Effects of Al3+ and La3+ Trivalent Metal Ions on Tomato Fruit Proteomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatment
2.2. Proteomic Analysis
2.2.1. Protein Extraction
2.2.2. iTRAQ Labeling Procedure
2.2.3. Cleaning of the Peptides
2.2.4. High pH First Dimension UPLC Separation
2.2.5. Low pH Second Dimension RP Separation
2.2.6. Mass Spectrometric Analysis
2.2.7. Data Processing
2.2.8. Database Searching/iTRAQ Quantitation
2.3 Statistical Analysis
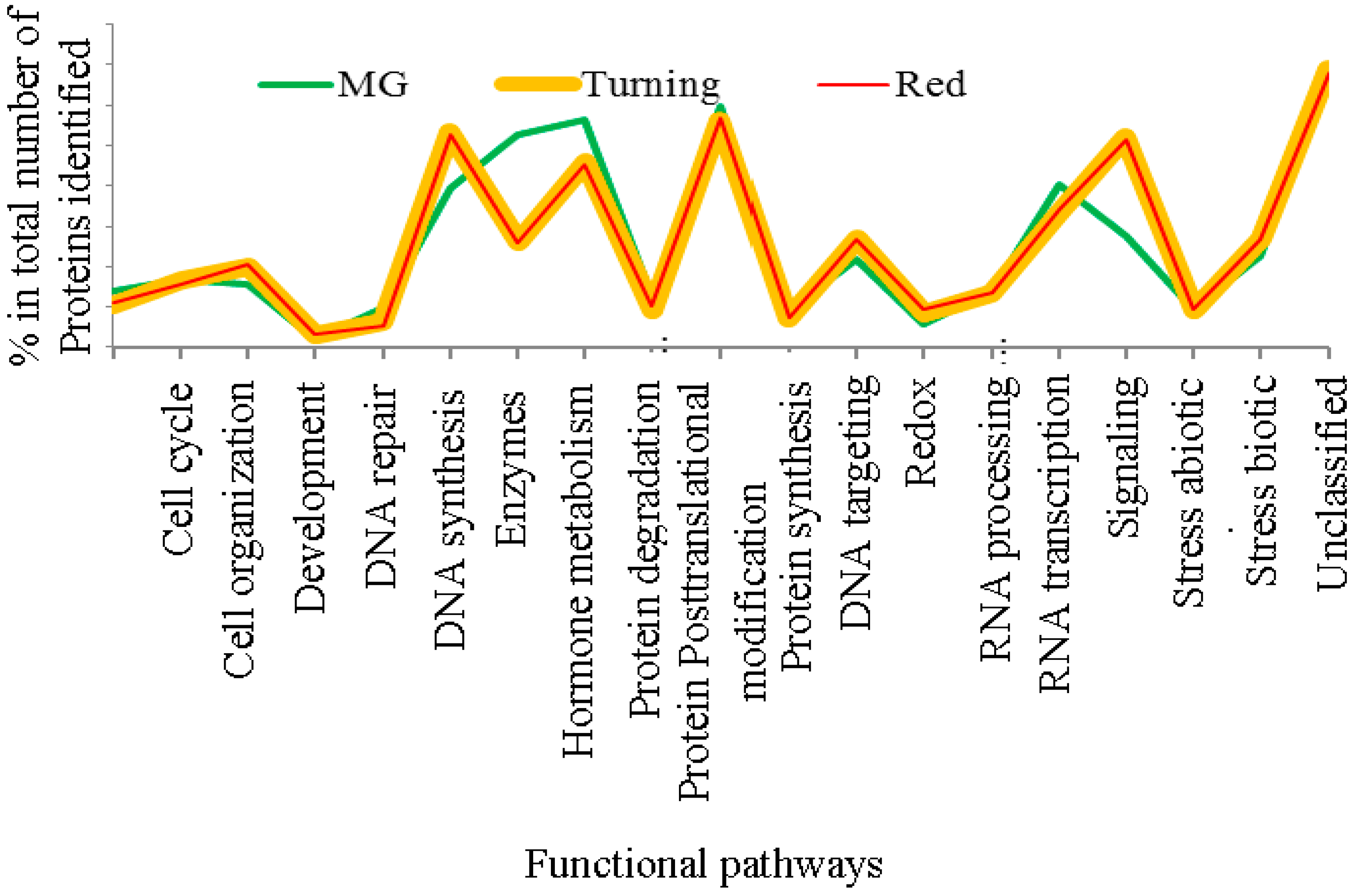
3. Results
3.1. The Al-Treatment-Induced Proteome Composition in Tomatoes
3.2. Differentially-Expressed Proteins in Tomatoes under Al and La Treatments
3.3. Patterns of Proteome Changes during the Tomato Ripening Process Associated with Al and La Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- United States Department of Agriculture. Vegetables, and Florist and Nursery Crops (3rd Edition). Handbook 66. USDA/ARS, Washington. 2003; Subsection: Commodity, Tomato. Available online: www.ba.ars.usda.gov/hb66/contents.html (accessed on 3 October 2016). [Google Scholar]
- Carrari, F.; Fernie, A.R. Metabolic regulation underlying tomato fruit development. J. Exp. Bot. 2006, 57, 1883–1897. [Google Scholar] [CrossRef]
- Tohge, T.; Fernie, A.R. Metabolomics-inspired insight into developmental, environmental and genetic aspects of tomato fruit chemical composition and quality. Plant Cell Physiol. 2015, 56, 1681–1696. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; Alseekh, S.; Fernie, A.R. On the regulation and function of secondary metabolism during fruit development and ripening. J. Exp. Bot. 2014, 65, 4599–4611. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, K.; Ju, Z.; Cao, D.; Fu, D.; Zhu, H.; Zhu, B.; Luo, Y. Genome-wide analysis of tomato NF-Y factors and their role in fruit ripening. BMC Genomics 2016, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extremes 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Shennan, C. Tomato fruit yields and quality under water deficit and salinity. J. Am. Soc. Hortic. Sci. 1991, 116, 215–221. [Google Scholar]
- Hede, A.R.; Skovmand, B.; López-Cesati, J. Application of Physiology in Wheat Breeding, Acid Soil and Aluminum Toxicity; Reynolds, M.P., Ortiz-Monasterio, J.J., Mchab, A., Eds.; International Maize and Wheat Improvement Center (CIMMYT) Staff Publications Collection: Mexico D.F., Mexico, 2001; pp. 172–182. [Google Scholar]
- Searcy, K.B.; Mulcahy, D.L. Comparison of the response to aluminum toxicity in gametophyte and sporophyte of four tomato (Lycopersicon esculentum Mill.) cultivars. Theor. Appl Genet. 1990, 80, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Smalley, T.J.; Jones, J.B., Jr.; Lasseigne, F.T. Aluminum toxicity in tomato. Part 1. Growth and mineral nutrition. J. Plant Nutr. 1994, 17, 293–306. [Google Scholar] [CrossRef]
- Simon, L.; Kieger, M.; Sung, S.S.; Smalley, T.J. Aluminum toxicity in tomato. Part 2. Leaf gas exchange, chlorophyll content, and invertase activity. Plant Nutr. 1994, 17, 307–317. [Google Scholar] [CrossRef]
- Okekeogbu, I.; Ye, Z.; Sangireddy, S.R.; Li, H.; Bhatti, S.; Hui, D.F.; Zhou, S.; Howe, K.J.; Fish, T.; Yang, Y.; et al. Effect of aluminum treatment on proteomes of radicles of seeds derived from Al treated tomato plants. Proteomes 2014, 2, 169–190. [Google Scholar] [CrossRef]
- Zhou, S.; Sauvé, R.; Thannhauser, T.W. Proteome changes induced by aluminium stress in tomato roots. J. Exp. Bot. 2009, 60, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, H.; Bhatti, S.; Zhou, S.; Yang, Y.; Fish, T.; Thannhauser, T.W. Development of a laser-capture-microdissection-based single-cell-type proteomics tool for studying proteomes of individual cell layers of plant roots. Horticult. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Foy, C.D.; Sadeghi, A.M.; Ritchie, J.C.; Krizek, D.T.; Davis, J.R.; Kemper, W.D. Aluminum toxicity and high bulk density: Role in limiting shoot and root growth of selected aluminum indicator plants and eastern gamagrass in an acid soil. J. Plant Nutr. 1999, 22, 1551–1566. [Google Scholar] [CrossRef]
- Larsen, P.B.; Degenhardt, J.; Tai, C.-Y.; Stenzler, L.M.; Howell, S.H.; Kochian, L.V. Aluminum-resistant Arabidopsis mutants that exhibit altered patterns of aluminum accumulation and organic acid release from roots. Plant Physiol. 1998, 117, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.R.; Skerrett, M.; Findlay, G.P.; Delhaize, E.; Tyerman, S.D. Aluminum activates an anion channel in the apical cells of wheat roots. Proc. Nat. Acad. Sci. 1997, 94, 6547–6552. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Wagatsuma, T.; Ikarashi, T. Comparative toxicity of Al3+, Yb3+, and La3+ to root-tip cells differing in tolerance to high Al3+ in terms of ionic potentials of dehydrated trivalent cations. Soil Sci. Plant Nutr. 1996, 42, 613–625. [Google Scholar] [CrossRef]
- Kinraide, T.B.; Ryan, P.R.; Kochian, L.V. Interactive effects of Al3+, H+, and other cations on root elongation considered in terms of cell-surface electrical potential. Plant Physiol. 1992, 99, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Bennet, R.J.; Breen, C.M. The use of lanthanum to delineate the aluminum signalling mechanisms functioning in the roots of Zea mays L. Environ. Exp. Bot. 1992, 32, 365–376. [Google Scholar] [CrossRef]
- Wheeler, D.M.; Power, I.L.; Edmeades, D.C. Effect of various metal ions on growth of two wheat lines known to differ in aluminum tolerance. In Plant Nutrition-from Genetic Engineering to Field Practice; Barrow, N.J., Ed.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1993; pp. 723–726. [Google Scholar]
- Menakbi, C.; Quignard, F.; Mineva, T. Complexation of trivalent metal cations to mannuronate type of alginate models from a density functional study. J. Phys. Chem. B 2016, 120, 3615–3623. [Google Scholar] [CrossRef] [PubMed]
- Millaleo, R.; Reyes-Diaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef]
- Ye, Z.; Sangrieddy, S.; Okekeogbu, I.; Zhou, S.; Yu, C.L.; Hui, D.; Howe, H.J.; Fish, T.; Thannhauser, T.W. Drought-induced leaf proteome changes in Switchgrass seedlings. Int. J. Mol. Sci. 2016, 17, 1251. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Okekeogbu, I.; Sangireddy, S.; Ye, Z.; Li, H.; Bhatti, S.; Hui, D.; McDonald, D.W.; Yang, Y.; Giri, S.; et al. Proteome modification in tomato plants upon long-term aluminum treatment. J. Proteome Res. 2016, 15, 1670–1684. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sauve, R.; Thannhauser, TW. Aluminum induced proteome changes in tomato cotyledons. Plant Signal. Behav. 2009, 4, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Magnavaca, R.; Gardner, C.; Clark, R. Evaluation of inbred maize lines for aluminum tolerance in nutrient solution. In Genetic Aspects of Plant Mineral Nutrition; Gabelman, H.L.B., Ed.; Martinus Nijhoff: Dordrecht, The Netherlands, 1987; pp. 255–265. [Google Scholar]
- Hecht-Buchholz, C.H.; Brady, D.J.; Asher, C.J.; Edwards, D.G. Effects of low activities of aluminum on soybean (Glycine max). II. Root cell structure and root hair development. In Plant Nutrition-Physiology and Applications; Van Beusichem, M.L., Ed.; Kluwer Academic Publishers: Dordrecht, the Netherlands, 1990; pp. 335–343. [Google Scholar]
- Thimm, O.; Bläsing, O.; Gibon, Y.; Nagel, A.; Meyer, S.; Krüger, P.; Selbig, J.; Müller, L.A.; Rhee, S.Y.; Stitt, M. MAPMAN: A user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 2004, 37, 914–939. [Google Scholar] [CrossRef] [PubMed]
- Usadel, B.; Nagel, A.; Thimm, O.; Redestig, H.; Blaesing, O.E.; Palacios-Rojas, N.; Selbig, J.; Hannemann, J.; Piques, M.C.; Steinhauser, D.; et al. Extension of the visualization tool MapMan to allow statistical analysis of arrays, display of corresponding genes, and comparison with known responses. Plant Physiol. 2005, 138, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Palmer, M.; Zhou, J.; Bhatti, S.; Howe, K.; Fish, T.; Thannhauser, T.W. Differential root proteome expression in tomato genotypes with contrasting drought tolerance exposed to dehydration. J. Am. Soc. Hort. Sci. 2013, 138, 131–141. [Google Scholar]
- Hetherington, S.E.; Smillie, R.M.; Davies, W.J. Photosynthetic activities of vegetative and fruiting tissues of tomato. J. Exp. Bot. 1998, 49, 1173–1181. [Google Scholar] [CrossRef]
- Weissbach, H.; Etienne, F.; Hoshi, T.; Heineman, S.H.; Lowther, W.T.; Matthews, B.; St John, G.; Nathan, C.; Brot, N. Peptide methionine sulfoxide reductase: Structure, mechanism of action, and biological function. Arch. Biochem. Biophys. 2002, 397, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Cocaliadis, M.F.; FernándezMuñoz, R.; Pons, C.; Orzaez, D.; Granell, A. Increasing tomato fruit quality by enhancing fruit chloroplast function. A double-edged sword? J. Exp. Bot. 2014, 65, 4589–4598. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Bradley, K.; Pyke, G.; Ball, C.; Lu, R.; Fray, A.; Marshall, S.; Jayasuta, C.; Baxter, R.; van Wijk, R. Network inference analysis identifies an APRR2-like gene linked to pigment accumulation in tomato and pepper fruits. Plant Physiol. 2013, 161, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.L.; Nguyen, C.V.; Hill, T.; Cheng, K.L.; Figueroa-Balderas, R.; Aktas, H.; Ashrafi, H.; Pons, C.; Fernandez-Munoz, R.; Vicente, A. Uniform ripening encodes a Golden 2-like transcription factor regulating tomato fruit chloroplast development. Science 2012, 336, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Sagar, M.; Chervin, C.; Mila, I.; Hao, Y.; Roustan, J.P.; Benichou, M.; Gibon, Y.; Biais, B.; Maury, P.; Latche, A. SlARF4, an auxin response factor involved in the control of sugar metabolism during tomato fruit development. Plant Physiol. 2013, 161, 1362–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lytovchenko, A.; Eickmeier, I.; Pons, C.; Osorio, S.; Szecowka, M.; Lehmberg, K.; Arrivault, S.; Tohge, T.; Pineda, B.; Teresa, M.A.; et al. Tomato fruit photosynthesis is seemingly unimportant in primary metabolism and ripening but plays a considerable role in seed development. Plant Physiol. 2011, 157, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Rontein, D.; Basset, G.; Hanson, A.D. Metabolic engineering of osmoprotectant accumulation in plants. Metab. Eng. 2002, 4, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Takehiro, T.; Yoko, I.; Koh, A.; Daisuke, H.; Shigeo, H.; Chiaki, M. Metabolic alterations in organic acids and γ-Aminobutyric acid in developing tomato (Solanum lycopersicum L.). fruits. Plant Cell Physiol. 2010, 51, 1300–1314. [Google Scholar] [CrossRef] [PubMed]
- Gillaspy, G.; Ben-David, H.; Gruissem, W. Fruits: A developmental perspective. Plant Cell 1993, 5, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J.; Giovannoni, J.J. Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Bastías, A.; Yañez, M.; Osorio, S.; Arbona, V.; Gómez-Cadenas, A.; Fernie, A.R.; Casaretto, J.A. The transcriptional factor AREB1 regulates primary metabolic pathways in tomato fruits. J. Exp. Bot. 2014, 65, 2351–2363. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Yoza, K.I.; Nogata, Y.; Kusumoto, K.I.; Voragen, A.G.J.; Ohta, H. accumulation in banana fruit with ripening during storage. Phytochemistry 1997, 46, 57–60. [Google Scholar] [CrossRef]
- Rastogi, R.; Davies, P.J. Polyamine metabolism in ripening tomato fruit. I. Identification of metabolites of putrescine and spermidine. Plant Physiol. 1990, 94, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Saftner, R.A.; Baldi, B.G. Polyamine levels and tomato fruit development: possible interaction with ethylene. Plant Physiol. 1990, 92, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, A.K.; Chung, S.H.; Goyal, R.K.; Fatima, T.; Solomos, T.; Srivastav, A.; Handa, A.K. Overaccumulation of higher polyamines in ripening transgenic tomato fruit revives metabolic memory, upregulates anabolism-related genes, and positively impacts nutritional quality. J. AOAC Int. 2007, 90, 1456–1464. [Google Scholar] [PubMed]
- Iusem, N.D.; Bartholomew, D.M.; Hitz, W.D.; Scolnik, P.A. Tomato(Lycopersicon esculentum) transcript induced by water deficit and ripening. Plant Physiol. 1993, 2, 1353–1354. [Google Scholar] [CrossRef]
- Amitai-Zeigerson, H.; Scolnik, P.A.; Bar-Zvi, D. Tomato Asr1 mRNA and protein are transiently expressed following salt stress, osmotic stress and treatment with abscisic acid. Plant Sci. 1995, 110, 205–213. [Google Scholar] [CrossRef]
- Golan, I.; Dominguez, P.G.; Konrad, Z.; Shkolnik-Inbar, D.; Carrari, F.; Bar-Zvi, D. Tomato ABSCISIC ACID STRESS RIPENING (ASR) gene family revisited. PLoS ONE 2014, 9, e107117. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kumar, A.; Salunke, D.M. Crystal structure of the vicilin from Solanum melongena reveals existence of different anionic ligands in structurally similar pockets. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]


| Log2 Fold (Al/La) b | FDR (p-Value) | Protein Accession Number c | Protein Description |
|---|---|---|---|
| Mature green (MG) tomatoes | |||
| −1.44 | 0.05 | Solyc11g012320.1.1 | Unknown protein |
| −1.36 | 0.02 | Solyc10g075090.1.1 | Non-specific lipid-transfer protein |
| −1.27 | 0.03 | Solyc05g051260.2.1 | Endo-1 4-beta-xylanase |
| −0.97 | 0.04 | Solyc02g094470.2.1 | Mitochondrial phosphate carrier protein |
| −0.93 | 0.01 | Solyc01g104590.2.1 | Ribosomal protein L3 |
| −0.90 | 0.04 | Solyc01g007500.2.1 | Photosystem II CP47 chlorophyll apoprotein |
| −0.89 | 0.03 | Solyc01g109970.2.1 | DNA repair protein |
| −0.89 | 0.03 | Solyc00g035010.1.1 | C2H2 finger domain-containing protein |
| −0.89 | 0.03 | Solyc11g069660.1.1 | Nbs-lrr, resistance protein |
| −0.89 | 0.03 | Solyc07g007760.2.1 | Defensin protein |
| −0.88 | 0.03 | Solyc12g009300.1.1 | Sucrose synthase |
| −0.84 | 0.03 | Solyc03g122310.2.1 | Aldehyde dehydrogenase |
| −0.81 | 0.01 | Solyc06g071720.1.1 | 60S ribosomal protein L27A |
| −0.80 | 0.02 | Solyc02g090560.2.1 | Calcium-transporting ATPase |
| −0.74 | 0.00 | Solyc01g007330.2.1 | Ribulose bisphosphate carboxylase large chain |
| −0.71 | 0.03 | Solyc11g065240.1.1 | Saccharopine dehydrogenase |
| −0.70 | 0.01 | Solyc06g060340.2.1 | Chloroplast photosystem II-associated protein |
| −0.70 | 0.04 | Solyc07g053650.2.1 | 26S proteasome regulatory subunit |
| −0.69 | 0.00 | Solyc11g062130.1.1 | Mitochondrial ADP/ATP carrier proteins |
| −0.68 | 0.04 | Solyc04g063290.2.1 | 30S ribosomal protein S5 |
| −0.68 | 0.02 | Solyc07g066610.2.1 | Phosphoglycerate kinase |
| −0.66 | 0.00 | Solyc06g009190.2.1 | Pectinesterase |
| −0.66 | 0.04 | Solyc01g107590.2.1 | Cinnamyl alcohol dehydrogenase |
| −0.65 | 0.00 | Solyc05g054580.2.1 | 60S acidic ribosomal protein P0 |
| −0.63 | 0.01 | Solyc12g044600.2.1 | NADP-dependent malic enzyme, chloroplastic |
| −0.62 | 0.01 | Solyc02g078570.2.1 | Epoxide hydrolase 3 |
| −0.61 | 0.01 | Solyc10g018300.1.1 | Transketolase 1 |
| 0.60 | 0.00 | Solyc03g083170.2.1 | Defective in meristem silencing 3 (chromatin remodeling) |
| 0.60 | 0.00 | Solyc09g082330.1.1 | 7S vicilin |
| 0.61 | 0.05 | Solyc01g060070.2.1 | Pore protein homolog |
| 0.63 | 0.02 | Solyc06g065940.2.1 | Protein FAR1-RELATED SEQUENCE 6 |
| 0.65 | 0.02 | Solyc03g005650.1.1 | Cc-nbs-lrr, resistance protein |
| 0.72 | 0.00 | Solyc07g063120.2.1 | WD-40 repeat protein |
| 0.89 | 0.00 | Solyc04g073990.2.1 | Annexin |
| 0.94 | 0.03 | Solyc01g079160.2.1 | GDSL esterase/lipase |
| 0.99 | 0.01 | Solyc09g082350.1.1 | Vicilin-like protein |
| 1.26 | 0.04 | Solyc05g054900.2.1 | Blue copper protein |
| Turning tomatoes | |||
| −0.61 | 0.01 | Solyc10g049800.1.1 | Legume lectin beta domain |
| −0.60 | 0.00 | Solyc03g083910.2.1 | Acid beta-fructofuranosidase |
| 0.60 | 0.00 | Solyc04g071610.2.1 | ABSCISIC ACID STRESS RIPENING 1 |
| 0.90 | 0.00 | Solyc11g022590.1.1 | Kunitz trypsin inhibitor |
| Red tomatoes | |||
| −0.90 | 0.00 | Solyc10g049800.1.1 | Legume lectin beta domain |
| −0.66 | 0.00 | Solyc06g072130.2.1 | Aquaporin |
| −0.61 | 0.00 | Solyc03g111720.2.1 | Peptide methionine sulfoxide reductase msrA |
| 0.61 | 0.02 | Solyc10g085480.1.1 | 60S ribosomal protein L24 |
| 0.89 | 0.00 | Solyc11g022590.1.1 | Kunitz trypsin inhibitor 4 |
| Cluster | Protein Accessions a | Log2 Fold (Al/La) b | Protein Description | ||
|---|---|---|---|---|---|
| MG c | Turning | Red | |||
| Cluster 1 | solyc01g060070.2.1 | 0.61d | 0.01 | 0.02 | Mitochondrial Tim17/22 |
| Cluster 2 | solyc01g007920.2.1 | 0.09 | 0.61d | 0.84d | Isochorismatase |
| solyc06g065940.2.1 | 0.63d | 0.23 | 0.31 | FRS6 MULE transposase | |
| solyc04g073990.2.1 | 0.89d | 0.34 | 0.17 | Annexin | |
| solyc11g022590.1.1 | 0.06 | 0.9d | 0.89d | Kunitz trypsin inhibitor 4 | |
| solyc04g071610.2.1 | 0.01 | 0.6d | 0.4 | ABA/WDS induced protein | |
| Cluster 3 | solyc06g060340.2.1 | −0.7d | 0.18 | 0.01 | Photosystem II 22 kDa protein |
| solyc01g007500.2.1 | −0.9d | 0.02 | 0.08 | Photosystem II, PsbB | |
| solyc01g007330.2.1 | −0.74d | 0 | 0.01 | RUBISCO, large subunit. | |
| solyc11g062130.1.1 | −0.69d | 0.01 | −0.02 | Mitochondrial ADP/ATP carrier proteins | |
| solyc06g073190.2.1 | −0.63d | 0.01 | 0.1 | Carbohydrate/purine kinase, PfkB | |
| solyc03g122310.2.1 | −0.84d | −0.09 | −0.09 | Aldehyde dehydrogenase | |
| solyc04g039850.1.1 | −0.93d | 0.2 | 0.17 | ATP synthase subunit | |
| solyc05g051260.2.1 | −1.27d | 0.1 | 0.16 | glycosyl hydrolase | |
| solyc06g009190.2.1 | −0.66d | −0.02 | 0 | Pectinesterase | |
| solyc05g051260.2.1 | −1.27 | 0.1 | 0.16 | Endo-1 4-beta-xylanase | |
| solyc01g073970.2.1 | −1.03 | −0.18 | 0.19 | Histone H3 | |
| solyc02g085840.2.1 | −0.66d | −0.05 | −0.01 | UV excision repair protein RAD23 | |
| solyc12g096300.1.1 | −1.37d | 0.08 | 0.24 | Ribosomal protein S6 | |
| solyc08g074240.2.1 | −0.74d | 0.01 | 0.13 | Ribosomal protein S6 | |
| solyc01g104590.2.1 | −0.93d | 0.01 | 0.09 | Ribosomal protein L3 | |
| solyc02g086240.2.1 | −0.60d | −0.15 | 0.19 | Ribosomal protein L5 | |
| solyc06g009210.2.1 | −0.88d | 0.13 | 0.22 | Ribosomal protein L19/L19e | |
| solyc05g054580.2.1 | −0.65d | 0.22 | 0.14 | Ribosomal protein L10 | |
| solyc06g071720.1.1 | −0.81d | 0 | −0.09 | Ribosomal protein L15 | |
| solyc08g007620.1.1 | −0.66d | 0.06 | 0.09 | Peptidase S8, subtilisin-related | |
| solyc07g053650.2.1 | −0.7d | −0.04 | −0.13 | 26S proteasome regulatory subunit | |
| solyc02g094470.2.1 | −0.97d | 0.24 | 0.26 | Mitochondrial phosphate carrier protein | |
| solyc11g069430.1.1 | −0.67d | 0.24 | −0.16 | Aquaporin | |
| solyc09g092380.2.1 | −0.7d | −0.1 | 0.01 | Adenosylhomocysteinase | |
| solyc06g075540.2.1 | −0.8d | −0.12 | 0.08 | Phosphatidyl synthase | |
| cluster 5 | solyc09g065260.1.1 | 0 | −0.59 | −0.64d | Blue copper protein |
| solyc06g072130.2.1 | −0.13 | −0.41 | −0.66d | Aquaporin | |
| solyc06g034040.1.1 | 0.07 | −0.49 | −0.6d | Oleosin | |
| cluster 7 | solyc07g066610.2.1 | −0.68d | 0.4 | 0.28 | Phosphoglycerate kinase |
| solyc11g068540.1.1 | −0.51 | 0.6d | 0.54 | N-carbamoylputrescine amidase | |
| solyc04g063290.2.1 | −0.68d | 0.6d | 0.41 | Ribosomal protein S5 | |
| cluster 9 | solyc01g007380.1.1 | −0.62d | −0.1 | −0.15 | Cytochrome f |
| solyc12g044600.2.1 | −0.63d | −0.21 | −0.14 | NADP-dependent malic enzyme | |
| solyc01g094200.2.1 | −0.6d | 0.06 | −0.07 | NAD-dependent malic enzyme | |
| solyc09g057650.2.1 | −0.67d | −0.2 | −0.23 | Ribosomal protein S8e | |
| cluster 10 | solyc03g005650.1.1 | 0.65d | −0.07 | −0.2 | Cc-nbs-lrr, resistance protein |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangireddy, S.; Okekeogbu, I.; Ye, Z.; Zhou, S.; Howe, K.J.; Fish, T.; Thannhauser, T.W. Effects of Al3+ and La3+ Trivalent Metal Ions on Tomato Fruit Proteomes. Proteomes 2017, 5, 7. https://doi.org/10.3390/proteomes5010007
Sangireddy S, Okekeogbu I, Ye Z, Zhou S, Howe KJ, Fish T, Thannhauser TW. Effects of Al3+ and La3+ Trivalent Metal Ions on Tomato Fruit Proteomes. Proteomes. 2017; 5(1):7. https://doi.org/10.3390/proteomes5010007
Chicago/Turabian StyleSangireddy, Sasikiran, Ikenna Okekeogbu, Zhujia Ye, Suping Zhou, Kevin J. Howe, Tara Fish, and Theodore W. Thannhauser. 2017. "Effects of Al3+ and La3+ Trivalent Metal Ions on Tomato Fruit Proteomes" Proteomes 5, no. 1: 7. https://doi.org/10.3390/proteomes5010007
APA StyleSangireddy, S., Okekeogbu, I., Ye, Z., Zhou, S., Howe, K. J., Fish, T., & Thannhauser, T. W. (2017). Effects of Al3+ and La3+ Trivalent Metal Ions on Tomato Fruit Proteomes. Proteomes, 5(1), 7. https://doi.org/10.3390/proteomes5010007





