydrogen and oxygen atoms), or they were not consistent in using the same size for the same types of atoms.

Table 6.
Size of the different types of atoms: A—relative size according to the teaching model, B—same size for all types of atoms, C—sizes of the atoms do not match the teaching model or have different sizes for the same type of atom. The responses are indicated in percentages. The number of participating students is indicated in parentheses.
5. Discussion
To present a balanced chemical reaction formula, one must have a comprehensive understanding of the symbolic level of chemistry, as well as the particles that are represented by the symbols. This study sought to analyse students’ ability to translate between two levels in a chemical transformation. In order to attain the objective, the study participants were instructed to illustrate the combustion of methane through symbolic representation and subsequently transform these symbols into particles using modelling clay. The application of clay modelling prompts students to make choices pertaining to size, shape, dimensionality, and distinguishing among various atom models. A method to guide students away from using letters and towards an alternative form of abstraction.
The study included two distinct groups of students: a group with prior knowledge of chemistry and another group new to the subject. It is reasonable to assume that the students who decided to repeat their chemistry course did not excel on their first try. Substantial variations were found among the student groups, indicating the specific areas where repetition is highly advantageous for learning. An illustrative case was found in the association between the 2D written equation and the clay model, where students with a background in chemistry showed enhanced performance, indicating that the experienced students had less difficulty in linking the two.
To demonstrate the conservation of atoms and the redistribution of atoms students need to translate symbols to particles and assign different colours to different atom types, while using the same colour for the same atom type. Most students (85%) succeeded in this, regardless of whether they previously had studied chemistry or not.
The conservation of atoms during the transformation was also evident in the clay’s molecular models. Several students successfully constructed clay models with the accurate composition of atoms and molecules, but their written formulas were sometimes not balanced in terms of coefficient usage. Another frequent error observed within this group was the use of O2 + O2 and H2O + H2O instead of the correct 2O2 and 2H2O. The results indicate either that utilising particles or clay models was more convenient compared to symbols, or that the students had not yet started employing abbreviations and found it more logical to think in terms of distinct species. A significant number of students failed to indicate any coefficients in their reaction formula, or mistakenly placed parentheses around the molecular formulas and subsequently inserted the coefficients as 2(O2) and 2(H2O). This could potentially indicate that they perceive the chemical reaction formula as a mathematical formula, as stated by [28,29,30,31,32,33,34]. Due to the complex nature of converting between symbolic and particulate models, it is recommended that the integration of modelling be introduced early on in chemistry education.
The findings also highlight the significance of prioritising the relationship between molecular formulas by utilising coefficients to signify the quantity of formula units as well as emphasising the use of arrows in reaction formula. Although the use of the arrow symbol indicated that these students understand that some kind of transformation occurs during the process.
By enabling students to utilise modelling, they can visually represent chemical transformations and make the thought process behind molecules and chemical reactions more apparent. This method can be useful for students to practice differentiating between particle levels (atomic and molecular), atom redistribution in chemical reactions, physical transformations, as well as for differentiating between indices and coefficients. Differentiation between indices and coefficients is a frequently encountered challenge [41]. Models can also provide an understanding of the different representations of molecular models and how they relate to each other in a chemical transformation [15,16]. The various representations of matter pose several challenges as they can represent individual atoms, molecules, and large clusters of molecules [21,22,23,24].
The VSEPR model serves as the fundamental basis for predicting the three-dimensionality of molecules, which is crucial for comprehending various aspects of chemistry, such as chemical bonding and steric hindrance.
Out of all the models built by the students, only one-third followed the principles of the VSEPR model. There was no difference between the two different groups of students (Table 4). Among the various molecules, carbon dioxide (CO2) posed the greatest challenge for the students in terms of its three-dimensional structure. Regardless of the presence of a double bond between carbon and oxygen resulting in a linear shape, the structure of the molecule was commonly perceived as having an angular shape. The utilisation of an angular shape by numerous students implies a disregard for the VSEPR model, as they chose to rely on their familiarity with the shape of the water molecule to estimate the three-dimensional arrangement of carbon dioxide. The findings demonstrate the significance of facilitating the translation of symbolic representations into three-dimensional particles that are not predetermined molecular models and using multiple representations sparks insights: their meanings, connections, related knowledge areas, and similarities and differences [15,16,17,18]. This method enables students to actively manipulate and contemplate the distinction between two-dimensional and three-dimensional dimensions while also considering the molecular structure. It serves as a valuable tool for instructors to observe first-hand how students apply their comprehension of molecular formulas at both the symbolic and particle levels. An important part of the VSEPR theory is atomic size; it is noteworthy that only 39% of students with a background in chemistry were able to identify discrepancies in atom size. Results also suggest that learning these concepts is a time-consuming process. This could be attributed to the fact that the symbols in the chemical reaction formula do not convey atom size.
6. Conclusions
This approach facilitates the exploration of the translation between the symbolic level of chemistry and the particles they symbolize. The results demonstrate the significance of investigating the range of interpretations that emerge when students are granted artistic autonomy to actively select size, shape, and two- or three-dimensionality in their translations of the symbolic aspect of chemistry. The development of a deeper understanding of the stoichiometry field centres around the accuracy of the translation [15,17,18].
The incorporation of clay modelling in the learning process yields benefits for both instructors and students, as it fosters an understanding of the interpretations of underlying processes and promotes a heightened awareness of substance structure and the redistribution of mass within different substances.
Author Contributions
Conceptualization, G.A.-N. and K.A.; methodology, G.A.-N. and K.A.; software, G.A.-N. and K.A.; validation, G.A.-N. and K.A.; formal analysis, G.A.-N. and K.A.; investigation, G.A.-N. and K.A.; resources, G.A.-N. and K.A.; data curation, G.A.-N. and K.A.; writing—original draft preparation, G.A.-N. and K.A.; writing—review and editing, G.A.-N. and K.A.; visualization, G.A.-N. and K.A.; supervision, G.A.-N. and K.A.; project administration, G.A.-N. and K.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval was not required for the study involving human participants in accordance with the local legislation and institutional requirements. The participants provided written informed consent to participate in the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Borgman, C.L.; Abelson, H.; Dirks, L.; Johnson, R.; Koedinger, K.R.; Linn, M.C.; Lynch, C.A.; Oblinger, D.G.; Pea, R.; Salen, K.; et al. Fostering Learning in the Networked World: The Cyberlearning Opportunity and Challenge. A 21st Century Agenda for the National Science Foundation; National Science Foundation: Alexandria, VA, USA, 2008. [Google Scholar]
- Disessa, A. Changing Minds: Computers, Learning, and Literacy; MIT Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Fensham, P.J. Science Education Policy-Making: Eleven Emerging Issues; UNESCO: Paris, France, 2008. [Google Scholar]
- Osborne, J.; Dillon, J. Science Education in Europe: Critical Reflections; King’s College: London, UK, 2008; Volume 13. [Google Scholar]
- Tytler, R. Re-imagining science education: Engaging students in science for Australia’s future. Lab. Talk 2007, 51, 6–9. [Google Scholar]
- Burmeister, M.; Rauch, F.; Eilks, I. Education for Sustainable Development (ESD) and chemistry educationThis article is part of a themed issue on sustainable development and green chemistry in chemistry education. Chem. Educ. Res. Pract. 2012, 13, 59–68. [Google Scholar] [CrossRef]
- Johnson, P. Progression in children’s understanding of a “basic” particle theory: A longitudinal study. Int. J. Sci. Educ. 1998, 20, 393–412. [Google Scholar] [CrossRef]
- Gilbert, J.K.; Treagust, D.F. Introduction: Macro, Submicro and Symbolic Representations and the Relationship between Them: Key Models in Chemical Education. In Multiple Representations in Chemical Education; Models and Modeling in Science Education Series; Springer: Milton Keynes, UK, 2009; pp. 1–8. ISBN 978-1-4020-8871-1. [Google Scholar]
- Talanquer, V. Macro, Submicro, and Symbolic: The many faces of the chemistry “triplet”. Int. J. Sci. Educ. 2011, 33, 179–195. [Google Scholar] [CrossRef]
- Johnstone, A.H. Macro- and micro-chemistry. Sch. Sci. Rev. 1982, 64, 377–379. [Google Scholar]
- Taber, K.S. Learning at the Symbolic Level. In Multiple Representations in Chemical Education; Models and Modeling in Science Education Series; Springer: Milton Keynes, UK, 2009; pp. 75–108. ISBN 978-1-4020-8871-1. [Google Scholar]
- Acevedo-Díaz, J.A.; García-Carmona, A.; del Mar Aragón-Méndez, M.; Oliva-Martínez, J.M. Scientific models: Meaning and role in scientific practice. Rev. Cient. Cent. Investig. Desarro. Cient. Univ. Distral Francisco Jose Caldas 2017, 3, 155–166. [Google Scholar] [CrossRef]
- Kind, P.; Osborn, J. Styles of Scientific Reasoning: A Cultural Rationale for Science Education? Sci. Educ. 2017, 101, 8–31. [Google Scholar] [CrossRef]
- de Vries, E.; Demetriadis, S.; Ainsworth, S. External Representations for Learning: Headed Towards a Digital Culture. In Technology-Enhanced Learning; Springer: Dordrecht, The Netherlands, 2009; pp. 137–153. ISBN 140209826X. [Google Scholar]
- Ainsworth, S. DeFT: A conceptual framework for considering learning with multiple representations. Learn. Instr. 2006, 16, 183–198. [Google Scholar] [CrossRef]
- Seufert, T. Supporting coherence formation in learning from multiple representations. Learn. Instr. 2003, 13, 227–237. [Google Scholar] [CrossRef]
- Van der Meij, J. Support for Learning with Multiple Representations Designing Simulation-Based Learning Environments. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2007. [Google Scholar]
- Seufert, T.; Brünken, R. Cognitive load and the format of instructional aids for coherence formation. Appl. Cogn. Psychol. 2006, 20, 321–331. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Markos, A.; Zarkadis, N. Understanding the atom and relevant misconceptions: Students’ profiles in relation to three cognitive variables. Sci. Educ. Int. 2016, 27, 464–488. [Google Scholar]
- Adbo, K.; Vidal Carulla, C. Learning About Science in Preschool: Play-Based Activities to Support Children’s Understanding of Chemistry Concepts. Int. J. Early Child. 2020, 52, 17–35. [Google Scholar] [CrossRef]
- Chittleborough, G.; Treagust, D. Correct interpretation of chemical diagrams requires transforming from one level of representation to another. Res. Sci. Educ. (Australas. Sci. Educ. Res. Assoc.) 2008, 38, 463–482. [Google Scholar] [CrossRef]
- Gabel, D. Improving Teaching and Learning through Chemistry Education Research: A Look to the Future. J. Chem. Educ. 1999, 76, 548–554. [Google Scholar] [CrossRef]
- Johnstone, A.H. The development of chemistry teaching: A changing response to changing demand. J. Chem. Educ. 1993, 70, 701–705. [Google Scholar] [CrossRef]
- Treagust, D.F.; Chittleborough, G. Chemistry: A matter of understanding representations. In Advances in Research on Teaching; Emerald Group Publishing Limited: Bingley, UK, 2001; Volume 8, pp. 239–267. ISBN 1479-3687. [Google Scholar]
- Taber, K. Teaching Secondary Chemistry, 2nd ed.; Hodder: London, UK, 2012; ISBN 9781444124323. [Google Scholar]
- Cheng, M.M.W. Students’ visualisation of chemical reactions-insights into the particle model and the atomic model. Chem. Educ. Res. Pract. 2018, 19, 227–239. [Google Scholar] [CrossRef]
- Niaz, M.; Lawson, A.E. Balancing chemical equations: The role of developmental level and mental capacity. J. Res. Sci. Teach. 1985, 22, 41–51. [Google Scholar] [CrossRef]
- Ben-Zvi, R.; Silberstein, J.; Eylon, B. Students’ visualization of a chemical reaction. Educ. Chem. 1987, 24, 117–120. [Google Scholar]
- Chiu, M.-H. A National Survey of Students’ Conceptions of Chemistry in Taiwan. Int. J. Sci. Educ. 2007, 29, 421–452. [Google Scholar] [CrossRef]
- Gabel, D.L.; Bunce, D.M. Research on Problem Solving: Chemistry. In Handbook of Research on Science Teaching and Learning; Gabel, D.L., Ed.; Macmillan: New York, NY, USA; Maxwell Macmillan Canada: Toronto, ON, Canada; Maxwell Macmillan International: New York, NY, USA, 1994; pp. 301–321. ISBN 978-0-02-897005-9. [Google Scholar]
- Krajcik, J.S. Developing understanding of chemical concepts. In The Psychology of Learning Science; Glynn, S.M., Yeany, R.H., Brittons, B.K., Eds.; Erlbaum: Hillsdale, MI, USA, 1991; pp. 117–147. [Google Scholar]
- Laugier, A.; Dumon, A. The Equation of Reaction: A Cluster of Obstacles Which are Difficult to Overcome. Chem. Educ. Res. Pract. 2004, 5, 327–342. [Google Scholar] [CrossRef]
- Lee, O.; Eichinger, D.C.; Anderson, C.W.; Berkheimer, G.D.; Blakeslee, T.D. Changing middle school students’ conceptions of matter and molecules. J. Res. Sci. Teach. 1993, 30, 249–270. [Google Scholar] [CrossRef]
- Osborne, R.J.; Cosgrove, M.M. Children’s Conceptions of the Changes of State of Water. J. Res. Sci. Teach. 1983, 20, 825. [Google Scholar] [CrossRef]
- Davidowitz, B.; Chittleborough, G.; Murray, E. Student-generated submicro diagrams: A useful tool for teaching and learning chemical equations and stoichiometry. Chem. Educ. Res. Pract. 2010, 11, 154–164. [Google Scholar] [CrossRef]
- Nakhleh, M.B. Why some students don’t learn chemistry: Chemical misconceptions. J. Chem. Educ. 1992, 69, 191–196. [Google Scholar] [CrossRef]
- Papaphotis, G.; Tsaparlis, G. Conceptual versus algorithmic learning in high school chemistry: The case of basic quantum chemical concepts. Part 1. Statistical analysis of a quantitative study. Chem. Educ. Res. Pract. 2008, 9, 323–331. [Google Scholar] [CrossRef]
- Salta, K.; Tzougraki, C. Conceptual Versus Algorithmic Problem-solving: Focusing on Problems Dealing with Conservation of Matter in Chemistry. Res. Sci. Educ. 2011, 41, 587–609. [Google Scholar] [CrossRef]
- Tasker, R.; Dalton, R. Visualizing the molecular world—Design, evaluation, and use of animations. In Visualization: Theory and Practice in Science Education; Gilbert, J.K., Reiner, M., Nakhleh, M., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 103–131. [Google Scholar]
- Gilbert, J.K.; Treagust, D. Multiple Representations in Chemical Education; Auflage 1/2009; Springer: Dordrecht, The Netherlands, 2009; Volume 4, ISBN 9781402088711. [Google Scholar]
- Marais, P.; Jordaan, F. Are We Taking Symbolic Language for Granted? J. Chem. Educ. 2000, 77, 1355–1357. [Google Scholar] [CrossRef]
- Wood, C.; Breyfogle, B. Interactive Demonstrations for Mole Ratios and Limiting Reagents. J. Chem. Educ. 2006, 83, 741–748. [Google Scholar] [CrossRef]
- Andersson, B. Pupils’ conceptions of matter and its transformations (age 12–16). Stud. Sci. Educ. 1990, 18, 53–85. [Google Scholar] [CrossRef]
- Barker, V.; Millar, R. Students’ reasoning about chemical reactions: What changes occur during a context-based post-16 chemistry course? Int. J. Sci. Educ. 1999, 21, 645–665. [Google Scholar] [CrossRef]
- Hartley, L.M.; Wilke, B.J.; Schramm, J.W.; D’Avanzo, C.; Anderson, C.W. College Students’ Understanding of the Carbon Cycle: Contrasting Principle-Based and Informal Reasoning. BioScience 2011, 61, 65–75. [Google Scholar] [CrossRef]
- Devetak, I.; Urbančič, M.; Wissiak Grm, K.S.; Krnel, D.; Glažar, S.A. Submicroscopic representations as a tool for evaluating students’ chemical conceptions. Acta Chim. Slov. 2004, 51, 799–814. [Google Scholar]
- Mulford, D.R.; Robinson, W.R. An Inventory for Alternate Conceptions among First-Semester General Chemistry Students. J. Chem. Educ. 2002, 79, 739–744. [Google Scholar] [CrossRef]
- Sanger, M.J. Evaluating Students’ Conceptual Understanding of Balanced Equations and Stoichiometric Ratios Using a Particulate Drawing. J. Chem. Educ. 2005, 82, 131–134. [Google Scholar] [CrossRef]
- Ainsworth, S.; Prain, V.; Tytler, R. Drawing to Learn in Science. Sci. Am. (Assoc. Adv. Sci.) 2011, 333, 1096–1097. [Google Scholar] [CrossRef] [PubMed]
- Ardac, D.; Akaygun, S. Effectiveness of multimedia-based instruction that emphasizes molecular representations on students’ understanding of chemical change. J. Res. Sci. Teach. 2004, 41, 317–337. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Quintana, C.; Krajcik, J.S. The impact of designing and evaluating molecular animations on how well middle school students understand the particulate nature of matter. Sci. Educ. 2010, 94, 73–94. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Linn, M.C. Can generating representations enhance learning with dynamic visualizations? J. Res. Sci. Teach. 2011, 48, 1177–1198. [Google Scholar] [CrossRef]
- Adbo, K.; AAkesson-Nilsson, G. Moving beyond the language–Visualizing chemical concepts through one’s own creative expression. Front. Educ. 2022, 7, 1034140. [Google Scholar] [CrossRef]
- Gilbert, J.K.; Justi, R. Modelling-Based Teaching in Science Education; Springer International Publishing AG: Cham, Switzerland, 2016; Volume 9, ISBN 9783319290386. [Google Scholar]
- Halloun, I.A. Modeling Theory in Science Education; Auflage 1/2006; Springer: Dordrecht, The Netherlands, 2006; Volume 24, ISBN 1402051514. [Google Scholar]
- Nersessian, N.J. Abstraction via generic modeling in concept formation in science. Mind Soc. 2002, 3, 129–154. [Google Scholar] [CrossRef]
- Treagust, D.; Harrison, A. The Genesis of Effective Scientific Explanations for the Classroom. In Researching Teaching Methodologies and Practices for Understanding Pedagogy; Routledge: London, UK, 1999. [Google Scholar]
- Gilbert, J.K. Models and modelling: Routes to more authentic science education. Int. J. Sci. Math. Educ. 2004, 2, 115–130. [Google Scholar] [CrossRef]
- Widing, L.; Nilsson, P.; Enochson, P.G. Modeling as a Tool to Improve Second Language Learners’ Descriptions of Non-Spontaneous Chemistry Concepts. Sci. Educ. Int. 2022, 33, 181–191. [Google Scholar] [CrossRef]
- Dori, Y.J.; Kaberman, Z. Assessing high school chemistry students’ modeling sub-skills in a computerized molecular modeling learning environment. Instr. Sci. 2012, 40, 69–91. [Google Scholar] [CrossRef]
- Hedegaard, M. The qualitative analysis of the development of a child’s theoretical knowledge and thinking. In Sociocultural Psychology; Cambridge University Press: Cambridge, UK, 1995; pp. 293–325. ISBN 0521089182. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).