Macrophage Proteome Analysis at Different Stages of Mycobacterium avium Subspecies paratuberculosis Infection Reveals a Mechanism of Pathogen Dissemination
Abstract
:1. Introduction
2. Methods and Materials
2.1. Bacteria
2.2. Mammalian Cell Culture
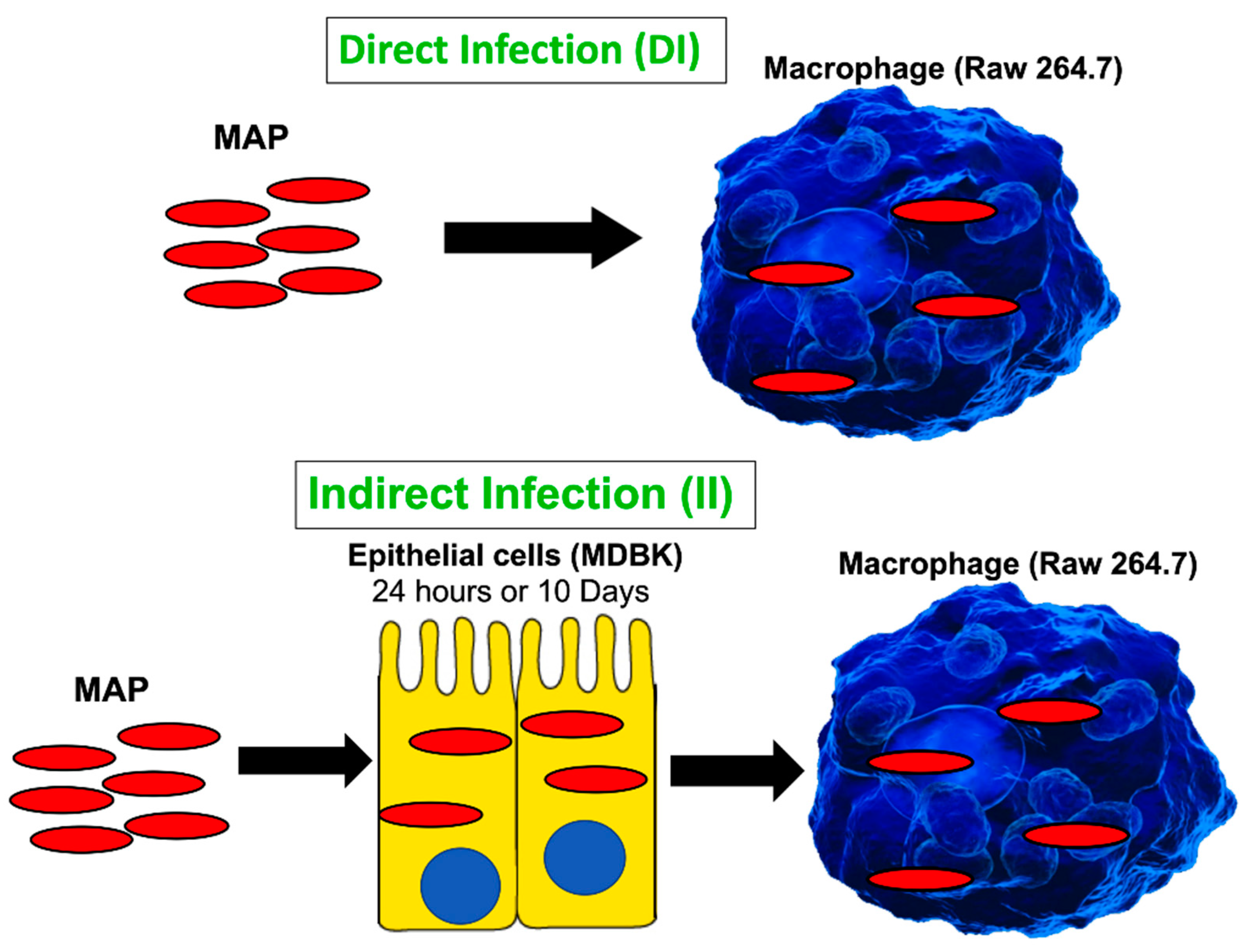
2.3. Invasion and Survival of MAP in RAW 264.7 Macrophages in the Direct Infection Assay
2.4. Invasion and Survival of MAP in RAW 264.7 Macrophages in the Indirect Infection Assay
2.5. Protein Sample Preparation and Mass Spectrometric Analysis
2.6. The Endothelial Cell Binding Assay
2.7. Polarized Endothelial Cell Transwell Assay
2.8. The Endothelial Cell Binding and Migration Assays via Integrins
2.9. Statistical Analysis and Data Interpretation
3. Results
3.1. The Cell Passage Model for Studying a Mechanism of MAP Persistence and Dissemination
3.2. MAP Passage through Epithelial Cells Increases Bacterial Invasion and Survival in Phagocytic Cells
3.3. Macrophage Proteome of Indirect/Passaged MAP Significantly Differs from the Direct Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Research Council. Diagnosis and Control of Johne’s Disease; The National Academies Press: Washington, DC, USA, 2003. [Google Scholar] [CrossRef]
- Barratt, A.S.; Arnoult, M.H.; Ahmadi, B.V.; Rich, K.M.; Gunn, G.J.; Stott, A.W. A framework for estimating society’s economic welfare following the introduction of an animal disease: The case of Johne’s disease. PLoS ONE 2018, 13, e0198436. [Google Scholar] [CrossRef]
- Carter, M.A. Prevalence and prevention of paratuberculosis in North America. Jpn J. Vet. Res. 2012, 60, S9–S18. [Google Scholar] [PubMed]
- Kovich, D.A.; Wells, S.J.; Friendshuh, K. Evaluation of the Voluntary Johne’s Disease Herd Status Program as a source of replacement cattle. J. Dairy Sci. 2006, 89, 3466–3470. [Google Scholar] [CrossRef] [Green Version]
- Bermudez, L.E.; Petrofsky, M.; Sommer, S.; Barletta, R.G. Peyer’s patch-deficient mice demonstrate that Mycobacterium avium subsp. paratuberculosis translocates across the mucosal barrier via both M cells and enterocytes but has inefficient dissemination. Infect. Immun. 2010, 78, 3570–3577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pott, J.; Basler, T.; Duerr, C.U.; Rohde, M.; Goethe, R.; Hornef, M.W. Internalization-dependent recognition of Mycobacterium avium ssp. paratuberculosis by intestinal epithelial cells. Cell Microbiol. 2009, 11, 1802–1815. [Google Scholar] [CrossRef] [PubMed]
- Antognoli, M.C.; Garry, F.B.; Hirst, H.L.; Lombard, J.E.; Dennis, M.M.; Gould, D.H.; Salman, M.D. Characterization of Mycobacterium avium subspecies paratuberculosis disseminated infection in dairy cattle and its association with antemortem test results. Vet. Microbiol. 2008, 127, 300–308. [Google Scholar] [CrossRef]
- Hines, M.E., 2nd; Stabel, J.R.; Sweeney, R.W.; Griffin, F.; Talaat, A.M.; Bakker, D.; Benedictus, G.; Davis, W.C.; de Lisle, G.W.; Gardner, I.A.; et al. Experimental challenge models for Johne’s disease: A review and proposed international guidelines. Vet. Microbiol. 2007, 122, 197–222. [Google Scholar] [CrossRef]
- Everman, J.L.; Eckstein, T.M.; Roussey, J.; Coussens, P.; Bannantine, J.P.; Bermudez, L.E. Characterization of the inflammatory phenotype of Mycobacterium avium subspecies paratuberculosis using a novel cell culture passage model. Microbiology 2015, 161, 1420–1434. [Google Scholar] [CrossRef] [Green Version]
- Koets, A.P.; Eda, S.; Sreevatsan, S. The within host dynamics of Mycobacterium avium ssp. paratuberculosis infection in cattle: Where time and place matter. Vet. Res. 2015, 46, 61. [Google Scholar] [CrossRef] [Green Version]
- McBride, J.W.; Walker, D.H. Progress and obstacles in vaccine development for the ehrlichioses. Expert Rev. Vaccines 2010, 9, 1071–1082. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.; Danelishvili, L.; Yamazaki, Y.; Alonso, M.; Paustian, M.L.; Bannantine, J.P.; Meunier-Goddik, L.; Bermudez, L.E. The ability of Mycobacterium avium subsp. paratuberculosis to enter bovine epithelial cells is influenced by preexposure to a hyperosmolar environment and intracellular passage in bovine mammary epithelial cells. Infect. Immun. 2006, 74, 2849–2855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, T.; Squillantea, E.; Gillespieb, M.; Shao, J. Transepithelial electrical resistance is not a reliable measurement of the Caco-2 monolayer integrity in Transwell. Drug Deliv. 2004, 11, 11–18. [Google Scholar] [CrossRef]
- Deutsch, E.W.; Bandeira, N.; Sharma, V.; Perez-Riverol, Y.; Carver, J.J.; Kundu, D.J.; Garcia-Seisdedos, D.; Jarnuczak, A.F.; Hewapathirana, S.; Pullman, B.S.; et al. The ProteomeXchange consortium in 2020: Enabling ‘big data‘ approaches in proteomics. Nucleic Acids Res. 2020, 48, D1145–D1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef] [PubMed]
- Wesolowski, J.; Paumet, F. SNARE motif: A common motif used by pathogens to manipulate membrane fusion. Virulence 2010, 1, 319–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dingjan, I.; Linders, P.T.A.; Verboogen, D.R.J.; Revelo, N.H.; Ter Beest, M.; van den Bogaart, G. Endosomal and Phagosomal SNAREs. Physiol. Rev. 2018, 98, 1465–1492. [Google Scholar] [CrossRef]
- Fratti, R.A.; Chua, J.; Deretic, V. Cellubrevin alterations and Mycobacterium tuberculosis phagosome maturation arrest. J. Biol. Chem. 2002, 277, 17320–17326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, D.J.; Evanson, O.A.; Moritz, A.; Deng, M.Q.; Abrahamsen, M.S. Differential responses of bovine macrophages to Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium. Infect. Immun. 2002, 70, 5556–5561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathnaiah, G.; Zinniel, D.K.; Bannantine, J.P.; Stabel, J.R.; Grohn, Y.T.; Collins, M.T.; Barletta, R.G. Pathogenesis, Molecular Genetics, and Genomics of Mycobacterium avium subsp. paratuberculosis, the Etiologic Agent of Johne’s Disease. Front. Vet. Sci. 2017, 4, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begg, D.J.; Whittington, R.J. Experimental animal infection models for Johne’s disease, an infectious enteropathy caused by Mycobacterium avium subsp. paratuberculosis. Vet. J. 2008, 176, 129–145. [Google Scholar] [CrossRef]
- Bannantine, J.P.; Everman, J.L.; Rose, S.J.; Babrak, L.; Katani, R.; Barletta, R.G.; Talaat, A.M.; Grohn, Y.T.; Chang, Y.F.; Kapur, V.; et al. Evaluation of eight live attenuated vaccine candidates for protection against challenge with virulent Mycobacterium avium subspecies paratuberculosis in mice. Front. Cell. Infect. Microbiol. 2014, 4, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marfell, B.J.; O’Brien, R.; Griffin, J.F. Global gene expression profiling of monocyte-derived macrophages from red deer (Cervus elaphus) genotypically resistant or susceptible to Mycobacterium avium subspecies paratuberculosis infection. Dev. Comp. Immunol. 2013, 40, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Casey, M.E.; Meade, K.G.; Nalpas, N.C.; Taraktsoglou, M.; Browne, J.A.; Killick, K.E.; Park, S.D.; Gormley, E.; Hokamp, K.; Magee, D.A.; et al. Analysis of the Bovine Monocyte-Derived Macrophage Response to Mycobacterium avium Subspecies Paratuberculosis Infection Using RNA-seq. Front. Immunol. 2015, 6, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLoughlin, K.E.; Nalpas, N.C.; Rue-Albrecht, K.; Browne, J.A.; Magee, D.A.; Killick, K.E.; Park, S.D.; Hokamp, K.; Meade, K.G.; O’Farrelly, C.; et al. RNA-seq Transcriptional Profiling of Peripheral Blood Leukocytes from Cattle Infected with Mycobacterium bovis. Front. Immunol. 2014, 5, 396. [Google Scholar] [CrossRef] [Green Version]
- Arsenault, R.J.; Li, Y.; Bell, K.; Doig, K.; Potter, A.; Griebel, P.J.; Kusalik, A.; Napper, S. Mycobacterium avium subsp. paratuberculosis inhibits gamma interferon-induced signaling in bovine monocytes: Insights into the cellular mechanisms of Johne’s disease. Infect. Immun. 2012, 80, 3039–3048. [Google Scholar] [CrossRef] [Green Version]
- Tessema, M.Z.; Koets, A.P.; Rutten, V.P.; Gruys, E. How does Mycobacterium avium subsp. paratuberculosis resist intracellular degradation? Vet. Q. 2001, 23, 153–162. [Google Scholar] [CrossRef]
- Arsenault, R.J.; Maattanen, P.; Daigle, J.; Potter, A.; Griebel, P.; Napper, S. From mouth to macrophage: Mechanisms of innate immune subversion by Mycobacterium avium subsp. paratuberculosis. Vet. Res. 2014, 45, 54. [Google Scholar] [CrossRef] [Green Version]
- Garg, H.; Joshi, A. SNAREs in HIV-1 assembly. Commun. Integr. Biol 2012, 5, 172–174. [Google Scholar] [CrossRef]
- Becken, U.; Jeschke, A.; Veltman, K.; Haas, A. Cell-free fusion of bacteria-containing phagosomes with endocytic compartments. Proc. Natl. Acad. Sci. USA 2010, 107, 20726–20731. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Woo, Y.; Hahn, T.W.; Jung, Y.M.; Jung, Y.J. Formation and Maturation of the Phagosome: A Key Mechanism in Innate Immunity against Intracellular Bacterial Infection. Microorganisms 2020, 8, 1298. [Google Scholar] [CrossRef]
- Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Rao, C.L.; Tang, M.L.; Zhang, Y.; Lu, X.X.; Chen, J.G.; Mao, C.; Deng, L.; Li, Q.; Mao, X.H. Rab32 GTPase, as a direct target of miR-30b/c, controls the intracellular survival of Burkholderia pseudomallei by regulating phagosome maturation. PLoS Pathog. 2019, 15, e1007879. [Google Scholar] [CrossRef]
- Danelishvili, L.; Bermudez, L.E. Mycobacterium avium MAV_2941 mimics phosphoinositol-3-kinase to interfere with macrophage phagosome maturation. Microbes Infect. 2015, 17, 628–637. [Google Scholar] [CrossRef] [Green Version]
- Podinovskaia, M.; Descoteaux, A. Leishmania and the macrophage: A multifaceted interaction. Future Microbiol. 2015, 10, 111–129. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, Y.; Weimbs, T.; Yaffe, M.B.; Zhou, D. Bacteria-generated PtdIns(3)P recruits VAMP8 to facilitate phagocytosis. Traffic 2007, 8, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Campoy, E.M.; Mansilla, M.E.; Colombo, M.I. Endocytic SNAREs are involved in optimal Coxiella burnetii vacuole development. Cell Microbiol. 2013, 15, 922–941. [Google Scholar] [CrossRef]
- Podolnikova, N.P.; Kushchayeva, Y.S.; Wu, Y.; Faust, J.; Ugarova, T.P. The Role of Integrins alphaMbeta2 (Mac-1, CD11b/CD18) and alphaDbeta2 (CD11d/CD18) in Macrophage Fusion. Am. J. Pathol. 2016, 186, 2105–2116. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.Q.; Hu, X.W.; Liu, Y.L.; Ye, Z.J.; Gui, Y.H.; Zhou, D.L.; Qi, C.L.; He, X.D.; Wang, H.; Wang, L.J. CD11b deficiency suppresses intestinal tumor growth by reducing myeloid cell recruitment. Sci. Rep. 2015, 5, 15948. [Google Scholar] [CrossRef]
- Park, Y.M.; Febbraio, M.; Silverstein, R.L. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Invest. 2009, 119, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Guy, E.; Kuchibhotla, S.; Silverstein, R.; Febbraio, M. Continued inhibition of atherosclerotic lesion development in long term Western diet fed CD36o /apoEo mice. Atherosclerosis 2007, 192, 123–130. [Google Scholar] [CrossRef]
- Silverstein, R.L.; Febbraio, M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal. 2009, 2, re3. [Google Scholar] [CrossRef] [Green Version]




| Proteins | Infection Group | Function |
|---|---|---|
| Vamp8 | II-24 | Regulates autophagosome–lysosome fusion |
| Vamp3 | DI, II-24 | Involved in the vesicular transport from the late endosomes to the trans-Golgi network |
| Vps18 | II-24, II-10 | Protein trafficking, formation of early endosomes, late endosomes, and lysosomes |
| Lamtor1 | II-24 | Lysosomal motility, activation on the late endosome as well as endosomal biogenesis |
| Rab32 | II-24 | Regulators of membrane trafficking pathways in eukaryotic cells; Endosome-mediated membrane trafficking |
| Rab3iL1 | II-24 | Regulates synaptic vesicle exocytosis |
| Hpse | II-10 | Endoglycosidase that cleaves heparan sulfate proteoglycans |
| Glb1, Hexb, Gm2a | II-10 | Cleaves beta-linked terminal galactosyl residues from gangliosides |
| Cathepsin D | II -24, II-10 | Acid protease intracellular protein breakdown activity |
| Arsb | II-10 | Lysosomal transport, autophagy, degranulation of neutrophils |
| CD63 | II-10 | Intracellular vesicular transport processes |
| SypL1 | II-10 | Regulates exocytosis, small cytoplasmic transport vesicles |
| Vps37c | DI | Required for the sorting of ubiquitinated transmembrane proteins into internal vesicles of multivesicular bodies |
| Litaf | DI | Lipopolysaccharide-induced tumor necrosis factor-alpha factor. Plays a role in endosomal protein trafficking and in targeting proteins for lysosomal degradation |
| SLCL5a3 | DI | Lysosome peptide/histidine transporter |
| Ar18a | DI | Plays a role in lysosome motility |
| Proteins | Infection Group | Function |
|---|---|---|
| Itga5 | DI, II-24, II-10 | Composed of an alpha subunit and a beta subunit that function in cell surface adhesion and signaling |
| Adam8 | DI (Inhibited in II-24 and II 10) | A disintegrin and metalloprotease domain implicated in a variety of biological processes involving cell–cell and cell–matrix interactions |
| Fibulin1 | II-24, II-10 | ECM protein that stabilizes collagen and other ECM proteins |
| Fibronectin/Fn1 | DI, II-24, II-10 | Cell adhesion and migration processes including embryogenesis, wound healing, blood coagulation, host defense, and metastasis |
| CCL4 | DI, II-24, II-10 | Secreted effector with chemokinetic and inflammatory functions |
| CD36 | II-24, II-10 | Involved in a variety of adhesive processes |
| Itgam/CD11b | II -24, II-10 (Inhibited in DI) | Adherence of neutrophils and monocytes to endothelium; involved in the phagocytosis of complement coated particles |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phillips, I.L.; Danelishvili, L.; Bermudez, L.E. Macrophage Proteome Analysis at Different Stages of Mycobacterium avium Subspecies paratuberculosis Infection Reveals a Mechanism of Pathogen Dissemination. Proteomes 2021, 9, 20. https://doi.org/10.3390/proteomes9020020
Phillips IL, Danelishvili L, Bermudez LE. Macrophage Proteome Analysis at Different Stages of Mycobacterium avium Subspecies paratuberculosis Infection Reveals a Mechanism of Pathogen Dissemination. Proteomes. 2021; 9(2):20. https://doi.org/10.3390/proteomes9020020
Chicago/Turabian StylePhillips, Ida L., Lia Danelishvili, and Luiz E. Bermudez. 2021. "Macrophage Proteome Analysis at Different Stages of Mycobacterium avium Subspecies paratuberculosis Infection Reveals a Mechanism of Pathogen Dissemination" Proteomes 9, no. 2: 20. https://doi.org/10.3390/proteomes9020020






