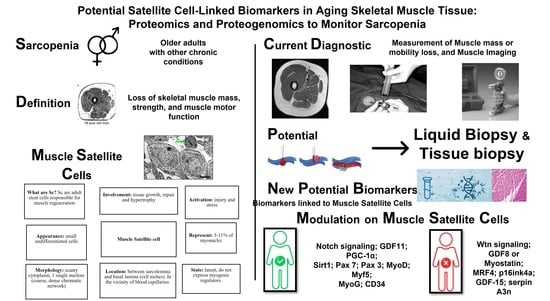
Potential Satellite Cell-Linked Biomarkers in Aging Skeletal Muscle Tissue: Proteomics and Proteogenomics to Monitor Sarcopenia
Abstract
:1. Introduction
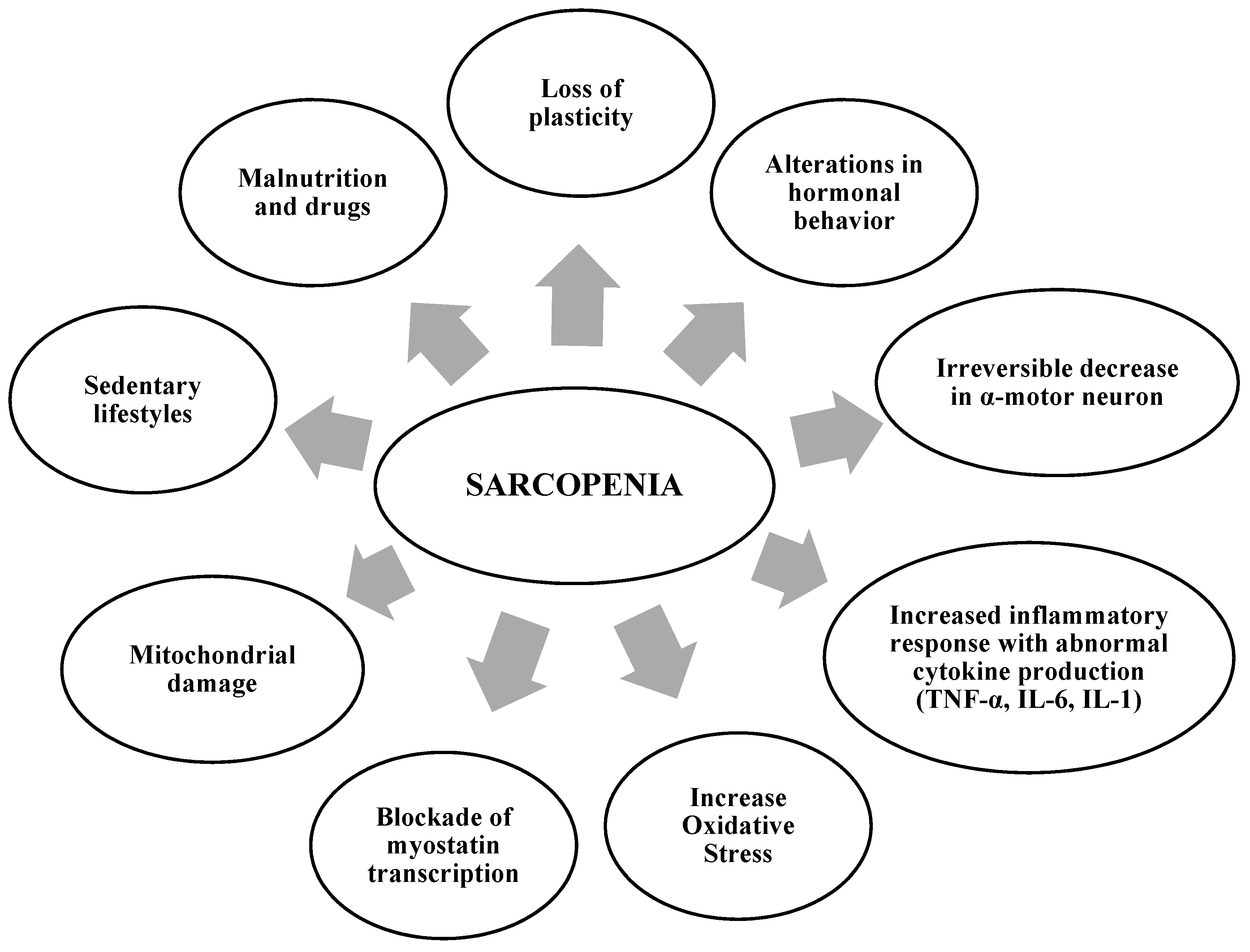
2. Factors That Contribute to the Development of Sarcopenia
3. Biological Basis of Skeletal Muscle in Aging
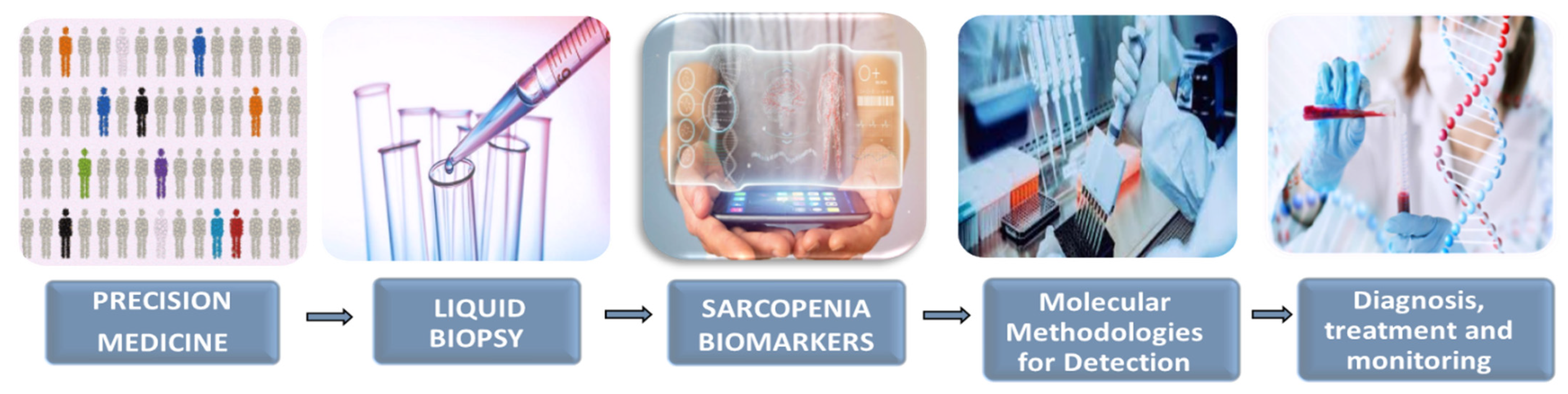
4. Liquid Biopsy as a New Tool in Precision Medicine: Potential Application in Sarcopenia
5. Potential Proteogenomic Biomarkers Involved in Sarcopenia
5.1. Notch Signaling
5.2. Wtn Signaling
5.3. Growth Differentiation Factor 11 (GDF11)
5.4. Myostatin or Growth Differentiation Factor 8 (GDF8)
5.5. Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1α (PGC-1α)
5.6. Sirtuin 1 (Sirt1)
5.7. Paired Box 7 (Pax 7)
5.8. Paired Box 3 (Pax 3)
5.9. Myogenic Regulatory Factors (MRFs)
5.10. CD34
5.11. p16ink4a: P16 Cyclin-Dependent Kinase 2A Inhibitor
6. Musculoskeletal Tissue Biopsy Samples Are a Key Diagnostic Tool
Characterized the Human Muscle Proteome
7. Role of the Proteome in Aging
Changes in the Proteome after Physical Exercise
8. Potential Proteomic Biomarkers Involved in Aging
8.1. Growth Differentiation Factor 15 (GDF-15)
8.2. Protein Alterations in Skeletal Muscle Atrophy
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Khan, S.S.; Singer, B.D.; Vaughan, D.E. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell 2017, 16, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and Clinical Relevance. Clin. Geriatr. Med. 2011, 27, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Papadopoulou, S.K. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Sakuma, K.; Yamaguchi, A. Sarcopenia and Age-Related Endocrine Function. Int. J. Endocrinol. 2012, 2012, 127362. [Google Scholar] [CrossRef]
- Kwon, Y.N.; Yoon, S.S. Sarcopenia: Neurological Point of View. J. Bone Metab. 2017, 24, 83–89. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Williams, F.R.; Quinlan, J.I.; Allen, S.L.; Greig, C.; Filer, A.; Raza, K.; Ghosh, S.; Lavery, G.G.; Newsome, P.N.; et al. Evaluation of the mechanisms of sarcopenia in chronic inflammatory disease: Protocol for a prospective cohort study. Skelet. Muscle 2021, 11, 27. [Google Scholar] [CrossRef]
- Campos, F.; Abrigo, J.; Aguirre, F.; Garcés, B.; Arrese, M.; Karpen, S.; Cabrera, D.; Andía, M.E.; Simon, F.; Cabello-Verrugio, C. Sarcopenia in a mice model of chronic liver disease: Role of the ubiquitin–proteasome system and oxidative stress. Pflüg. Arch. Eur. J. Physiol. 2018, 470, 1503–1519. [Google Scholar] [CrossRef] [PubMed]
- Welle, S. Cellular and molecular basis of age-related sarcopenia. Can. J. Appl. Physiol. 2002, 27, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Yakabe, M.; Akishita, M. Age-related sarcopenia and its pathophysiological bases. Inflamm. Regen. 2016, 36, 17. [Google Scholar] [CrossRef]
- Masanés Torán, F.; Navarro López, M.; Sacanella Meseguer, E.; López Soto, A. What is sarcopenia? Semin. Fund. Esp. Reum. 2010, 11, 14–23. [Google Scholar]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults—Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lazaro, C.I.; Adams, D.P.; Fernandez-Lazaro, D.; Garcia-González, J.M.; Caballero-Garcia, A.; Miron-Canelo, J.A. Medication adherence and barriers among low-income, uninsured patients with multiple chronic conditions. Res. Soc. Adm. Pharm. 2019, 15, 744–753. [Google Scholar] [CrossRef]
- Lexell, J.; Henriksson-Larsén, K.; Winblad, B.; Sjöström, M. Distribution of different fiber types in human skeletal muscles: Effects of aging studied in whole muscle cross sections. Muscle Nerve 1983, 6, 588–595. [Google Scholar] [CrossRef]
- Yu, J. The etiology and exercise implications of sarcopenia in the elderly. Int. J. Nurs. Sci. 2015, 2, 199–203. [Google Scholar] [CrossRef]
- Cho, M.-R.; Lee, S.; Song, S.-K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef]
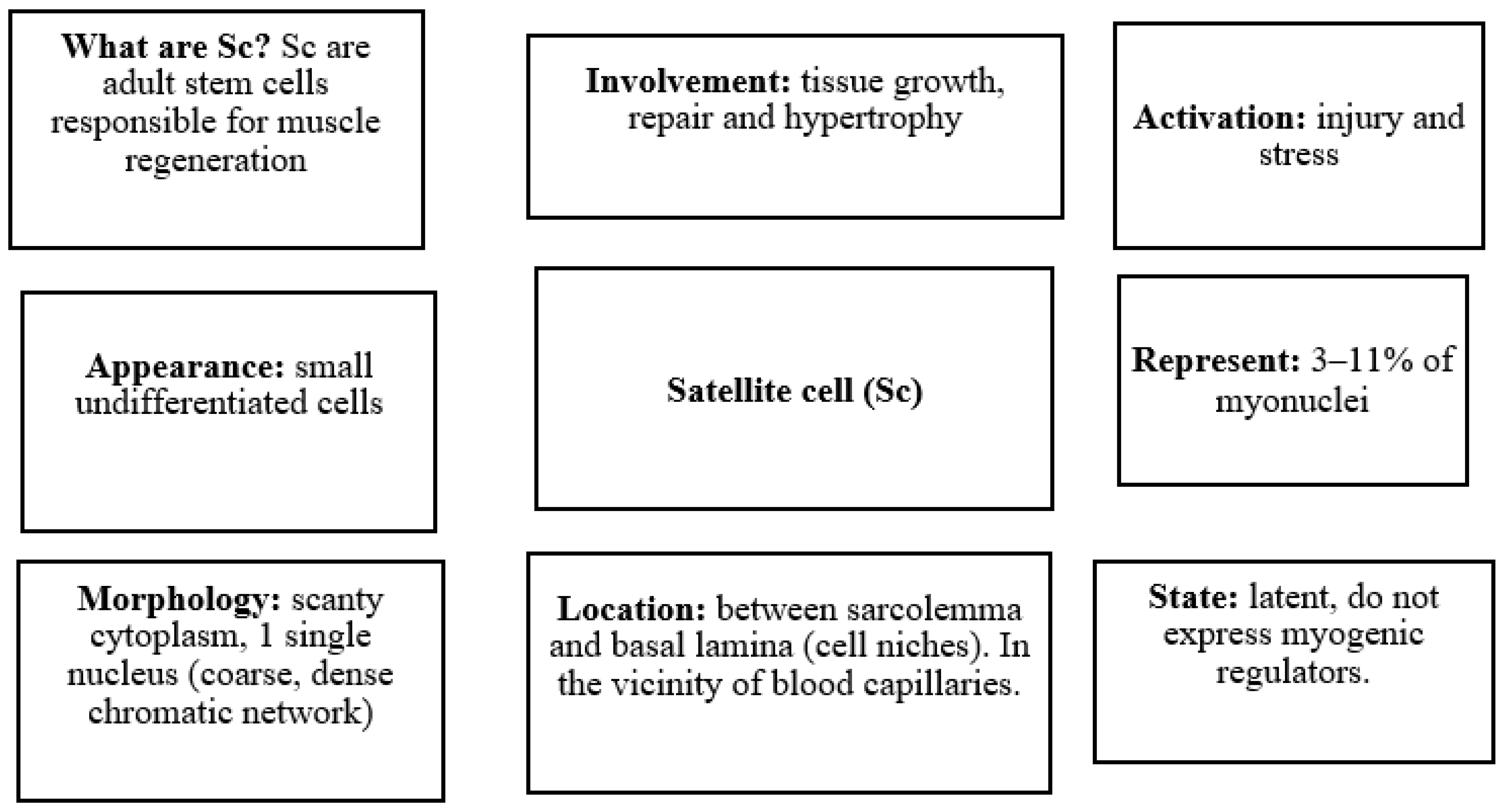
- Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493–495. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite Cells and the Muscle Stem Cell Niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.E.; Partridge, T.A. Muscle satellite cells. Int. J. Biochem. Cell Biol. 2003, 35, 1151–1156. [Google Scholar] [CrossRef]
- Fuchs, E.; Blau, H.M. Tissue Stem Cells: Architects of Their Niches. Cell Stem Cell 2020, 27, 532–556. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Victor, P.; García-Prat, L.; Muñoz-Cánoves, P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat. Rev. Mol. Cell Biol. 2021, 23, 204–226. [Google Scholar] [CrossRef] [PubMed]
- Fry, C.S.; Lee, J.D.; Mula, J.; Kirby, T.J.; Jackson, J.R.; Liu, F.; Yang, L.; Mendias, C.L.; Dupont-Versteegden, E.E.; McCarthy, J.J.; et al. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat. Med. 2014, 21, 76–80. [Google Scholar] [CrossRef]
- Sharpless, N.E.; DePinho, R.A. How stem cells age and why this makes us grow old. Nat. Rev. Mol. Cell. Biol. 2007, 8, 703–713. [Google Scholar] [CrossRef]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar]
- Fernández-Lázaro, D.; Hernández, J.L.G.; García, A.C.; Martínez, A.C.; Mielgo-Ayuso, J.; Cruz-Hernández, J.J. Liquid Biopsy as Novel Tool in Precision Medicine: Origins, Properties, Identification and Clinical Perspective of Cancer’s Biomarkers. Diagnostics 2020, 10, 215. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Hernández, J.L.G.; García, A.C.; del Castillo, A.C.; Hueso, M.V.; Cruz-Hernández, J.J. Clinical Perspective and Translational Oncology of Liquid Biopsy. Diagnostics 2020, 10, 443. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Hwang, H.; Kim, S.K.; Choi, J.Y.; Lee, S.M.; Bang, H.; Kwon, E.S.; Lee, K.P.; Chung, S.G.; Kwon, K.S. Prediction of sarcopenia using a combination of multiple serum biomarkers. Sci. Rep. 2018, 8, 8574. [Google Scholar] [CrossRef]
- Conboy, I.M.; Conboy, M.J.; Smythe, G.M.; Rando, T.A. Notch-Mediated Restoration of Regenerative Potential to Aged Muscle. Science 2003, 302, 1575–1577. [Google Scholar] [CrossRef] [PubMed]
- Buas, M.F.; Kadesch, T. Regulation of skeletal myogenesis by Notch. Exp. Cell Res. 2010, 316, 3028–3033. [Google Scholar] [CrossRef] [PubMed]
- Tsivitse, S. Notch and Wnt signaling, physiological stimuli and postnatal myogenesis. Int. J. Biol. Sci. 2010, 6, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Arthur, S.T.; Cooley, I.D. The Effect of Physiological Stimuli on Sarcopenia; Impact of Notch and Wnt Signaling on Impaired Aged Skeletal Muscle Repair. Int. J. Biol. Sci. 2012, 8, 731–760. [Google Scholar] [CrossRef] [PubMed]
- Brack, A.S.; Conboy, M.J.; Roy, S.; Lee, M.; Kuo, C.J.; Keller, C.; Rando, T.A. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 2007, 317, 807–810. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y.; Han, F.; Qiu, H.; Shi, J.; Huang, N.; Hou, N.; Sun, X. Growth differentiation factor 11: A “rejuvenation factor” involved in regulation of age-related diseases? Aging 2021, 13, 12258–12272. [Google Scholar] [CrossRef]
- Tominaga, K.; Suzuki, H.I. TGF-β Signaling in Cellular Senescence and Aging-Related Pathology. Int. J. Mol. Sci. 2019, 20, 5002. [Google Scholar] [CrossRef]
- Sinha, M.; Jang, Y.C.; Oh, J.; Khong, D.; Wu, E.Y.; Manohar, R.; Miller, C.; Regalado, S.G.; Loffredo, F.S.; Pancoast, J.R.; et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 2014, 344, 649–652. [Google Scholar] [CrossRef]
- Guerfali, I.; Manissolle, C.; Durieux, A.C.; Bonnefoy, R.; Bartegi, A.; Freyssenet, D. Calcineurin A and CaMKIV transactivate PGC-1alpha promoter, but differentially regulate cytochrome c promoter in rat skeletal muscle. Pflüg. Arch. Eur. J. Physiol. 2007, 454, 297–305. [Google Scholar] [CrossRef]
- Wright, D.C.; Han, D.H.; Garcia-Roves, P.M.; Geiger, P.C.; Jones, T.E.; Holloszy, J.O. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J. Biol. Chem. 2007, 282, 194–199. [Google Scholar] [CrossRef]
- Anderson, R.; Prolla, T. PGC-1α in aging and anti-aging interventions. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2009, 1790, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Dinulovic, I.; Furrer, R.; Beer, M.; Ferry, A.; Cardel, B.; Handschin, C. Muscle PGC-1α modulates satellite cell number and proliferation by remodeling the stem cell niche. Skelet. Muscle 2016, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Vignaud, A.; Cebrian, J.; Martelly, I.; Caruelle, J.P.; Ferry, A. Effect of anti-inflammatory and antioxidant drugs on the long-term repair of severely injured mouse skeletal muscle. Exp. Physiol. 2005, 90, 487–495. [Google Scholar] [CrossRef]
- Alway, S.E.; Myers, M.J.; Mohamed, J.S. Regulation of satellite cell function in sarcopenia. Front. Aging Neurosci. 2014, 6, 246. [Google Scholar] [CrossRef]
- Myers, M.J.; Shepherd, D.L.; Durr, A.J.; Stanton, D.S.; Mohamed, J.S.; Hollander, J.M.; Alway, S.E. The role of SIRT1 in skeletal muscle function and repair of older mice. J. Cachexia Sarcopenia Muscle 2019, 10, 929–949. [Google Scholar] [CrossRef] [PubMed]
- Paixão-Côrtes, V.R.; Salzano, F.M.; Bortolini, M.C. Origins and evolvability of the PAX family. Semin. Cell Dev. Biol. 2015, 44, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, J.M.; Hawrot, K.; Sharpe, C.; Noble, A.; Wood, W.M.; Jorge, E.C.; Goldhamer, D.J.; Kardon, G.; Dietrich, S. The emergence of Pax7-expressing muscle stem cells during vertebrate head muscle development. Front. Aging Neurosci. 2015, 7, 62. [Google Scholar] [CrossRef]
- Lepper, C.; Partridge, T.A.; Fan, C.M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 2011, 138, 3639–3646. [Google Scholar] [CrossRef]
- Sambasivan, R.; Yao, R.; Kissenpfennig, A.; Van Wittenberghe, L.; Paldi, A.; Gayraud-Morel, B.; Guenou, H.; Malissen, B.; Tajbakhsh, S.; Galy, A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 2011, 138, 3647–3656. [Google Scholar] [CrossRef]
- Relaix, F.; Montarras, D.; Zaffran, S.; Gayraud-Morel, B.; Rocancourt, D.; Tajbakhsh, S.; Mansouri, A.; Cumano, A.; Buckingham, M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J. Cell Biol. 2006, 172, 91–102. [Google Scholar] [CrossRef]
- Wang, M.; Song, W.; Jin, C.; Huang, K.; Yu, Q.; Qi, J.; Zhang, Q.; He, Y. Pax3 and pax7 exhibit distinct and overlapping functions in marking muscle satellite cells and muscle repair in a marine teleost, sebastes schlegelii. Int. J. Mol. Sci. 2021, 22, 3769. [Google Scholar] [CrossRef] [PubMed]
- Filareto, A.; Rinaldi, F.; Arpke, R.W.; Darabi, R.; Belanto, J.J.; Toso, E.A.; Miller, A.Z.; Ervasti, J.M.; McIvor, R.S.; Kyba, M.; et al. Pax3-induced expansion enables the genetic correction of dystrophic satellite cells. Skelet. Muscle 2015, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Asfour, H.A.; Allouh, M.Z.; Said, R.S. Myogenic regulatory factors: The orchestrators of myogenesis after 30 years of discovery. Exp. Biol. Med. 2018, 243, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, T.; Toyono, T.; Inoue, A.; Matsubara, T.; Kawamoto, T.; Kokabu, S. Factors Regulating or Regulated by Myogenic Regulatory Factors in Skeletal Muscle Stem Cells. Cells 2022, 11, 1493. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Legendre, N.P.; Biswas, A.A.; Lawton, A.; Yamamoto, S.; Tajbakhsh, S.; Kardon, G.; Goldhamer, D.J. Loss of MyoD and Myf5 in Skeletal Muscle Stem Cells Results in Altered Myogenic Programming and Failed Regeneration. Stem Cell Rep. 2018, 10, 956–969. [Google Scholar] [CrossRef]
- Rudnicki, M.A.; Jaenisch, R. The MyoD family of transcription factors and skeletal myogenesis. Bioessays 1995, 17, 203–209. [Google Scholar] [CrossRef]
- Rudnicki, M.A.; Schnegelsberg, P.N.J.; Stead, R.H.; Braun, T.; Arnold, H.H.; Jaenisch, R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 1993, 75, 1351–1359. [Google Scholar] [CrossRef]
- Ganassi, M.; Badodi, S.; Wanders, K.; Zammit, P.S.; Hughes, S.M. Myogenin is an essential regulator of adult myofibre growth and muscle stem cell homeostasis. Elife 2020, 9, e60445. [Google Scholar] [CrossRef]
- Hyatt, J.P.K.; Roy, R.R.; Baldwin, K.M.; Edgerton, V.R. Nerve activity-independent regulation of skeletal muscle atrophy: Role of MyoD and myogenin in satellite cells and myonuclei. Am. J. Physiol. Cell Physiol. 2003, 285, C1161–C1173. [Google Scholar] [CrossRef]
- Shen, H.; Lv, Y.; Shen, X.Q.; Xu, J.H.; Lu, H.; Fu, L.C.; Duan, T. Implantation of muscle satellite cells overexpressing myogenin improves denervated muscle atrophy in rats. Braz. J. Med. Biol. Res. 2016, 49, e5124. [Google Scholar] [CrossRef]
- Hinterberger, T.J.; Sassoon, D.A.; Rhodes, S.J.; Konieczny, S.F. Expression of the muscle regulatory factor MRF4 during somite and skeletal myofiber development. Dev. Biol. 1991, 147, 144–156. [Google Scholar] [CrossRef]
- Schiaffino, S.; Dyar, K.A.; Calabria, E. Skeletal muscle mass is controlled by the MRF4–MEF2 axis. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Moretti, I.; Ciciliot, S.; Dyar, K.A.; Abraham, R.; Murgia, M.; Agatea, L.; Akimoto, T.; Bicciato, S.; Forcato, M.; Pierre, P.; et al. MRF4 negatively regulates adult skeletal muscle growth by repressing MEF2 activity. Nat. Commun. 2016, 7, 12397. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.S.; Fackler, M.J.; Civin, C.I.; Stratford May, W. CD34: Structure, biology, and clinical utility [see comments]. Blood 1996, 87, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, J.R.; Heslop, L.; Yu, D.S.; Tajbakhsh, S.; Kelly, R.G.; Wernig, A.; Buckingham, M.E.; Partridge, T.A.; Zammit, P.S. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J. Cell Biol. 2000, 151, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, L.A.; Dick, S.A.; Siegel, A.L.; Anonuevo, A.S.; McNagny, K.M.; Megeney, L.A.; Cornelison, D.D.; Rossi, F.M. CD34 promotes satellite cell motility and entry into proliferation to facilitate efficient skeletal muscle regeneration. Stem Cells 2011, 29, 2030–2041. [Google Scholar] [CrossRef]
- LaPak, K.M.; Burd, C.E. The Molecular Balancing Act of p16INK4a in Cancer and Aging. Mol. Cancer Res. 2014, 12, 167–183. [Google Scholar] [CrossRef]
- Molofsky, A.V.; Slutsky, S.G.; Joseph, N.M.; He, S.; Pardal, R.; Krishnamurthy, J.; Sharpless, N.E.; Morrison, S.J. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 2006, 443, 448–452. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Walters, J.; Baborie, A. Muscle biopsy: What and why and when? Pract. Neurol. 2020, 20, 385–395. [Google Scholar] [CrossRef]
- Ohlendieck, K. Skeletal muscle proteomics: Current approaches, technical challenges and emerging techniques. Skelet. Muscle 2011, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Højlund, K.; Yi, Z.; Hwang, H.; Bowen, B.; Lefort, N.; Flynn, C.R.; Langlais, P.; Weintraub, S.T.; Mandarino, L.J. Characterization of the human skeletal muscle proteome by one-dimensional gel electrophoresis and HPLC-ESI-MS/MS. Mol. Cell. Proteom. 2008, 7, 257–267. [Google Scholar] [CrossRef]
- Tombline, G.; Gigas, J.; Macoretta, N.; Zacher, M.; Emmrich, S.; Zhao, Y.; Seluanov, A.; Gorbunova, V. Proteomics of Long-Lived Mammals. Proteomics 2020, 20, e1800416. [Google Scholar] [CrossRef]
- Isfort, R.J. Proteomic analysis of striated muscle. J. Chromatogr. B 2002, 771, 155–165. [Google Scholar] [CrossRef]
- Mobasheri, A. Proteomic profiling and its applications to muscle aging and sarcopenia. Front. Physiol. 2012, 2, 117. [Google Scholar] [CrossRef] [PubMed]
- Lourenço dos Santos, S.; Baraibar, M.A.; Lundberg, S.; Eeg-Olofsson, O.; Larsson, L.; Friguet, B. Oxidative proteome alterations during skeletal muscle ageing. Redox Biol. 2015, 5, 267–274. [Google Scholar] [CrossRef]
- Ohlendieck, K. Proteomic profiling of fast-to-slow muscle transitions during aging. Front. Physiol. 2011, 2, 105. [Google Scholar] [CrossRef]
- Ubaida-Mohien, C.; Lyashkov, A.; Gonzalez-Freire, M.; Tharakan, R.; Shardell, M.; Moaddel, R.; Semba, R.D.; Chia, C.W.; Gorospe, M.; Sen, R.; et al. Discovery proteomics in aging human skeletal muscle finds change in spliceosome, immunity, proteostasis and mitochondria. Elife 2019, 8, e49874. [Google Scholar] [CrossRef]
- Ebhardt, H.A.; Degen, S.; Tadini, V.; Schilb, A.; Johns, N.; Greig, C.A.; Fearon, K.C.H.; Aebersold, R.; Jacobi, C. Comprehensive proteome analysis of human skeletal muscle in cachexia and sarcopenia: A pilot study. J. Cachexia Sarcopenia Muscle 2017, 8, 567–582. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Steenberg, D.E.; Hostrup, M.; Birk, J.B.; Larsen, J.K.; Santos, A.; Kjøbsted, R.; Hingst, J.R.; Schéele, C.C.; Murgia, M.; et al. Deep muscle-proteomic analysis of freeze-dried human muscle biopsies reveals fiber type-specific adaptations to exercise training. Nat. Commun. 2021, 12, 304. [Google Scholar] [CrossRef]
- Candia, J.; Cheung, F.; Kotliarov, Y.; Fantoni, G.; Sellers, B.; Griesman, T.; Huang, J.; Stuccio, S.; Zingone, A.; Ryan, B.M.; et al. Assessment of Variability in the SOMAscan Assay. Sci. Rep. 2017, 7, 14248. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Biancotto, A.; Moaddel, R.; Moore, A.Z.; Gonzalez-Freire, M.; Aon, M.A.; Candia, J.; Zhang, P.; Cheung, F.; Fantoni, G.; et al. Plasma proteomic signature of age in healthy humans. Aging Cell 2018, 17, e12799. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rodríguez, A.; Avanzas, P.; González-González, J.; Belleyo-Belkasem, C.; Abreu-González, P. Growth differentiation factor 15, a new prognostic marker in diabetic cardiomyopathy. Rev. Esp. Cardiol. 2016, 69, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Lehallier, B.; Gate, D.; Schaum, N.; Nanasi, T.; Lee, S.E.; Yousef, H.; Moran Losada, P.; Berdnik, D.; Keller, A.; Verghese, J.; et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 2019, 25, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Florin, A.; Lambert, C.; Sanchez, C.; Zappia, J.; Durieux, N.; Tieppo, A.M.; Mobasheri, A.; Henrotin, Y. The secretome of skeletal muscle cells: A systematic review. Osteoarthr. Cartil. Open 2020, 2, 100019. [Google Scholar] [CrossRef]
- Gueugneau, M.; d’Hose, D.; Barbé, C.; de Barsy, M.; Lause, P.; Maiter, D.; Bindels, L.B.; Delzenne, N.M.; Schaeffer, L.; Gangloff, Y.G.; et al. Increased Serpina3n release into circulation during glucocorticoid-mediated muscle atrophy. J. Cachexia Sarcopenia Muscle 2018, 9, 929–946. [Google Scholar] [CrossRef]

| Biomarker | Physiological and Biological Interaction with Sc | Modulation on Sc | |
|---|---|---|---|
| Notch signaling | Master regulator of Sc function and its balance controls Sc self-renewal and myogenic differentiation in a coordinated manner | + | |
| Wtn signaling | Dysregulation of Wnt signaling during aging has been proposed to contribute to the loss of Sc function in sarcopenic skeletal muscle | − | |
| Growth differentiation factor 11 (GDF11) | Sc frequency and function were observed to increase, along with the number of Sc with intact DNA, and the number of Sc with damaged DNA was reduced | + | |
| Growth differentiation factor 8 (GDF8) or Myostatin | Negative regulator of skeletal muscle development and size and Sc | − | |
| Peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α) | Prevent Sc cell apoptosis and markedly increase the myogenic potential of skeletal muscle Sc | + | |
| Sirtuin 1 (Sirt1) | Increases Sc proliferation, inhibits myoblast differentiation, and reduces myogenin expression | + | |
| Paired box 7 (Pax 7) | Sc Pax7(+) repair damaged muscle tissue | + | |
| Paired box 3 (Pax 3) | Play an essential role in adult muscle homeostasis and skeletal muscle repair, promoting the contribution of muscle Sc to the balance of muscle structure and/or function | + | |
| Myogenic regulatory factors (MRFs) | MyoD and Myf5 | Transformation of Sc into muscle cells | + |
| Myogenin (MyoG) | Beneficial relationship between Sc and niche by stimulating differentiation signals and transformation of Sc to myocytes | + | |
| MRF4 | Block Sc activity by delaying the regeneration of muscle fibers | − | |
| CD34 | Necessary for the progression of Sc to efficient muscle tissue in response to acute and chronic damage | + | |
| P16 cyclin-dependent kinase 2A inhibitor (p16ink4a) | Limits the proliferation and renewal of Sc | − | |
| Growth differentiation factor 15 (GDF-15) | A strong association between age and plasma values | − | |
| Serpin Family A Member 3 (serpin A3n) | Increased secretion by atrophied muscle cells | − | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Lázaro, D.; Garrosa, E.; Seco-Calvo, J.; Garrosa, M. Potential Satellite Cell-Linked Biomarkers in Aging Skeletal Muscle Tissue: Proteomics and Proteogenomics to Monitor Sarcopenia. Proteomes 2022, 10, 29. https://doi.org/10.3390/proteomes10030029
Fernández-Lázaro D, Garrosa E, Seco-Calvo J, Garrosa M. Potential Satellite Cell-Linked Biomarkers in Aging Skeletal Muscle Tissue: Proteomics and Proteogenomics to Monitor Sarcopenia. Proteomes. 2022; 10(3):29. https://doi.org/10.3390/proteomes10030029
Chicago/Turabian StyleFernández-Lázaro, Diego, Evelina Garrosa, Jesús Seco-Calvo, and Manuel Garrosa. 2022. "Potential Satellite Cell-Linked Biomarkers in Aging Skeletal Muscle Tissue: Proteomics and Proteogenomics to Monitor Sarcopenia" Proteomes 10, no. 3: 29. https://doi.org/10.3390/proteomes10030029
APA StyleFernández-Lázaro, D., Garrosa, E., Seco-Calvo, J., & Garrosa, M. (2022). Potential Satellite Cell-Linked Biomarkers in Aging Skeletal Muscle Tissue: Proteomics and Proteogenomics to Monitor Sarcopenia. Proteomes, 10(3), 29. https://doi.org/10.3390/proteomes10030029