Abstract
This paper presents a Problem-Based Learning (PBL) electrochemistry course contextualized within a real-world problem of wastewater treatment, designed to enhance students’ subject matter knowledge. The sample was a group of chemistry and chemical engineering undergraduate students who were taking an electrochemical course. The research outlines various activities and analyzes five cases of team learning outcomes using Atlas.ti(TM) 22 software. The analysis identifies and describes eight categories of scientific knowledge and practices derived from student reports. The results are represented using a Sankey diagram to show the complexity of students’ thinking after solving their problem. The findings indicate significant progress in students’ conceptual understanding of electrochemistry, the development of complex thinking, and the recognition of its relevance in solving everyday problems.
1. Introduction
Electrochemistry is a versatile scientific discipline with applications in energy, technology, and environmental sustainability, contributing to a lower carbon footprint (Brett & Oliveira-Brett, 2020). Extensive research in areas such as battery technology, environmental remediation, electrosynthesis, electroanalysis, and neuroscience, to name a few, has driven innovation in this field. Consequently, it is crucial to equip students with a solid foundation in electrochemistry.
However, electrochemistry is often perceived as one of the most challenging subjects for students (Johnstone, 2010; De Jong & Treagust, 2002), with numerous documented alternative conceptions (Brandriet & Lowery, 2014; Rahayu et al., 2022; Lu et al., 2020; Sanger & Greenbowe, 1997; Sanmartín & Sanjosé, 2014; De Jong & Treagust, 2002; Taber, 2019; Barke et al., 2009; Tsaparlis, 2019; Turner et al., 2024). While many students can solve quantitative problems on exams, few can answer questions that require deeper conceptual knowledge (Sanger & Greenbowe, 1997). The challenge is that electrochemical systems are inherently complex, both spatially and dynamically, generally requiring several models to describe and understand what is happening in the electrochemical cell (Faulkner, 2014). This demands an understanding of chemical reactions, elements of electricity, mass and charge transport, elements of electrical circuits, and knowledge about the nature of electrically conductive materials (Kempler et al., 2021).
Traditional undergraduate theoretical electrochemistry courses are developed predominantly from a symbolic conceptual level. The electrochemical structuring concepts rely on the order of topics presented in the physical chemistry textbook, which emphasizes mathematical development. Specialized texts, such as Bard and Faulkner (2000), result in additional challenges for students due to their high analytical and mathematical rigor (Kempler et al., 2021). Furthermore, the use of general physical chemistry textbooks can lead to the emergence of alternative conceptions (Turner et al., 2024; Sanger & Greenbowe, 1999). For instance, the Daniell cell, presented in textbooks as the initial model, with which both language and general description of the electrochemical cell are addressed—cathode, anode, electrolyte, semi-reactions, or salt bridge—often fails to help students perceive the electrochemical cell as a system (Boulabiar et al., 2004).
Contextualization is scarce in this kind of traditional course, with few examples of real-world applications like batteries and well-known industrial electrosynthesis processes. Generally, the questions that the student answers are as follows: define, enunciate, and explain; all of which can be framed in low-level thinking requirements or declarative knowledge. Written exercises, often selected from end-of-chapter problems, can be solved using algorithmic strategies without necessarily understanding phenomenological or mathematical models. These exercises are usually disconnected, presenting a fragmented and irrelevant view of electrochemical practices. Students frequently refer to the Nernst equation as a “formula”, which requires certain data that the teacher provides to solve it rather than recognizing it as a model. In general, the students have no opportunity to use it as a tool for thinking about reality, which can help them understand and solve scientific problems.
This research proposes that a theoretical electrochemistry course should not be based on textbook structure. Instead, it should be designed to produce complex scientific thinking by incorporating a guided inquiry learning orientation and experimental disciplinary practice as cognitive tools in the sense that Guile (2006) proposes: to conceive theoretical concepts as cultural tools that we apply in practice to see these tools as resources to evaluate practice critically. Also, the context and the application of knowledge in relevant practices and in learning communities based on inquiry can produce more interrelated and practical learning. Thus, students have a more significant opportunity to develop scientific thinking skills, such as inquiry, modeling, or argumentation.
This way, students are presented with an electrochemical problem that requires them to build ideas and concepts of the subject matter while developing simple, homemade, and low-cost electrochemical cells. This hands-on approach encourages them in the construction of the apparatus and the performance of the necessary experiments. They are challenged to link the macroscopic world, the construction of the cell, and the function of its elements, with the models and symbolic representations they develop to solve its functioning.
Since the course outset, all learning activities and assessments are designed around a problem to be solved, which is disciplinary centered and intended to develop students’ content knowledge while orienting them to build a product: the electrochemical apparatus. That is, with a Problem-Based Learning (PBL) approach and a project orientation. The course features a flexible classroom setup, internet connection through mobile devices, and virtual space integration with the classroom platform.
Active learning environments have been shown to outperform traditional science classes. They promote improved performance, reduce failure rates, and do better at serving large groups (Freeman et al., 2014). PBL is an active, socio-constructivist learning approach based on inquiry. It integrates theory and practice and is designed based on context. The goal is to provide students with a deep and transformative learning experience that fosters scientific thinking through an authentic problem of their interest (Savery, 2006; Ramos-Mejía, 2020). This approach aims to develop critical and complex thinking, high-level cognitive skills, metacognition, collaborative work, and, in the context of scientific disciplines, an experience in which the student can build an adequate notion of the nature of science.
PBL, a student-centered approach, has been widely used in higher education, particularly in the sciences (Hung & Amida, 2020). It started in the medical training context in Canada, but now it is applied to many disciplines, including chemistry (Varadarajan & Ladage, 2024). Compared to traditional instruction, PBL has been shown to enhance students’ remembering of the acquired knowledge (Dochy et al., 2003) and valuing it as powerful, specifically in enhancing learning (Dochy et al., 2005).
Problem- and project-based learning engages students in solving real-world problems and questions that are aligned with curriculum goals, fostering deeper learning and relevance (Hmelo-Silver, 2004). These problems must be significant to students but ill-defined, so they are motivated by the task. Because the challenge fulfills them, they are willing to invest themselves in the inquiry and in managing their own learning until they achieve a conceptual understanding. Such settings enable STEM students to recognize the interdisciplinary nature of complex problems, preparing them for workplace requirements through teamwork, collaboration for a common outcome, and peer critique of each other’s work (Odell & Pedersen, 2020).
In chemistry education, PBL has been used successfully, for example, in sustainable development (Günter et al., 2017) and a range of transferable skills (Overton & Randles, 2015), environmental chemistry (Jansson et al., 2015), improvement in students’ self-efficacy beliefs (Mataka & Kowalske, 2015), and students’ critical thinking and problem-solving skills (Aidoo et al., 2016). In electrochemistry courses, Günter and Alpat (2017) found that students in PBL contexts were better at understanding the topic and its structure compared to students in traditional settings.
The student learning outcome of this course is to develop a conceptual model of the electrochemical cell, with which they can understand the basic principles of its operation and the variables that can modify its efficiency. This is referred to as “electrochemical thinking”. As students build their models, they also realize that the context and purpose for which the electrochemical cell is used play an important role in understanding its design and operation. The model can then function as “a hub where heterogeneous information (scientific and empirical knowledge, relevant variables and parameters, measurement methods, pragmatic criteria concerning the solution) is collected and integrated into a coherent whole” (Boon et al., 2022, p. 15). If students are allowed to venture into designing devices and performing multiscale modeling or applying analytical thinking, this can contribute to consolidating their understanding of the basic concepts of thermodynamics, kinetics, transport, and materials science (Kempler et al., 2021), all of which are fundamental to electrochemical thinking.
Students should approach the study of building an electrochemical cell, galvanic or electrolytic, depending on their chosen problem. Figure 1 presents the problems offered to students, who must justify their choice of materials and operating details by applying electrochemical knowledge acquired in the course. As they build their devices, students develop the necessary skills to elucidate how the electrochemical device works. When theoretical predictions do not match experimental observations and data, students must be able to explain the set conditions that could be involved. Their challenge is to develop electrochemical thinking that incorporates the complexity of real systems.

Figure 1.
List of problems offered to students. They must choose one to solve in teams throughout the course.
The course was designed considering three skill dimensions:
1. Development of Lower-Order Thinking Skills (LOTS) through a flipped classroom approach, where students review video materials or short readings and complete a self-grading Google questionnaire. They also deliver a weekly activity that addresses various examples. Thus, as the course progresses, the focus is on using the problem selected by each team as the example with which knowledge is explored. All work is incorporated into their final written report, ensuring that the problem is built in class rather than treated as an extra activity.
2. Development of higher-order thinking skills (HOTS), which includes critical thinking, question formulation, problem-solving, decision-making, systemic and evaluative thinking, and transfer, according to Zoller and Nahum (2012). To solve the problem, the students’ task begins with the questions they elaborate on to explore its difficulty and thus achieve the problem-solving thinking scheme: What do I know? And what do I need to know?
Students must develop the following aspects: description of materials choice and their justification based on materials’ fit to function in the electrochemical cell; description of electrochemical cell configuration; description and explanation of conductivity; description and explanation of thermodynamics; kinetic description and explanation; experimental discussion and explanation regarding discrepancies with theoretical calculations; waste treatment and/or disposal; and bibliographic references.
The problem serves as an integrative activity that puts into practice the knowledge and skills obtained so far. Hence, students can develop scientific know-how in a learning community. In this electrochemistry introductory course, students recognize macroscopic phenomena instead of starting with a discussion of redox reactions at the symbolic level. Students build concepts according to the following structure: (1) recognize the macroscopic system, (2) relate with the symbolic representation, and (3) model the interface and bulk phenomena at the nanoscopic level.
Using simulators is fundamental to exploring and linking macroscopic, symbolic, and nanoscopic representations (Lee & Osmam, 2017). Students use simulators available online and develop their own using the Nernst equation for thermodynamics and the Butler–Volmer equation for a kinetic approach; they also represent double-layer electrochemical reaction phenomena through x-y diagrams and particle sketches. Simulators help students visualize the synchrony between detectable signals and nanoscopic/kinematic behaviors in electrodes (Wang & Wang, 2022).
Development of Soft Skills: collaborative work and activity planning. Students work in teams, experiencing and valuing collaboration, positive interdependence, role-based engagement, and quality criteria to reach general agreements. They engage in a “Team Contract”, establishing their commitments and goals, and evaluating themselves during the activities with their own rubric.
The course identifies scientific knowledge and practices that students develop from the implementation of PBL as previously described:
1. Content Knowledge: Students demonstrate subject matter knowledge, ranging from definitions to the recognition of electrochemical cell elements and their functioning.
2. Modelling: Students use models to explain some issues related to electrochemistry or make graphical simulations to represent data.
3. Engineering Practice: Students design and build requested equipment related to the subject. The solution to technological problems regarding design concepts is based on functional interpretations of phenomena (Boon et al., 2020).
4. Electrochemical Thinking: There is evidence of both recognition and understanding of the interrelatedness of electrochemical processes in students’ work.
5. Argumentation: Students must justify their choice of materials, show practical and professional project communication elements, and relate different kinds of knowledge that support their affirmations.
The course focuses on developing HOTS through one of the problems the students selected to show the knowledge and thinking results after solving it: Cans out of the trash! This problem centers on the wastewater treatment process, mainly from grey water produced in washing machines. Given the water shortage in Mexico City and other parts of the country, there are students who have suffered from this problem at home, and, therefore, it becomes relevant to show how electrochemistry can help improve wastewater quality. Electrochemistry offers different technological solutions for wastewater treatment, and electrocoagulation is one of them. This technique is mainly used in wastewater contaminated with surfactants from detergents, soaps, and organic residues such as oils or greases, which can form very stable emulsions of water–oil–surfactant systems. In sewage, the physicochemical coagulation–flocculation method is one of the most widely used within primary treatments.
The coagulants used are iron and aluminum salts, where polyvalent cations cause the destabilization of colloids’ double layers. In electrocoagulation (Shahedi et al., 2020; Das et al., 2022), the coagulant is generated in situ in a proportion that can be regulated through parameters controlled by Faraday’s laws in an electrolytic cell. That is, through the oxidation of an Al or Fe electrode, which functions as an anode, the polyvalent ions Al3+ or Fe2+ are generated, and the latter can be subsequently oxidized to Fe3+ with the action of oxygen dissolved in water. Colloids are destabilized by the interaction of charges, agglomerating and acting with insoluble basic species of metal cations formed when the pH of the system becomes alkaline; they form flocs, on which organic and inorganic substances adsorb. The floc can precipitate or be washed to the surface by hydrogen gas bubbles formed from hydrogen cation reduction at the cathode (electroflotation). Cathodically generated hydrogen is also responsible for pH increase and metal hydroxide formation, so there is no need for extra reagents to adjust the pH. Thus, the amount of reagents added is very low or non-existent, produces less sludge than normal coagulation, and organic matter is effectively removed; it has high current efficiencies, treatment is achieved in shorter times than in other methods, and in places where electricity is cheap, costs are lower than in conventional methods.
Although electrocoagulation is very complex, it is a simple wastewater treatment option, but it represents a comprehension challenge due to all physicochemical phenomena occurring. However, extensive research has been performed on surface phenomena, electrolysis, methods of chemical determination, and water treatment quality monitoring. It also involves approaching the environmental standards field, not as recipes to be followed but as methods with reproducible protocols established by professionals in the discipline. Wastewater treatment by electrocoagulation allows the incorporation of conceptual and procedural content that is significant for the student and has important motivational content. The result is predetermined and implies a deductive approach to the object of study; that is, the problem posed is a well-documented system, solved for the teacher but still unknown to the students, which makes it a project with an open answer for them, but a controlled environment for the teacher.
This problem imposes several elements that contribute to the contextualization of electrochemical practice increasing its complexity, unlike an example of electrochemical cells in ideal conditions. In this sense, in addition to the scientific knowledge and practices described above, the following categories are added:
- Transversal Knowledge: There is evidence that students incorporate information from other disciplines to describe complex electrochemical processes.
- Sustainability: Students demonstrate an understanding of environmental issues and sustainable practices.
- Context: Students develop projects using everyday materials, ensuring practical applicability in real-world settings.
2. Methodology
Electrochemistry is a mandatory theoretical course for the Chemistry and Chemical Engineering bachelor of the UNAM School of Chemistry. The cohort is 250 students and does not have an associated laboratory. Electrochemistry is taught in the second year when students have taken the conceptual elements necessary to complete this course successfully.
The study was conducted during the first school period of 2024, with one class of 50 students using the PBL approach by the instructor, who is also one of this paper’s authors. Students were divided into teams of 3 or 4 members, each selecting a problem of their liking from the list provided (see Figure 1). They worked on their chosen problem theoretically and experimentally throughout the school period, presenting their solutions in a final report. This report had the following structure: introduction, objectives, justification of the chosen problem, materials selection explanation, construction process of the electrochemical cell that would solve the problematic situation, a theoretical discussion that provided a basis for the experiment, discussion, and conclusions.
Student assessments were designed considering the three skills dimensions: LOTS, HOTS, and soft skills. All students passed the course. This study focuses on analyzing whether students developed electrochemical knowledge and electrochemical thinking in the context of complex thinking, by inspecting the reports of five teams that chose wastewater treatment problems. The teams considered are Aquacan, Aluminiums, Electrovives, Electrocoagulation, and Electrolytes.
The reports written by the students were assessed using the qualitative analysis program ATLAS.ti(R). This program allows for faster coding and more efficient results. Each report was analyzed using the categories indicated for scientific knowledge and scientific practices and elements related to sustainability and the environment listed and described above. Table 1 shows quotes from every category of scientific knowledge and scientific practices as they appear in the students’ documents.

Table 1.
Categories and codes used for the analysis, as well as quotes from students.1
Table 1.
Categories and codes used for the analysis, as well as quotes from students.1
| Category | Code | Quotes |
|---|---|---|
| Knowledge of the discipline (Electrochemical knowledge) | CKnw | “…to prepare the electrolyte solution. A typical concentration can be around 0.1 M to 0.5 M. The main half-reactions are as follows: oxidation: Al → Al3+ + 3e Reduction: 2H2O + 2e → H+ + 2OH− Global reaction: 2Al + 6H2O → 2Al(OH)3 + 3H2(g)” |
| Transversal knowledge | TransKnw | “biological treatment, it should be taken into account that the effluent is not suitable for consumption due to some bacteria, viruses or pathogens that were not eliminated in the previous process…” |
| Modeling | Mod | In this case, students represent their electrochemical cell using graphs, equations, and interphase sub-micro representations (see Figure 2) |
| Argumentation | Arg | “Water is a substance with a high dielectric constant, which allows the dissociation of inorganic salts and allows the solutions to conduct electricity; in this case, the use of water should ideally be drinkable, but because other electrolytes are also available, this can increase the probability of finding the desired final product” |
| Context | Ctx | “Given the high amounts of water used only in domestic activities, like washing clothes, dishes, or hands, the concern arises that, in most cases, this water has only one use and is then disposed of into the drainage system or even the ground on the street” |
| Electrochemical thinking | EchThink | “The conductivity of an electrochemical cell is crucial for its efficient operation, especially in processes such as electrocoagulation. Understanding the difference between electrode as an electronic conductor and electrolyte as an ionic conductor is critical to optimizing cell performance” |
| Engineering Practice | EngP | “Although the concept may sound simple, its execution requires careful design and rigorous testing to ensure its efficacy and safety” |
| Sustainability | Sust | “The problems of water stress, water pollution, poor waste management, and the lack of awareness about the consumption of single-use objects that we discard by turning valuable materials into garbage are urgent global challenges”. |
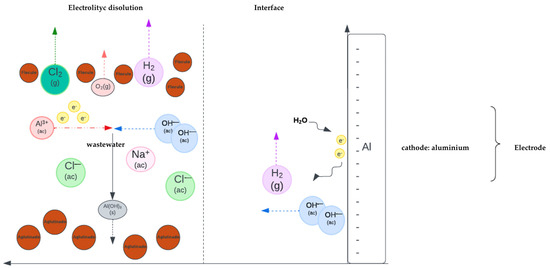
Figure 2.
Students’ representation of molecules and ions at the bulk and cathode interface shows hydrogen evolution on Al(s) in an alkaline environment. It uses particulate representations, arrows to show interactions and movement, and double-layer x-y graphic model representation.
Figure 2.
Students’ representation of molecules and ions at the bulk and cathode interface shows hydrogen evolution on Al(s) in an alkaline environment. It uses particulate representations, arrows to show interactions and movement, and double-layer x-y graphic model representation.
3. Analysis of Results
The analysis using Atlas.ti(R) revealed the frequency of different codes and their co-occurrences, providing insights into the relationships between categories described in Table 1. Word Clouds diagrams elicit the language the students know and use, while Sankey exhibits conceptual relations that could gather complex thinking.
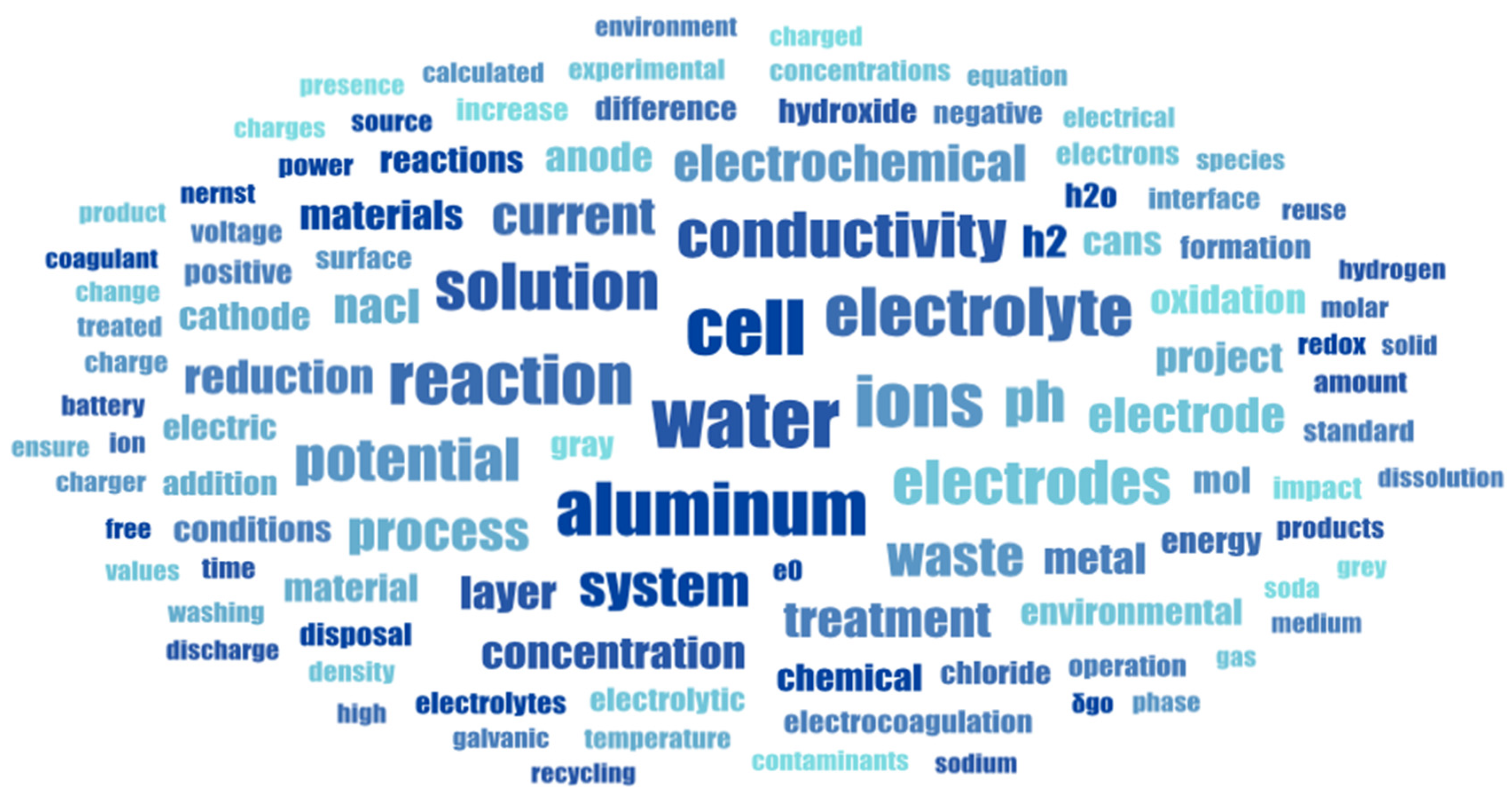
The Word Cloud in Figure 3 illustrates the most frequent terms used by students, after leaving only those words directly related to the problem and content knowledge. It shows the students’ language in all five teams on their final report. Subject matter language can be recognized, as well as elements associated with the different categories of analysis. The most frequent words show a complete picture of the electrochemical discussion in the specific context of wastewater treatment using aluminum electrodes.
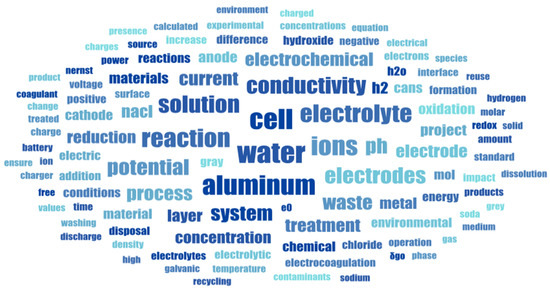
Figure 3.
Word Cloud outcome of the language used on the report of selected teams.
Atlas.ti(R) allows us to identify the occurrence of the different codes analyzed and bring forth the relationships that happen between them, that is, the co-occurrences. For this reason, the most frequent codes will be reviewed for the whole group and for three specific teams: Aquacan, Aluminiums, and Electrocoagulation.
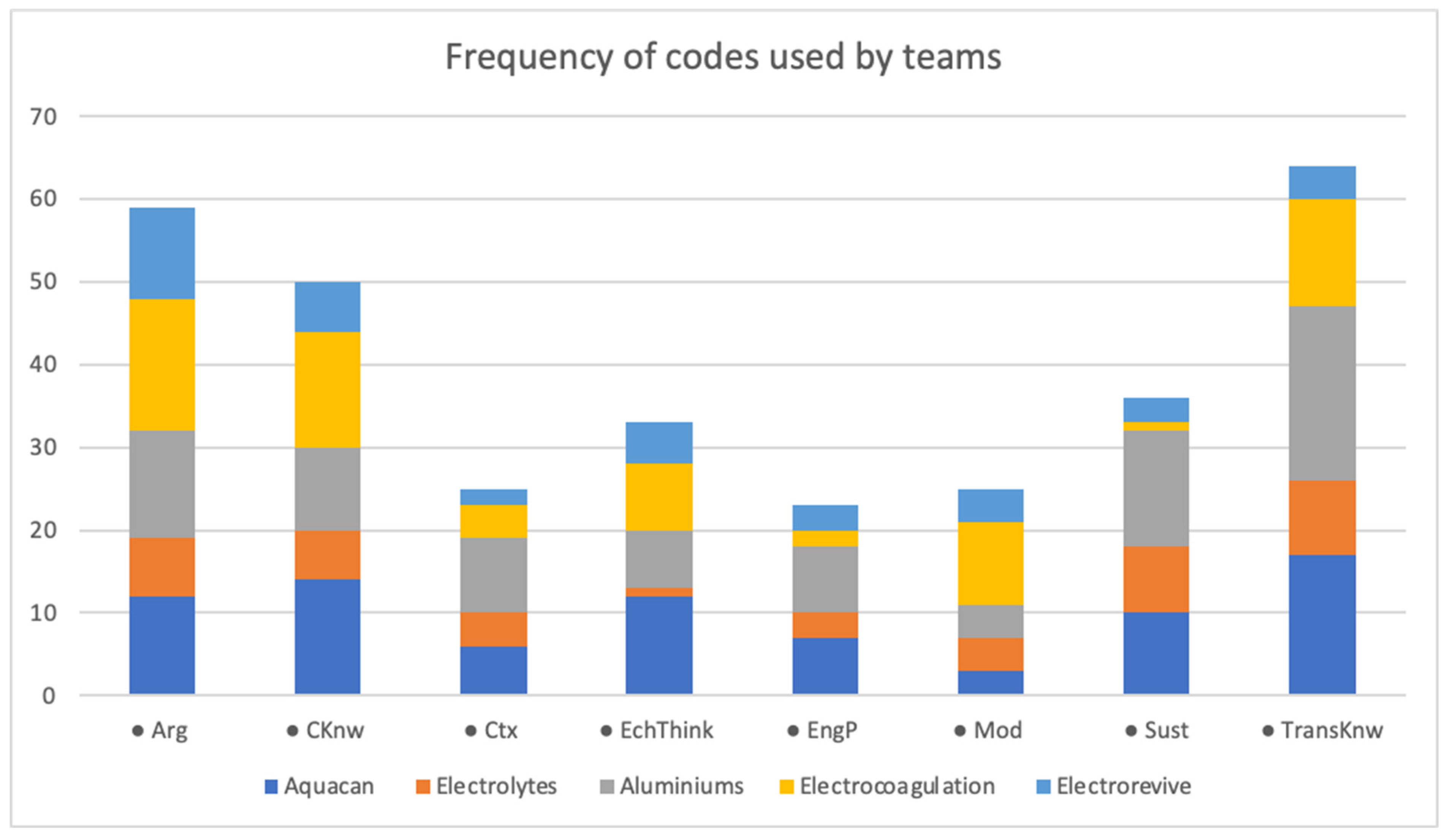
Figure 4 shows the general case of occurrences. All teams exhibit the presence of all codes; however, some developed more robust knowledge than others. The sizes of color bars indicate the occurrence for every code; for example, the “Aluminiums” and “Aquacan” teams obtained more robust knowledge than “Electrovives” and “Electrolytes”. This is also related to the reports presented; some were longer and argumentative, while others just showed the content knowledge and some elements of argumentation and transversal knowledge.
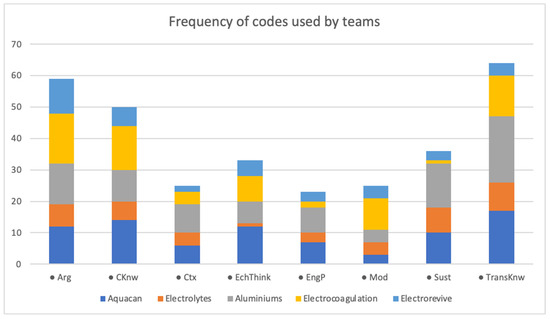
Figure 4.
Bar diagrams of codes for every team. Codes are the following: Arg (argumentation), CKnw (electrochemical knowledge), Ctx (context), EcThink (electrochemical thinking), EngP (engineering practices), Mod (models), Sust (sustainability), TransKnow (transversal know).
Moreover, three code categories appear more frequently: transversal knowledge (TransKnow), scientific argumentation practice (Arg), and content knowledge or electrochemical knowledge (CKnow). To a greater or lesser extent, all five teams show a high occurrence of these codes. This frequency is expected since students must use content knowledge to address their problems. Also, they must be able to justify and/or argue in such terms the relevance of the proposed solution; however, it is interesting that transversal knowledge appears the most. This category involves the knowledge students acquire in previous courses and in the inquiry phase, which allows them to strengthen their learning and use it practically in this subject.
PBL proposals highlight the relevance of knowledge thinking and application in disciplinary contexts. As students’ views become more complex, they need knowledge from other courses (TransKnow) for better contextualization. As a result, they develop a thought of electrochemistry inquiry much more like electrochemistry professional thinking. In contrast, traditional electrochemistry courses taught at the university are focused on subject matter knowledge only, with large amounts of mathematical foundation but without necessarily developing chemical and electrochemical thinking. Therefore, for these types of courses, this study would expect codes mainly related to content knowledge, without a clear context or a relationship of transversal knowledge.
On the other hand, from the perspective of problem-solving within a context, students must use elements, such as those described in Table 1, as ways to integrate them to formulate better arguments. Complex thinking means applying all the elements of scientific thinking within a context and with elements related to the environmental problem trying to be solved. Likewise, it requires using argumentation in a way there is evidence of critical thinking, question formulation, problem-solving, decision-making, systemic and evaluative thinking, and transfer, as Zoller and Nahum (2012) indicate as HOTS.
Sustainability and electrochemical thinking were the second most common codes. Since the problem posed is for wastewater treatment, sustainability is expected to appear frequently in student discourse. Nevertheless, some teams were better at this issue than others, highlighting that their work not only integrates sustainability but also elements of the environment. However, students do not necessarily consider this; there is not much evidence of this code in some cases.
Electrochemical thinking (ElechThink) shows how students understand and use electrochemical cells, an essential and necessary content of this course. This code is particularly relevant for the study as it shows that students are getting a real, complex understanding of the subject and how this can help solve different environmental and energetic issues.
It is also interesting to note that engineering practice (EngP) is an element that, in many cases, is taken for granted. Teachers do not explain to students how to build apparatuses or equipment, but it is mandatory in this course, the first author posits. This code appears less in some teams, suggesting how difficult it could be for students.
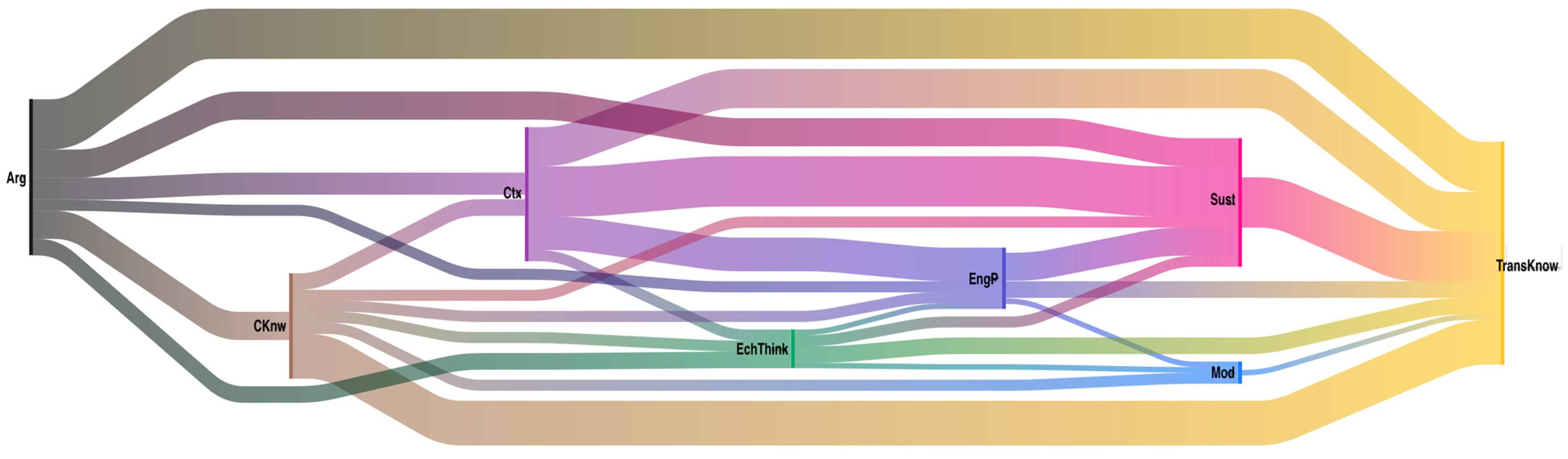
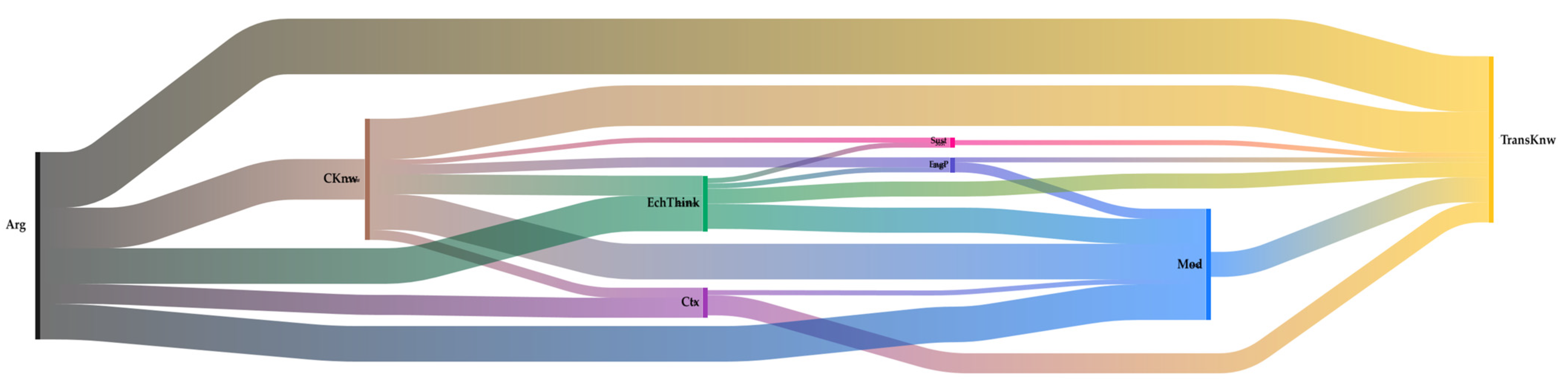
Another element that can be analyzed is how codes are interrelated; this phenomenon is called co-occurrence. To perform this, the Sankey diagrams represent two general ideas: the frequency of each code (occurrence), indicated by the thickness of the flow lines, and how these codes relate to each other (co—occurrences). Figure 5 shows the Sankey diagram for the co-occurrences of the “Aluminiums” team. This type of diagram represents the complexity of thinking the students acquire. In this case, as in all teams, the most recurrent codes were argumentation (Arg) and transversal knowledge (TransKnw), so it was of interest to know which other codes were related to these.
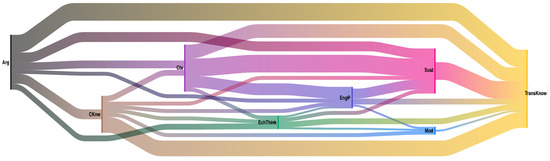
Figure 5.
Co-occurrence diagram for the Aluminums team. The colors represent the different codes: black (argumentation), yellow (transversal knowledge), pink (sustainability), brown (content knowledge), light blue (modeling), dark blue (engineering practice), green (electrochemical thinking), and purple (context).
A direct relationship of co-occurrence between argumentative practice and transversal knowledge is evident. Students justify and/or argue whenever they use transversal knowledge. However, a closer look reveals relationships that go through other codes, for example, Arg—EngP—Sust—TransKnow; this means that students used these four codes interrelated simultaneously for insights. It is the same case for Arg—CKnow—EchThink—Sust—TransKnow. It is possible to observe the complexity of the thinking structure that students are building, which, the authors believe, is directly related to implementing this PBL approach in the electrochemistry course.
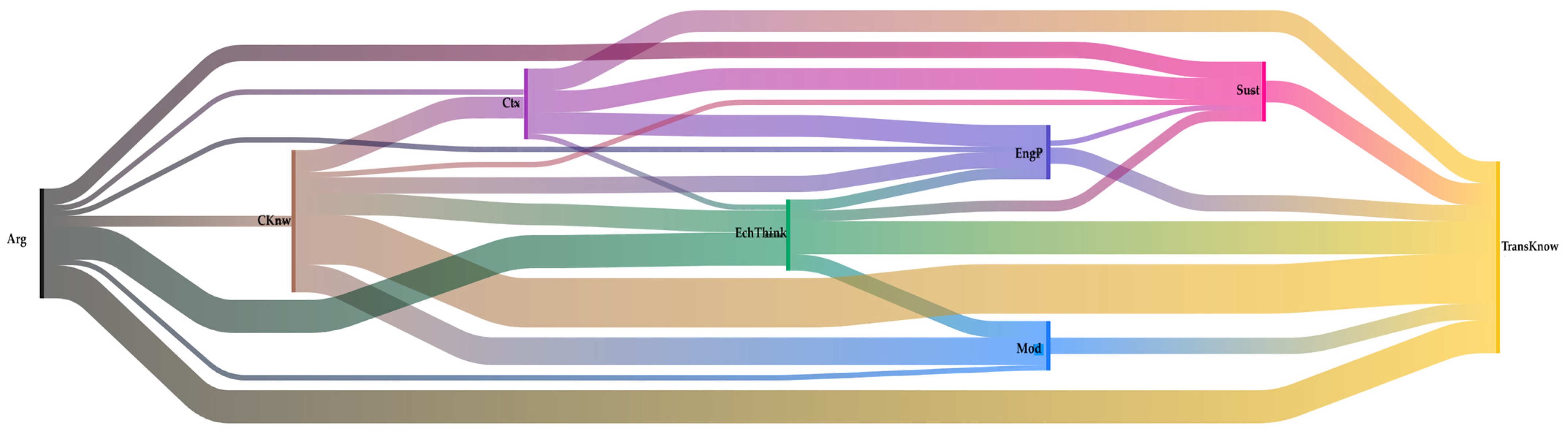
Figure 6 shows a diagram of co-occurrences for the Aquacan team, which differs significantly from the Aluminiums. For Aquacan, codes such as electrochemical thinking and modeling have a higher frequency than sustainability. However, both argumentation and transversal knowledge are the most frequent. In this case, it is possible to visualize relationships between Arg—EchThink—TransKnw, but there is also a complex relationship between CKnw—Mod—TransKnw. Another noticeable aspect is the more significant occurrence of model code, presenting relationships mainly with subject matter knowledge, as well as electrochemical thinking and transversal knowledge.
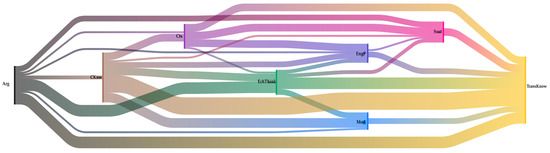
Figure 6.
Sankey diagram over co-occurrences of the Aquacan team. We can see how students’ thinking is complex but different from the Aluminiums team. The colors represent the various codes: black (argumentation), yellow (TransKnow. transversal knowledge), pink (sustainability), brown (content knowledge), light blue (modeling), dark blue (engineering practice), green (electrochemical thinking), and purple (context).
The fact that electrochemical thinking becomes relevant to the findings is essential to this work. It could mean that students are integrating the whole content knowledge into more complex possibilities, such as solving problematic situations and recognizing that electrochemistry is not just a subject to be memorized but to be understood. For the Aquacan team, as well as for the Electrocoagulation team (see Figure 7), electrochemical thinking appears frequently linked with other knowledge and practices. This is evidence of students explaining how they constructed their apparatus (EngP), how it worked, and how it was related to their theoretical calculations (CKnw), while at the same time, they applied a scientific perspective using modeling (Mod) and transversal knowledge (TransKnow) to support their electrochemical knowledge (CKnow) arguments (Arg). All of this is justified around context (Ctx) and with a sustainability perspective (Sust).
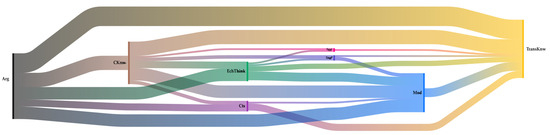
Figure 7.
Sankey diagram over co-occurrences of the Electrocoagulation team.
Students achieved a model that acted as a “hub” for incorporating scientific and empirical knowledge and pragmatic criteria concerning the solution, integrating everything into a coherent whole. They posed questions and solved a problem using critical scientific thinking. Also, they made decisions while evaluating their empirical results and justified them using systemic thinking because they not only considered content-electrochemical knowledge but also incorporated context, transversal knowledge, and sustainability. Thus, students attained electrochemical thinking (EchThink) using HOTS. In other words, they achieved complex scientific thinking.
Figure 7 shows the co-occurrence diagram for the Electrocoagulation team. An important feature is the significant presence of model code, which is more frequently related to argumentation and knowledge of the discipline. Still, there is also a complex interrelatedness between Arg—Cknw—EchThink—Mod—TransKnw. In this case, codes related to context and sustainability appear with minimal occurrence, indicating that although students could develop some complex thinking, they only prioritized those elements related to scientific thinking. Unlike the other teams, where the context and sustainability look more robust, this team approaches the problem’s solution from a more superficial perspective.
The colors represent the different codes: black (argumentation), yellow (transversal knowledge), pink (sustainability), brown (content knowledge), light blue (modeling), dark blue (engineering practice), green (electrochemical thinking), and purple (context).
The findings show evidence that implementing PBL courses in undergrad studies could help students develop content knowledge and, at the same time, some scientific skills. Both let students develop different levels of critical thinking focused on the subject matter, electrochemistry, but in a contextualized way.
In the literature, the studies related to electrochemistry teaching using the PBL approach assess how much content is learned by students after being taught through this approach. One example is Acar and Tarhan (2007); these authors analyze how much students in 11th grade improve their understanding of some concepts related to electrochemistry. They found that students remediate common misconceptions when implementing a cooperative learning group approach. Another study by Günter and Alpat (2017) shows the results of executing the PBL approach in an analytical chemistry course while teaching electrochemistry. The authors identified that students understood subject matter knowledge better if they felt more motivated.
Tsaparlis (2019) also conducted a review of the electrochemistry teaching–learning process where he shows some of the few papers about electrochemistry PBL; and only one of them presents an undergrad strategy, albeit Tsaparlis (2019) mentioned that one of these papers did not show evidence of research results. Comparing these papers with this one, the authors could say that one significant difference is that the present research study assesses if students develop complex thinking when they must solve an electrochemical problem. The researchers considered that it is essential for students to improve their electrochemical understanding; however, they must provide more information about knowledge and skills related to scientific thinking and engineering practices, which is related to higher-order thinking skills.
4. Student Perceptions
It is challenging for teachers at the university level to get involved in courses with these characteristics. Students mainly focus on more traditional learning and achieving content knowledge. Therefore, knowing the students’ opinions at the end of the course became a priority for this study. In the comments, students highlight various aspects of the PBL approach, such as teamwork and the perspective of the context as the center of problem-solving. It is possible to distinguish that they perceive the development of elements of complexity in the structuring of the resolution of their problem. In addition, students recognize the importance of electrochemistry in an everyday context and not just seeing it as another subject. Below are two comments from students who belong to the studied teams:
Student 1. Aluminiums team: “Working on a team project to study an electrocoagulation cell intended for greywater treatment using aluminum electrodes has been a deeply enriching and challenging experience. In particular, the fact that the aluminum used was recovered from recycled cans added an extra layer of interest and sustainability to our project. This approach allowed us to apply theoretical principles of electrochemistry and contribute to the reuse of materials and the promotion of eco-friendly practices. One of the most significant learnings was understanding the complexity and variables involved in the electrocoagulation process. Each step required meticulous planning and in-depth knowledge of electrochemical phenomena, from preparing and treating recycled aluminum to optimizing the cell’s operating conditions. We learned how to evaluate and adjust parameters such as current density, water pH, and electrolyte concentration to maximize contaminant removal efficiency”.
Student 2. Electrovives team: “We address a crucial problem by seeking to revitalize wastewater through electrocoagulation. This innovative technique offers a practical solution for wastewater treatment and highlights the importance of sustainability and water conservation. It is a good proposal for students to apply the concepts seen in the course as if we were in the lab, in addition to the fact that to carry out the project, we acquire greater knowledge. These projects offer a tangible perspective on how electrochemistry has useful applications for solving real-life problems, which are significant topics today”.
5. Limitations of Study
This study is limited to grouping students’ work. However, the authors consider it possible to collect individual data and compare whether this complex thinking is also developed in each student.
6. Conclusions
This research study presents the implementation of an electrochemistry course contextualized in a problematic situation, electrocoagulation, which may allow students to develop complex thinking about disciplinary content. Such implementation requires that students build their electrochemical knowledge from the base of transversal knowledge that has to do with scientific practices other than electrochemistry, such as characterization methods and sustainability, as well as the context in which this problem is intended to be solved.
To analyze the results of the implementation, the scientific knowledge and practices that involved solving the problem were codified, recognizing the following: content (electrochemical) knowledge, transversal knowledge, electrochemical thinking, sustainability, context, model, and argumentation. The learning outcomes of five teams of students taking electrocoagulation as their problem were analyzed using the Atlas.ti(R) software. Significant advances were found in the students’ electrochemical conceptual understanding, complex way of thinking involving HOTS, and recognition of its relevance in solving real-world problems. This way of analysis recognizes how students think and, simultaneously, how they understand electrochemical knowledge from context and an environmental point of view.
In summary, this study demonstrates that a Problem-Based Learning approach in electrochemistry education can significantly enhance students’ conceptual understanding, complex thinking, and scientific skills. By contextualizing learning within real-world problems, students develop a deeper appreciation for the relevance of electrochemistry in addressing everyday challenges. The integration of theoretical knowledge, practical experimentation, and collaborative work prepares students for the interdisciplinary and complex nature of scientific inquiry in the modern world.
Author Contributions
All authors contributed to the study’s conception and design. A.R.-M. performed material preparation and data collection. Both authors performed the data analysis and wrote the first draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by DGAPA, UNAM grant number PAPIME PE2004223.
Institutional Review Board Statement
In this study, the ethics/human subject requirements of our institution were met when the data was collected.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
Note
| 1 | In column three are shown students’ answers; the authors decided not to change or correct them. |
References
- Acar, B., & Tarhan, L. (2007). Effect of cooperative learning strategies on students’ understanding of concepts in electrochemistry. International Journal of Science and Mathematics Education, 5(2), 349–373. [Google Scholar] [CrossRef]
- Aidoo, B., Boateng, S. K., Kissi, P. S., & Ofori, I. (2016). Effect of problem-based learning on students’ achievement in chemistry. Journal of Education and Practice, 7(33), 103–108. [Google Scholar]
- Bard, A. J., & Faulkner, L. R. (2000). Electrochemical methods: Fundamentals and applications. Wiley. [Google Scholar]
- Barke, H.-D., Hazari, A., & Yitbarek, S. (2009). Misconceptions in chemistry. Addressing perceptions in chemical education. Springer. [Google Scholar]
- Boon, M., Michelfelder, D., Doorn, N., & Philosophy. (2020). Scientific methodology in the engineering sciences. In D. P. Michelfelder, & N. Doorn. (Eds.), The routledge handbook of the philosophy of engineering. Routledge. [Google Scholar]
- Boon, M., Orozco, M., & Sivakumar, K. (2022). Epistemological and educational issues in teaching practice-oriented scientific research: Roles for philosophers of science. European Journal for Philosophy of Science, 12(1), 16. [Google Scholar] [CrossRef]
- Boulabiar, A., Bouraoui, K., Chastrette, M., & Abderrabba, M. (2004). A historical analysis of the daniell cell and electrochemistry teaching in french and tunisian textbooks. Journal of Chemical Education, 81(5), 754. [Google Scholar] [CrossRef]
- Brandriet, X., & Lowery, B. (2014). The development of the redox concept inventory as a measure of students’ symbolic and particulate redox understandings and confidence. Journal of Chemical Education, 91, 1132−1144. [Google Scholar] [CrossRef]
- Brett, C. M. A., & Oliveira-Brett, A. M. (2020). Future tasks of electrochemical research. Journal of Solid State Electrochemistry, 24(9), 2051–2052. [Google Scholar] [CrossRef]
- Das, P. P., Sharma, M., & Purkait, M. K. (2022). Recent progress on electrocoagulation process for wastewater treatment: A review. Separation and Purification Technology, 292, 121058. [Google Scholar] [CrossRef]
- De Jong, O., & Treagust, D. F. (2002). The teaching and learning of electrochemistry. In J. K. Gilbert, O. De Jong, R. Justi, D. Treagust, & J. Van Driel (Eds.), Chemical education: Towards research-based practice. Kluwer Academic Publishers. [Google Scholar]
- Dochy, F., Segers, M., Bossheand, P., & Struyven, K. (2005). Students’ perceptions of a problem-based learning environment. Learning Environments Research, 8, 41–66. [Google Scholar] [CrossRef]
- Dochy, F., Segers, M., Van den Bossche, P., & Gijbels, D. (2003). Effects of problem-based learning: A meta-analysis. Learning and Instruction, 13(5), 533–568. [Google Scholar] [CrossRef]
- Faulkner, L. R. (2014). Reflections on chemistry and electrochemistry after fifty years of practice. The Electrochemical Society Interface, 23(1), 37–38. [Google Scholar] [CrossRef]
- Freeman, S., Eddy, S. L., McDonough, M., Smith, M. K., Okoroafor, N., Jordt, H., & Wenderoth, M. P. (2014). Active learning increases student performance in science, engineering, and mathematics. Proceedings of the National Academy of Sciences United States of America, 111(23), 8410–8415. [Google Scholar] [CrossRef] [PubMed]
- Guile, D. (2006). Learning across contexts. Educational Philosophy and Theory, 38(3), 251–268. [Google Scholar] [CrossRef]
- Günter, T., & Alpat, S. K. (2017). The effects of problem-based learning (PBL) on the academic achievement of students studying ‘Electrochemistry’. Chemistry Education Research and Practice, 18(1), 78. [Google Scholar] [CrossRef]
- Günter, T., Akkuzu, N., & Alpat, Ş. (2017). Understanding ‘green chemistry’ and ‘sustainability’: An example of problem-based learning (PBL). Research in Science & Technological Education, 35(4), 500–520. [Google Scholar] [CrossRef]
- Hmelo-Silver, C. E. (2004). Problem based learning: What and how students learn? Educational Psychology Review, 16(3), 235–266. [Google Scholar] [CrossRef]
- Hung, W., & Amida, A. (2020). Problem-based learning in college science. In J. J. Mintzes, & E. M. Walter (Eds.), Active learning in college science. Springer. [Google Scholar] [CrossRef]
- Jansson, S., Söderströms, H., Andersson, P. L., & Nording, M. L. (2015). Implementation of problem-based learning in environ-mental chemistry. Journal of Chemical Education, 92, 2080−2086. [Google Scholar] [CrossRef]
- Johnstone, A. H. (2010). You can’t get there from here. Journal of Chemical Education, 87(1), 22–29. [Google Scholar] [CrossRef]
- Kempler, P. A., Boettcher, S. W., & Ardo, S. (2021). Reinvigorating electrochemistry education. IScience, 24(5), 102481. [Google Scholar] [CrossRef] [PubMed]
- Lee, T. T., & Osmam, K. (2017). Chapter 6. Misconceptions in electrochemistry: How do pedagogical agents help? In M. Karpudewan, A. Md Zain, & A. Chandrasegaran (Eds.), Overcoming students’ misconceptions in science. Springer. [Google Scholar] [CrossRef]
- Lu, H., Jiang, Y., & Bi, H. (2020). Development of a measurement instrument to assess student’s proficiency levels regarding galvanic cells. Chemistry Education Research and Practice, 21, 655–667. [Google Scholar] [CrossRef]
- Mataka, L. M., & Kowalske, M. G. (2015). The influence of PBL on students’ self-efficacy beliefs in chemistry. Chemistry Education Research and Practice, 16(4), 929–938. [Google Scholar] [CrossRef]
- Odell, M. R. L., & Pedersen, J. L. (2020). Chapter 23: Project and problem-based teaching and learning. In B. Akpan, & T. Kennedy (Eds.), Science education in theory and practice, springer texts in education. Springer. [Google Scholar]
- Overton, T. L., & Randles, C. A. (2015). Beyond problem-based learning: Using dynamic PBL in chemistry. Chemistry Education Research and Practice, 16(2), 251–259. [Google Scholar] [CrossRef]
- Rahayu, S., Treagust, D. F., & Chandrasegaran, A. L. (2022). High school and preservice chemistry teacher education students’ understanding of voltaic and electrolytic cell concepts: Evidence of consistent learning difficulties across years. International Journal of Science and Mathematics Education, 20(8), 1859–1882. [Google Scholar] [CrossRef]
- Ramos-Mejía, A. (2020). ¿Cómo se puede usar el celular como pretexto para enseñar la Tabla Periódica? Educación Química, 31(1), 49–61. [Google Scholar] [CrossRef]
- Sanger, M. J., & Greenbowe, T. J. (1997). Students’ misconceptions in electrochemistry: Current flow in electrolyte solutions and the salt bridge. Journal of Chemical Education, 74(7), 819. [Google Scholar] [CrossRef]
- Sanger, M. J., & Greenbowe, T. J. (1999). An analysis of college chemistry textbooks as sources of misconceptions and errors in electrochemistry. Journal of Chemical Education, 76(6). [Google Scholar] [CrossRef]
- Sanmartín, S.-P., & Sanjosé, V. (2014). An approach to the conceptions of pre-university and university students about galvanic batteries. Educación Química, 25(2), 139–147. [Google Scholar] [CrossRef]
- Savery, J. R. (2006). Overview of problem-based learning: Definitions and distinctions. Interdisciplinary Journal of Problem-Based Learning, 1(1), 5–15. [Google Scholar] [CrossRef]
- Shahedi, A., Darban, A., Taghipour, F., & Jamshidi-Zanjani, A. (2020). A review on industrial wastewater treatment via electrocoagulation processes. Current Opinion in Electrochemistry, 22, 154–169. [Google Scholar] [CrossRef]
- Taber, K. S. (2019). Alternative conceptions and the learning of chemistry. Israel Journal of Chemistry, 59, 450–469. [Google Scholar] [CrossRef]
- Tsaparlis, G. (2019). Teaching and learning electrochemistry. Israel Journal of Chemistry, 59(6–7), 478–492. [Google Scholar] [CrossRef]
- Turner, K. L., He, S., Marchegiani, B., Read, S., Blackburn, J., Miah, N., & Leketas, M. (2024). Around the world in electrochemistry: A review of the electrochemistry curriculum in high schools. Journal of Solid State Electrochemistry, 28(3–4), 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Varadarajan, S., & Ladage, S. (2024). Problem-based learning (PBL): A literature review of theory and practice in undergrad-uate chemistry laboratories. Journal of Chemical Education, 101, 3027−3038. [Google Scholar] [CrossRef]
- Wang, X., & Wang, Z. (2022). Animated electrochemistry simulation modules. Journal of Chemical Education, 99, 752–758. [Google Scholar] [CrossRef]
- Zoller, U., & Nahum, T. L. (2012). Chapter 16: From teaching to know to learning to think in science education. In B. J. Fraser, K. G. Tobin, & C. J. McRobbie (Eds.), Second international handbook of science education. Springer International Handbooks of Education. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).