Seasonal Proteome Variations in Orbicella faveolata Reveal Molecular Thermal Stress Adaptations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Locations
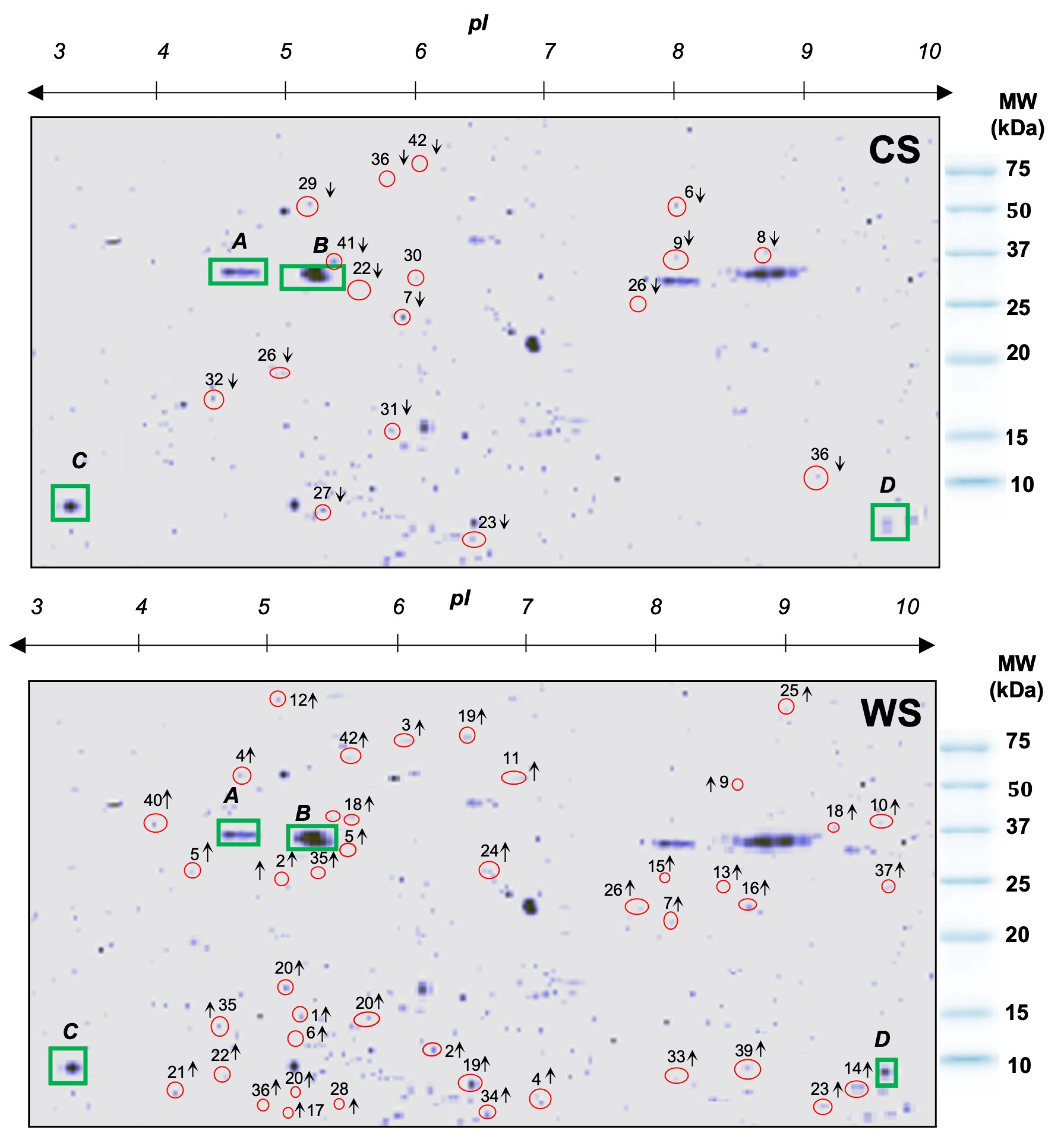
2.2. High-Resolution Two-Dimensional Gel Electrophoresis (2D-GE)
2.3. Gel Image and Statistical Analysis
2.4. Protein in-Gel Digestion and Data Analysis
2.5. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF/TOF MS)
2.6. RNA Extraction
2.7. Primer Design and Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR) Validation
3. Results
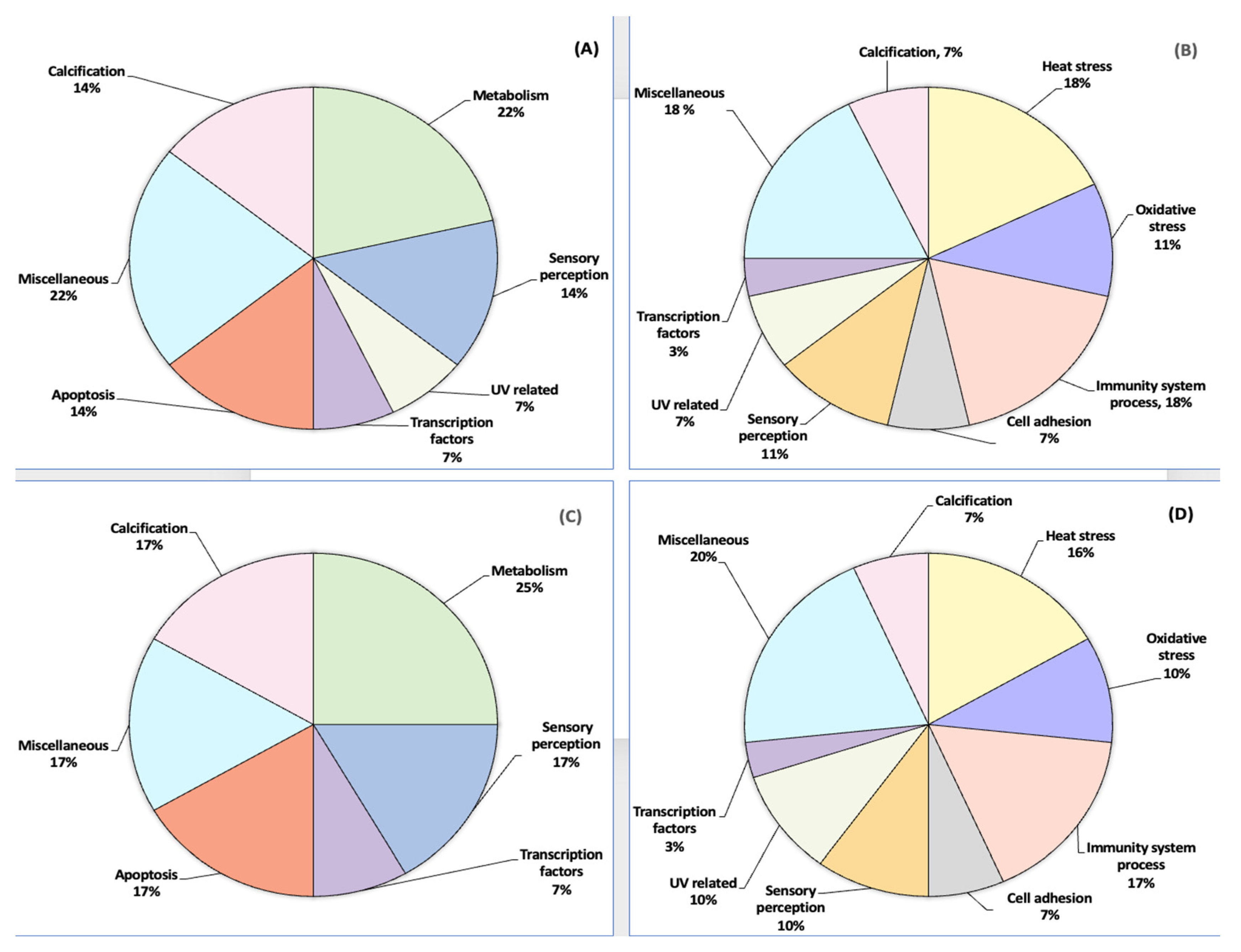
3.1. Upregulated and Downregulated Proteoforms
3.2. Orbicella faveolata Proteoforms Altered under Cold and Warm Seasonal Stress Conditions
3.3. Statistical Analysis
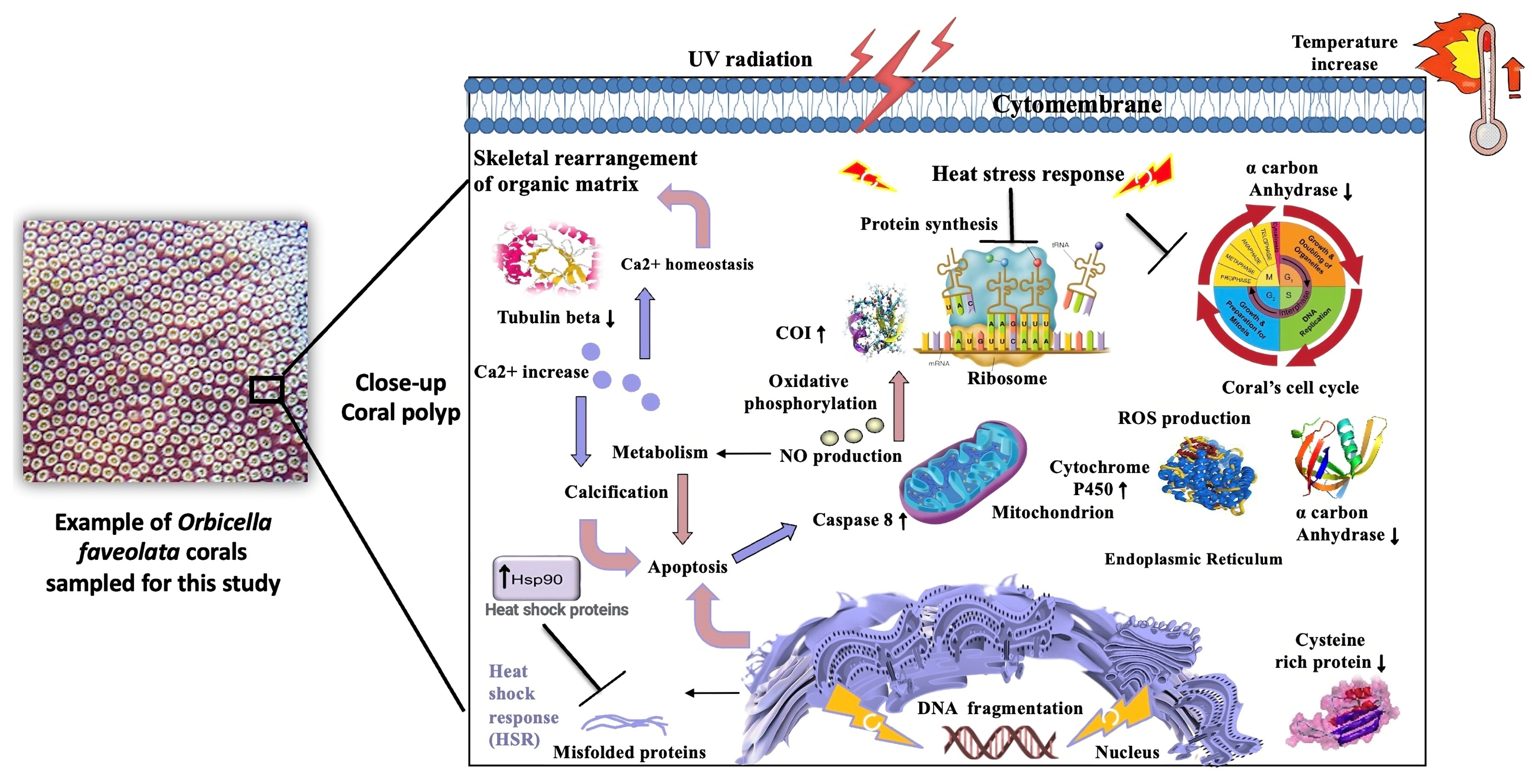
4. Discussion
4.1. Responsiveness to Stress in Orbicella faveolata
4.2. Expression Profile of the Reference Genes in Orbicella faveolata
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, J.B.C.; Donovan, M.K.; Cramer, K.L.; Lam, V.; Lam, W. (Eds.) Status and Trends of Caribbean Coral Reefs: 1970–2012; International Union for the Conservation of Nature: Gland, Switzerland, 2014. [Google Scholar]
- Aeby, G.S.; Ushijima, B.; Campbell, J.E.; Jones, S.; Williams, G.J.; Meyer, J.L.; Häse, C.; Paul, V.J. Pathogenesis of a Tissue Loss Disease Affecting Multiple Species of Corals Along the Florida Reef Tract. Front. Mar. Sci. 2019, 6, 678. [Google Scholar] [CrossRef]
- Sabine, A.M.; Smith, T.B.; Williams, D.E.; Brandt, M.E. Environmental Conditions Influence Tissue Regeneration Rates in Scleractinian Corals. Mar. Pollut. Bull. 2015, 95, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Viehman, T.S. Coral Decline and Reef Habitat Loss in the Caribbean: Modeling Abiotic Limitations on Coral Populations and Communities. Ph.D. Thesis, Duke University, Durham, NC, USA, 2017. [Google Scholar]
- Torres, J.L.; Armstrong, R.A.; Weil, E. Enhanced Ultraviolet Radiation Can Terminate Sexual Reproduction in the Broadcasting Coral Species Acropora cervicornis Lamarck. J. Exp. Mar. Bio. Ecol. 2008, 358, 39–45. [Google Scholar] [CrossRef]
- Eakin, C.M.; Morgan, J.A.; Heron, S.F.; Smith, T.B.; Liu, G.; Alvarez-Filip, L.; Baca, B.; Bartels, E.; Bastidas, C.; Bouchon, C.; et al. Caribbean Corals in Crisis: Record Thermal Stress, Bleaching, and Mortality in 2005. PLoS ONE 2010, 5, e13969. [Google Scholar] [CrossRef] [PubMed]
- Weil, E. Coral Reef Diseases in the Wider Caribbean. In Coral Health and Disease; Loya, Y., Rosenberg, E., Eds.; Springer: New York, NY, USA, 2002; Volume 1, pp. 35–68. [Google Scholar]
- Guest, J.R.; Baird, A.H.; Maynard, J.A.; Muttaqin, E.; Edwards, A.J.; Campbell, S.J.; Yewdall, K.; Affendi, Y.A.; Chou, L.M. Contrasting Patterns of Coral Bleaching Susceptibility in 2010 Suggest an Adaptive Response to Thermal Stress. PLoS ONE 2012, 7, e33353. [Google Scholar] [CrossRef]
- Hagedorn, M.; Page, C.; ONeill, K.; Flores, D.; Tichy, L.; Chamberland, V.; Lager, C.; Zuchowicz, N.; Lohr, K.; Blackburn, H.; et al. Successful demonstration of assisted gene flow in the threatened coral Acropora palmata across genetically-isolated caribbean populations using cryopreserved sperm. BioRxiv 2018, 492447. [Google Scholar] [CrossRef]
- Prada, C.; Hanna, B.; Budd, A.F.; Woodley, C.M.; Schmutz, J.; Grimwood, J.; Iglesias-Prieto, R.; Pandolfi, J.M.; Levitan, D.; Johnson, K.G.; et al. Empty Niches after Extinctions Increase Population Sizes of Modern Corals. Curr. Biol. 2016, 26, 3190–3194. [Google Scholar] [CrossRef] [PubMed]
- Ricaurte, M.; Schizas, N.V.; Ciborowski, P.; Boukli, N.M. Proteomic Analysis of leached and Unbleached Acropora palmata, a Threatened Coral Species of the Caribbean. Mar. Pollut. Bull. 2016, 107, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Gravinese, P.M.; Douwes, A.; Eaton, K.R.; Muller, E.M. Ephemeral Hypoxia Reduces Oxygen Consumption in the Caribbean Coral Orbicella Faveolata. Coral Reefs 2022, 41, 13–18. [Google Scholar] [CrossRef]
- Mayfield, A.B.; Lin, C. Field-Testing a Proteomics-Derived Machine-Learning Model for Predicting Coral Bleaching Susceptibility. Appl. Sci. 2023, 13, 1718. [Google Scholar] [CrossRef]
- McRae, C.J.; Mayfield, A.B.; Huang, W.B.; Côté, I.M.; Fan, T.Y. Contrasting Proteomic Responses of Adult and Larval Coral to High Temperatures. Front. Mar. Sci. 2021, 8, 716124. [Google Scholar] [CrossRef]
- Adjeroud, M.; Tsuchiya, M. Genetic variation and clonal structure in the scleractinian coral Pocillopora damicornis in the Ryukyu Archipelago, southern Japan. Mar. Biol. 1999, 134, 753–759. [Google Scholar] [CrossRef]
- Alvarado-Chacon, E.M.; Gómez-Lemos, L.A.; Sierra-Sabalza, N.P.; Hernández-Chamorro, A.M.; Lozano-Peña, J.P.; Valcárcel-Castellanos, C.A.; Pizarro, V.; García-Urueña, R.; Zárate-Arévalo, J.C.; Rojas, J.A. Early life history of the Caribbean coral Orbicella faveolata (Scleractinia: Merulinidae). Rev. Biol. Trop. 2020, 68, 1262–1274. [Google Scholar] [CrossRef]
- Ruiz-Diaz, C.P.; Toledo-Hernández, C.; Sánchez-González, J.L.; Betancourt, B. The Effects of Depth-Related Environmental Factors on Traits in Acropora cervicornis Raised in Nurseries. Water 2022, 14, 212. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Chomzynksi, P. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate–Phenol–Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J. Molecular Cloning: A Laboratory Manual/J., 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1987. [Google Scholar]
- Palumbi, S.R.; Vollmer, S.; Romano, S.; Oliver, T.; Ladner, J. The role of genes in understanding the evolutionary ecology of reef building corals. Evol. Ecol. 2012, 26, 317–335. [Google Scholar] [CrossRef]
- Fukami, H.; Chen, C.A.; Budd, A.F.; Collins, A.; Wallace, C.; Chuang, Y.-Y.; Chen, C.; Dai, C.-F.; Iwao, K.; Sheppard, C.; et al. Mitochondrial and Nuclear Genes Suggest that Stony Corals Are Monophyletic but Most Families of Stony Corals Are Not (Order Scleractinia, Class Anthozoa, Phylum Cnidaria). PLoS ONE 2008, 3, e3222. [Google Scholar] [CrossRef]
- Wirshing, H.H.; Baker, A.C. Molecular evolution of calcification genes in morphologically similar but phylogenetically unrelated scleractinian corals. Mol. Phylogenet. Evol. 2014, 77, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, S.; DeSalvo, M.K.; Voolstra, C.R.; Reyes-Bermudez, A.; Medina, M. Identification and Gene Expression Analysis of a Taxonomically Restricted Cysteine-Rich Protein Family in Reef-Building Corals. PLoS ONE 2009, 4, e4865. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. QBase Relative Quantification Framework and Software for Management and Automated Analysis of Real-Time Quantitative PCR Data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef]
- DeSalvo, M.; Sunagawa, S.; Voolstra, C.; Medina, M. Transcriptomic Responses to Heat Stress and Bleaching in the Elkhorn Coral Acropora palmata. Mar. Ecol. Prog. Ser. 2010, 402, 97–113. [Google Scholar] [CrossRef]
- Dávalos-Dehullu, E.; Hernández-Arana, H.; Carricart-Ganivet, J.P. On the Causes of Density Banding in Skeletons of Corals of the Genus Montastraea. J. Exp. Mar. Bio. Ecol. 2008, 365, 142–147. [Google Scholar] [CrossRef]
- Hofmann, G.E.; O’Donnell, M.J.; Todgham, A.E. Using Functional Genomics to Explore the Effects of Ocean Acidification on Calcifying Marine Organisms. Mar. Ecol. Prog. Ser. 2008, 373, 219–225. [Google Scholar] [CrossRef]
- Tchernov, D.; Gorbunov, M.Y.; de Vargas, C.; Narayan Yadav, S.; Milligan, A.J.; Häggblom, M.; Falkowski, P.G. Membrane Lipids of Symbiotic Algae Are Diagnostic of Sensitivity to Thermal Bleaching in Corals. Proc. Natl. Acad. Sci. USA 2004, 101, 13531–13535. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, L.; Gong, S.; Guo, M.; Yuan, X.; Zhou, G.; Lei, X.; Zhang, Y.; Yuan, T.; Lian, J.; et al. Impact of Ocean Warming and Acidification on Symbiosis Establishment and Gene Expression Profiles in Recruits of Reef Coral Acropora intermedia. Front. Microbiol. 2020, 11, 532447. [Google Scholar] [CrossRef]
- Huang, G.; Huang, S.; Yan, X.; Yang, P.; Li, J.; Xu, W.; Zhang, L.; Wang, R.; Yu, Y.; Yuan, S.; et al. Two Apextrin-like Proteins Mediate Extracellular and Intracellular Bacterial Recognition in Amphioxus. Proc. Natl. Acad. Sci. USA 2014, 111, 13469–13474. [Google Scholar] [CrossRef]
- Miller, D.J.; Hemmrich, G.; Ball, E.E.; Hayward, D.C.; Khalturin, K.; Funayama, N.; Agata, K.; Bosch, T.C.G. The Innate Immune Repertoire in Cnidaria—Ancestral Complexity and Stochastic Gene Loss. Genome Biol. 2007, 8, R59. [Google Scholar] [CrossRef]
- Galli, G.; Solidoro, C. ATP Supply May Contribute to Light-Enhanced Calcification in Corals More than Abiotic Mechanisms. Front. Mar. Sci. 2018, 5, 68. [Google Scholar] [CrossRef]
- Brown, B.E.; Dunne, R.P.; Somerfield, P.J.; Edwards, A.J.; Simons, W.J.F.; Phongsuwan, N.; Putchim, L.; Anderson, L.; Naeije, M.C. Long-Term Impacts of Rising Sea Temperature and Sea Level on Shallow Water Coral Communities over a ~40 Year Period. Sci. Rep. 2019, 9, 8826. [Google Scholar] [CrossRef]
- Edge, S.E.; Morgan, M.B.; Gleason, D.F.; Snell, T.W. Development of a Coral CDNA Array to Examine Gene Expression Profiles in Montastraea faveolata Exposed to Environmental Stress. Mar. Pollut. Bull. 2005, 51, 507–523. [Google Scholar] [CrossRef]
- Maor-Landaw, K.; Levy, O. Gene Expression Profiles during Short-Term Heat Stress; Branching vs. Massive Scleractinian Corals of the Red Sea. PeerJ 2016, 4, e1814. [Google Scholar] [CrossRef]
- Levy, O.; Karako-Lampert, S.; Ben-Asher, H.W.; Zoccola, D.; Pagès, G.; Ferrier-Pagès, C. Molecular Assessment of the Effect of Light and Heterotrophy in the Scleractinian Coral Stylophora pistillata. Proc. R. Soc. B Biol. Sci. 2016, 283, 20153025. [Google Scholar] [CrossRef]
- Weston, A.J.; Dunlap, W.C.; Beltran, V.H.; Starcevic, A.; Hranueli, D.; Ward, M.; Long, P.F. Proteomics Links the Redox State to Calcium Signaling During Bleaching of the Scleractinian Coral Acropora microphthalma on Exposure to High Solar Irradiance and Thermal Stress. Mol. Cell. Proteom. 2015, 14, 585–595. [Google Scholar] [CrossRef]
- Arora, P.D.; Wang, Y.; Bresnick, A.; Dawson, J.; Janmey, P.A.; McCulloch, C.A. Collagen Remodeling by Phagocytosis Is Determined by Collagen Substrate Topology and Calcium-Dependent Interactions of Gelsolin with Nonmuscle Myosin IIA in Cell Adhesions. Mol. Biol. Cell 2013, 24, 734–747. [Google Scholar] [CrossRef]
- Kaniewska, P.; Campbell, P.R.; Kline, D.I.; Rodriguez-Lanetty, M.; Miller, D.J.; Dove, S.; Hoegh-Guldberg, O. Major Cellular and Physiological Impacts of Ocean Acidification on a Reef Building Coral. PLoS ONE 2012, 7, e34659. [Google Scholar] [CrossRef]
- Hemond, E.M.; Kaluziak, S.T.; Vollmer, S. V The Genetics of Colony Form and Function in Caribbean Acropora Corals. BMC Genom. 2014, 15, 1133. [Google Scholar] [CrossRef]
- Takeuchi, T.; Yamada, L.; Shinzato, C.; Sawada, H.; Satoh, N. Stepwise Evolution of Coral Biomineralization Revealed with Genome-Wide Proteomics and Transcriptomics. PLoS ONE 2016, 11, e0156424. [Google Scholar] [CrossRef]
- Takahashi, S.; Murata, N. How Do Environmental Stresses Accelerate Photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Weis, V.M. Cellular Mechanisms of Cnidarian Bleaching: Stress Causes the Collapse of Symbiosis. J. Exp. Biol. 2008, 211, 3059–3066. [Google Scholar] [CrossRef]
- Mayfield, A.B.; Aguilar, C.; Kolodziej, G.; Enochs, I.C.; Manzello, D.P. Shotgun Proteomic Analysis of Thermally Challenged Reef Corals. Front. Mar. Sci. 2021, 8, 660153. [Google Scholar] [CrossRef]
- Rosic, N.; Ling, E.Y.S.; Chan, C.K.K.; Lee, H.C.; Kaniewska, P.; Edwards, D.; Dove, S.; Hoegh-Guldberg, O. Unfolding the Secrets of Coral-Algal Symbiosis. ISME J. 2015, 9, 844–856. [Google Scholar] [CrossRef]
- Rosic, N.N.; Pernice, M.; Dunn, S.; Dove, S.; Hoegh-Guldberg, O. Differential Regulation by Heat Stress of Novel Cytochrome P450 Genes from the Dinoflagellate Symbionts of Reef-Building Corals. Appl. Environ. Microbiol. 2010, 76, 2823–2829. [Google Scholar] [CrossRef]
- Polato, N.R.; Voolstra, C.R.; Schnetzer, J.; DeSalvo, M.K.; Randall, C.J.; Szmant, A.M.; Medina, M.; Baums, I.B. Location-Specific Responses to Thermal Stress in Larvae of the Reef-Building Coral Montastraea faveolata. PLoS ONE 2010, 5, e11221. [Google Scholar] [CrossRef]
- Tomanek, L. Environmental Proteomics: Changes in the Proteome of Marine Organisms in Response to Environmental Stress, Pollutants, Infection, Symbiosis, and Development. Ann. Rev. Mar. Sci. 2011, 3, 373–399. [Google Scholar] [CrossRef]
- Rosic, N.; Kaniewska, P.; Chan, C.-K.; Ling, E.Y.; Edwards, D.; Dove, S.; Hoegh-Guldberg, O. Early Transcriptional Changes in the Reef-Building Coral Acropora aspera in Response to Thermal and Nutrient Stress. BMC Genom. 2014, 15, 1052. [Google Scholar] [CrossRef]
- Byler, K.A.; Carmi-Veal, M.; Fine, M.; Goulet, T.L. Multiple Symbiont Acquisition Strategies as an Adaptive Mechanism in the Coral Stylophora pistillata. PLoS ONE 2013, 8, e59596. [Google Scholar] [CrossRef]
- Dunn, S.R.; Pernice, M.; Green, K.; Hoegh-Guldberg, O.; Dove, S.G. Thermal Stress Promotes Host Mitochondrial Degradation in Symbiotic Cnidarians: Are the Bat teries of the Reef Going to Run Out? PLoS ONE 2012, 7, e39024. [Google Scholar] [CrossRef]
- Roth, M.S.; Deheyn, D.D. Effects of Cold Stress and Heat Stress on Coral Fluorescence in Reef-Building Corals. Sci. Rep. 2013, 3, 1421. [Google Scholar] [CrossRef]
- Donner, S.D. An evaluation of the effect of recent temperature variability on the prediction of coral bleaching events. Ecol. Appl. 2011, 21, 1718–1730. [Google Scholar] [CrossRef]
- Lough, J.M. Small change, big difference: Sea surface temperature distributions for tropical coral reef ecosystems, 1950–2011. J. Geophys. Res. 2012, 117, C09018. [Google Scholar] [CrossRef]
- Brown, B.E. Coral bleaching: Causes and consequences. Coral Reefs 1997, 16, S129–S138. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res. 1999, 50, 839–866. [Google Scholar] [CrossRef]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.; Kleypas, J.; et al. Climate Change, Human Impacts, and the Resilience of Coral Reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef]
- Baker, A.C.; Glynn, P.W.; Riegl, B. Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coast. Shelf Sci. 2008, 80, 435–471. [Google Scholar] [CrossRef]
- Donner, S.D.; Knutson, T.R.; Oppenheimer, M. Model-based assessment of the role of human-induced climate change in the 2005 Caribbean coral bleaching event. Proc. Natl. Acad. Sci. USA 2007, 104, 5483–5488. [Google Scholar] [CrossRef]
- Wall, C.B.; Wallsgrove, N.J.; Gates, R.D.; Popp, B.N. Amino acidδ13C and δ15N analyses reveal distinct species-specific patterns of trophic plasticity in a marine symbiosis. Limnol. Amp; Oceanogr. 2021, 66, 2033–2050. [Google Scholar] [CrossRef]
- Stephens, T.G.; Ragan, M.A.; Bhattacharya, D.; Chan, C.X. Core Genes in Diverse Dinoflagellate Lineages Include a Wealth of Conserved Dark Genes with Unknown Functions. Sci. Rep. 2018, 8, 17175. [Google Scholar] [CrossRef]



| RT-PCR Primers for Orbicella faveolate | |||
|---|---|---|---|
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Citation |
| Actin (Housekeeping gene) | GAGATGAAGGCTGCATCTGGAGAG | ACAAAGCTTGGCTGGAACA | [21] |
| Alpha carbonic anhydrase | ACAATTTTCGATGCAGGGCT | GCTCCGAACCTTGCGAATTA | [22] |
| Cytochrome c oxidase subunit I | GCGCGATGTTAGGTGATGAT | TTCCTGCGCCTTGTTCAAC | [23] |
| Green fluorescent protein | TCTGCCCCATGGTAAGTGTT | GGCTTGCCTTTTCCTTCTCC | |
| Beta-tubulin | TCAGAGAAGAATATCCCGACAGA | GGTGTGGTGAGCTTCAGAGT | |
| Scleractinian cysteine-rich protein | GGCTGCCAAGTTTCATTTGTG | CAACGTTCATTTGGTGGCAC | [24] |
| Spot # | Category | Protein Name | Acc. No.a | GO Molecular Function | Theoretical pI/Mwb | Pc | MS/MSd | ENe | SCg | EN (+/−)i | SC (+/−)j |
|---|---|---|---|---|---|---|---|---|---|---|---|
| f.cf | f.ch | ||||||||||
| 1 | Heat stress | Galaxin-like 1 [Acropora millepora] | ADI50284 | Defense against oxidative stress | 5.06/17.93 | 7 | 56 | 4.40 | 2.62 | + | + |
| 2 | Heat shock transcription factor | ACH53605.1 | DNA-binding | 6.38/8.96 | 11 | 23 | 4.83 | 3.33 | + | + | |
| 3 | Heat shock protein 70 A1-like [Orbicella faveolata] | XP_020619238.1 | Stress response | 5.54/70.15 | 76 | 396 | 6.72 | 5.40 | + | + | |
| 4 | Heat shock protein HSP 90-beta-like [Orbicella faveolata] | XP_020618369.1 | Protein folding | 4.82/85.05 | 32 | 270 | 4.31 | 5.22 | + | + | |
| 5 | Endoplasmin-like [Orbicella faveolata] | XP_020606735.1 | Protein folding and sorting | 4.87/28.26 | 6 | 280 | 5.25 | 5.04 | + | + | |
| 6 | Metabolism | Cytochrome b [Montastraea faveolata] | Q762T3 | Ubiquinol-cytochrome-c reductase | 8.19/43.22 | 7 | 154 | 5.23 | 3.25 | − | − |
| 7 | Cytochrome oxidase subunit I, partial (mitochondrion) [Orbicella faveolata] | AHA90940.1 | Oxidative phosphorylation | 5.54/21.89 | 11 | 280 | 2.06 | 2.06 | − | − | |
| 8 | Cytochrome P450 74A [Acropora palmata] | ACD42778.1 | Oxidoreductase | 8.99/40.80 | 3 | 123 | 1.86 | 1.93 | − | − | |
| 9 | Oxidative stress | Activin 1 protein [Acropora digitifera] | BAQ19091.1 | Free radical binding | 8.71/48.39 | 9 | 78 | 4.84 | 2.28 | + | + |
| 10 | NADH dehydrogenase subunit 1 | Q4G6D2 | Oxidation-reduction | 9.10/35.04 | 8 | 86 | 4.50 | 4.23 | + | + | |
| 11 | Thioredoxin reductase [Acropora millepora] | AFI99106.2 | Oxidoreductase | 6.37/74.30 | 5 | 88 | 2.33 | 2.45 | + | + | |
| 12 | Immune system process | Apextrin [Acropora millepora] | ABK63971.2 | Innate immune response | 5.08/96.1 | 10 | 22 | 3.20 | 3.20 | + | + |
| 13 | ATP synthase F0 subunit 6 (mitochondrion) [Orbicella faveolata] | YP_271955.1 | Hydrogen ion transmembrane transporter | 8.46/25.07 | 7 | 38 | 2.60 | 2.56 | + | + | |
| 14 | ATP synthase subunit 8 | Q8SJB2 | Hydrogen ion transmembrane transporter | 9.51/8.52 | 6 | 214 | 3.93 | 2.87 | + | + | |
| 15 | ATPase subunit 6 (ATP synthase subunit 6) | Q9TBW1 | ATPase synthase subunit 6 | 7.92/25.37 | 11 | 87 | 15.45 | 15.43 | + | + | |
| 16 | Toll-like receptor 2 isoform X4 [Orbicella faveolata] | XP_020617122.1 | Innate immune response | 8.61/24.40 | 7 | 66 | 14.30 | 11.25 | + | + | |
| 17 | Cell adhesion | Mini-collagen, partial [Acropora palmata] | AAM74869.1 | Collagen Trimer | 5.6/6.18 | 8 | 465 | 9.22 | 8.89 | + | + |
| 18 | 2-acylglycerol O-acyltransferase 1-like [Orbicella faveolata] | XP_020602679.1 | Acyltransferase | 9.24/38.12 | 6 | 230 | 8.41 | 7.58 | + | + | |
| 19 | Sensory perception | Integrin subunit betaCn1 [Acropora millepora] | AAB66910.1 | Cell matrix adhesion | 6.41/87.84 | 7 | 254 | 1.60 | 2.47 | + | + |
| 20 | Actin, partial [Acropora millepora] | ABY40470.1 | Calcium regulation | 5.26/17.33 | 11 | 489 | 6.83 | 5.87 | + | + | |
| 21 | Beta-tubulin, partial [Orbicella faveolata] | AHZ61618.1 | GTPase | 4.57/8.99 | 6 | 336 | 4.24 | 3.24 | + | + | |
| 22 | PREDICTED: unconventional myosin-VIIa-like [Acropora digitifera] | XP_012559373.1 | Phagocytosis, engulfment | 5.83/40.51 | 13 | 455 | 7.31 | 6.48 | − | − | |
| 23 | Myosin-IIIb-like [Orbicella faveolata] | XP_020624818.1 | Calcium-regulation | 6.82/3.27 | 4 | 325 | 4.62 | 3.45 | − | − | |
| 24 | UV related | GFP-like fluorescent chromoprotein cFP484 [Orbicella faveolata] | XP_020605513.1 | Generation of precursor metabolites and energy | 6.51/25.68 | 4 | 325 | 6.51 | 5.24 | + | + |
| 25 | GPI inositol-deacylase-like [Orbicella faveolata] | XP_020615720.1 | GPI anchored proteins | 9.08/100.56 | 4 | 325 | 3.96 | 3.45 | + | + | |
| 26 | Green fluorescent protein [Orbicella faveolata] | ABC68475.1 | Photoprotection | 7.75/25.54 | 7 | 76 | 7.23 | 6.44 | − | + | |
| 27 | Transcription factors | PaxC, partial [Acropora palmata] | ACI29769.1 | DNA binding | 5.01/2.99 | 10 | 599 | 2.01 | 1.47 | − | − |
| 28 | BMP2/4, partial [Orbicella faveolata] | AHZ61804 | Growth factor | 5.31/5.00 | 7 | 456 | 2.60 | 1.98 | + | + | |
| 29 | Apoptosis | Caspase 8 [Acropora palmata] | ADG23096.1 | Programmed cell death | 5.94/50.79 | 4 | 654 | 3.30 | 2.56 | − | − |
| 30 | PREDICTED: ectonucleoside triphosphate diphosphohydrolase 2-like [Acropora digitifera] | XP_015779265 | DNA binding | 6.01/31.70 | 3 | 345 | 3.60 | 2.54 | − | − | |
| 31 | Calcification | Carbonic anhydrase 5A, mitochondrial-like [Orbicella faveolata] | XP_020613891.1 | Carbonate dehydratase | 5.81/15.74 | 4 | 180 | 6.40 | 5.34 | − | − |
| 32 | Calmodulin-like protein, partial [Acropora millepora] | ACY07618.1 | Calcium binding | 4.74/19.66 | 3 | 259 | 8.40 | 9.26 | − | − | |
| 33 | Small cysteine-rich protein 8 | B2ZG38 | Neurotoxin | 8.67/8.10 | 7 | 204 | 6.45 | 3.25 | + | + | |
| 34 | DnaJ homolog subfamily C member 5-like [Orbicella faveolata] | XP_020605856.1 | Protein folding | 6.9/8.26 | 4 | 45 | 9.23 | 3.42 | + | + | |
| 35 | Miscellaneous | Alpha i G protein [Acropora palmata] | AFZ78088.1 | Signal transducer | 5.72/40.35 | 9 | 573 | 9.22 | 9.20 | + | + |
| 36 | Small cysteine-rich protein 6 | C1KIZ5 | Calcification | 4.67/9.33 | 8 | 23 | 5.39 | 4.49 | − | − | |
| 37 | Zinc finger protein 768-like [Orbicella faveolata] | XP_020615964.1 | Transcriptional regulation | 9.59/25.29 | 6 | 26 | 2.64 | 2.51 | + | + | |
| 38 | Alpha q G protein [Acropora palmata] | AFZ78090 | Polypeptide binding | 5.32/41.88 | 6 | 736 | 2.31 | 2.31 | + | + | |
| 39 | Alpha carbonic anhydrase, partial [Orbicella faveolata] | AHZ61699.1 | Carbonate dehydratase | 8.24/11.20 | 4 | 456 | 7.46 | 6.45 | + | + | |
| 40 | Uncharacterized protein LOC110065766 [Orbicella faveolata] | XP_020628594.1 | Uncharacterized protein LOC110065766 [Orbicella faveolata] | 4.28/46.09 | 3 | 96 | 6.15 | 5.99 | + | + | |
| 41 | Galaxin-2-like [Orbicella faveolata] | D9IQ16.1 | Calcium ion transport | 5.45/35.12 | 8 | 126 | 8.03 | 7.25 | − | + | |
| 42 | Procollagen galactosyltransferase 1-like [Orbicella faveolata] | XP_020604034 | Iron-ion binding | 5.91/68.37 | 6 | 320 | 7.02 | 6.48 | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricaurte, M.; Schizas, N.V.; Weil, E.F.; Ciborowski, P.; Boukli, N.M. Seasonal Proteome Variations in Orbicella faveolata Reveal Molecular Thermal Stress Adaptations. Proteomes 2024, 12, 20. https://doi.org/10.3390/proteomes12030020
Ricaurte M, Schizas NV, Weil EF, Ciborowski P, Boukli NM. Seasonal Proteome Variations in Orbicella faveolata Reveal Molecular Thermal Stress Adaptations. Proteomes. 2024; 12(3):20. https://doi.org/10.3390/proteomes12030020
Chicago/Turabian StyleRicaurte, Martha, Nikolaos V. Schizas, Ernesto F. Weil, Pawel Ciborowski, and Nawal M. Boukli. 2024. "Seasonal Proteome Variations in Orbicella faveolata Reveal Molecular Thermal Stress Adaptations" Proteomes 12, no. 3: 20. https://doi.org/10.3390/proteomes12030020







