Effects of Diffusion-Induced Nonlinear Local Volume Change on the Structural Stability of NMC Cathode Materials of Lithium-Ion Batteries
Abstract
:1. Introduction
- 1.
- To construct a fully coupled finite element chemomechanical model in order to investigate the effects of a concentration-dependent partial molar volume on the mechanical response of NMC particle.
- 2.
- To study and compare the simulation results for the constant and concentration-dependent partial molar volume of the NMC particle during the charging (delithiation) process.
- 3.
- To investigate the simultaneous effects of the particle size or the charging rate and the concentration-dependent partial molar volume on the mechanical response of the NMC-active material.
2. Methodology
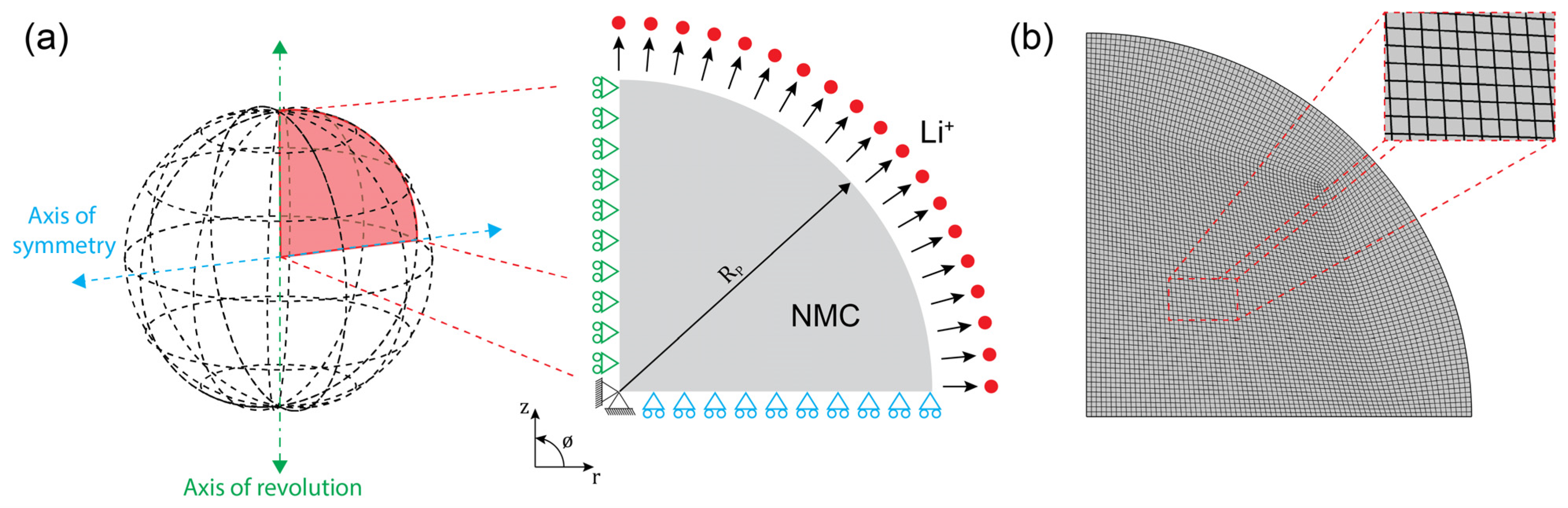
2.1. Fully Coupled Chemomechanical Model
2.1.1. Modeling of Lithium Ion Diffusion in an Active Particle
2.1.2. Modeling of Diffusion-Induced Stress
2.2. Material Properties and Numerical Simulations
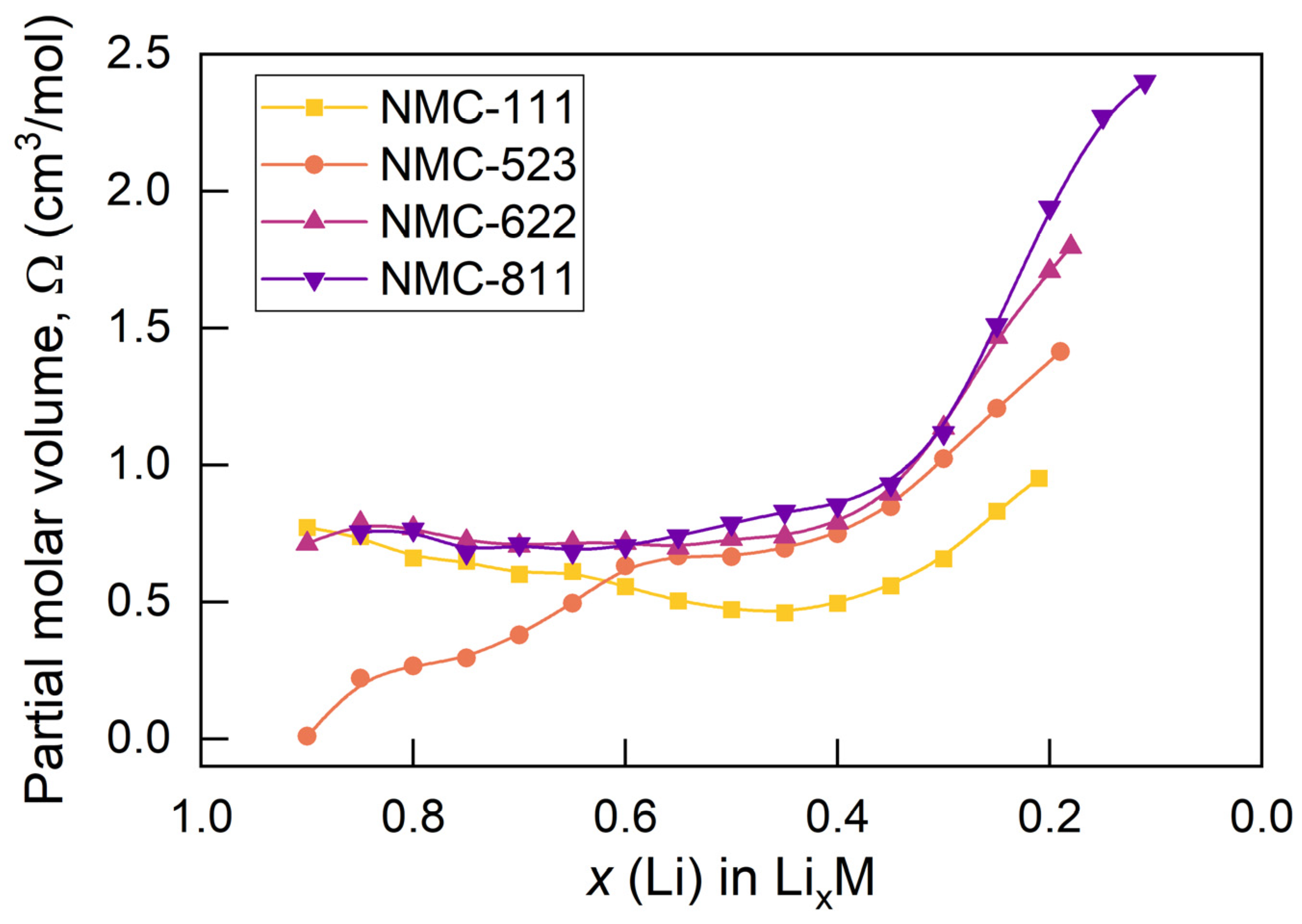
Concentration-Dependent Partial Molar Volume
3. Results and Discussions
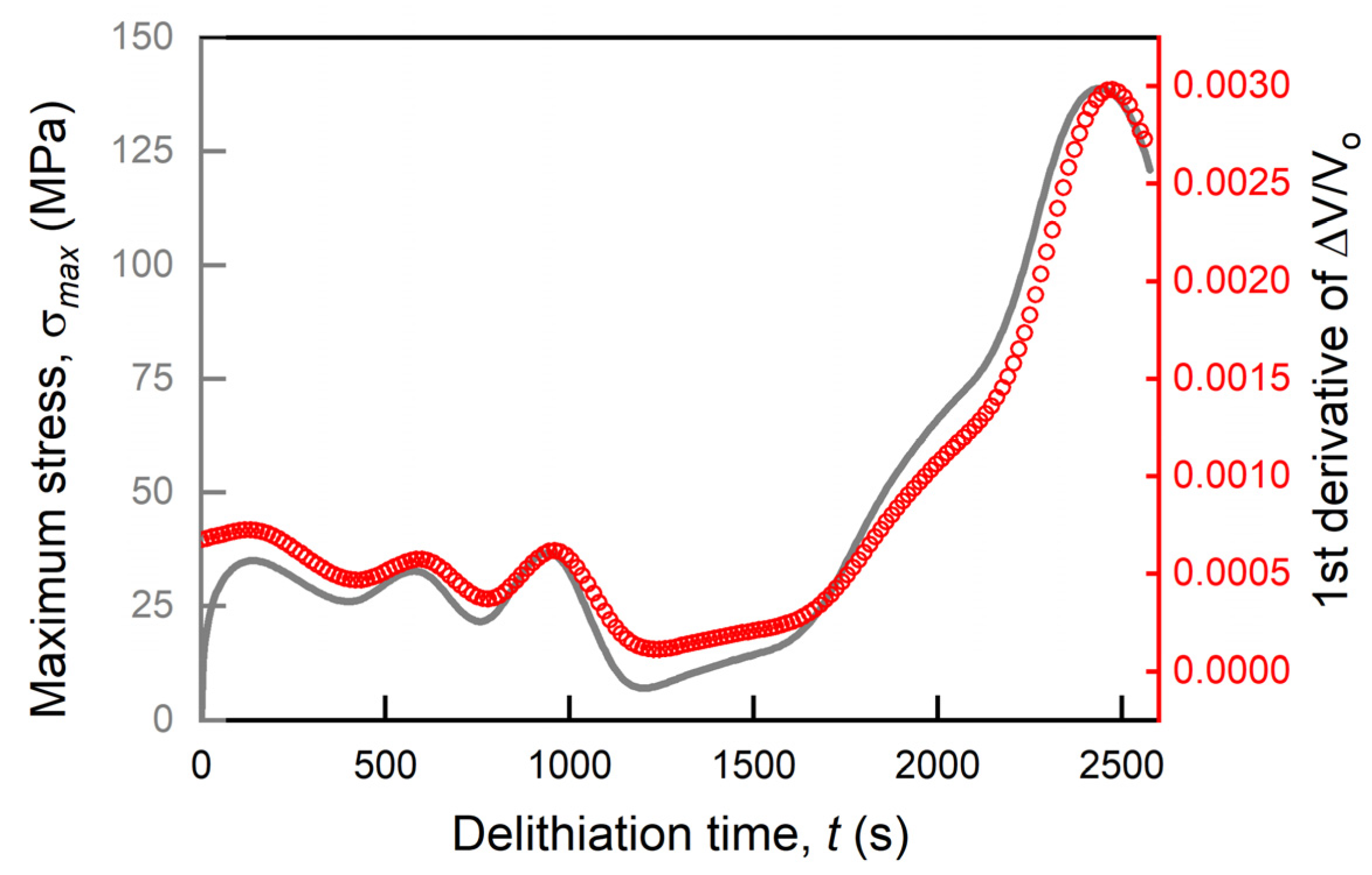
3.1. Validations of Numerical Results
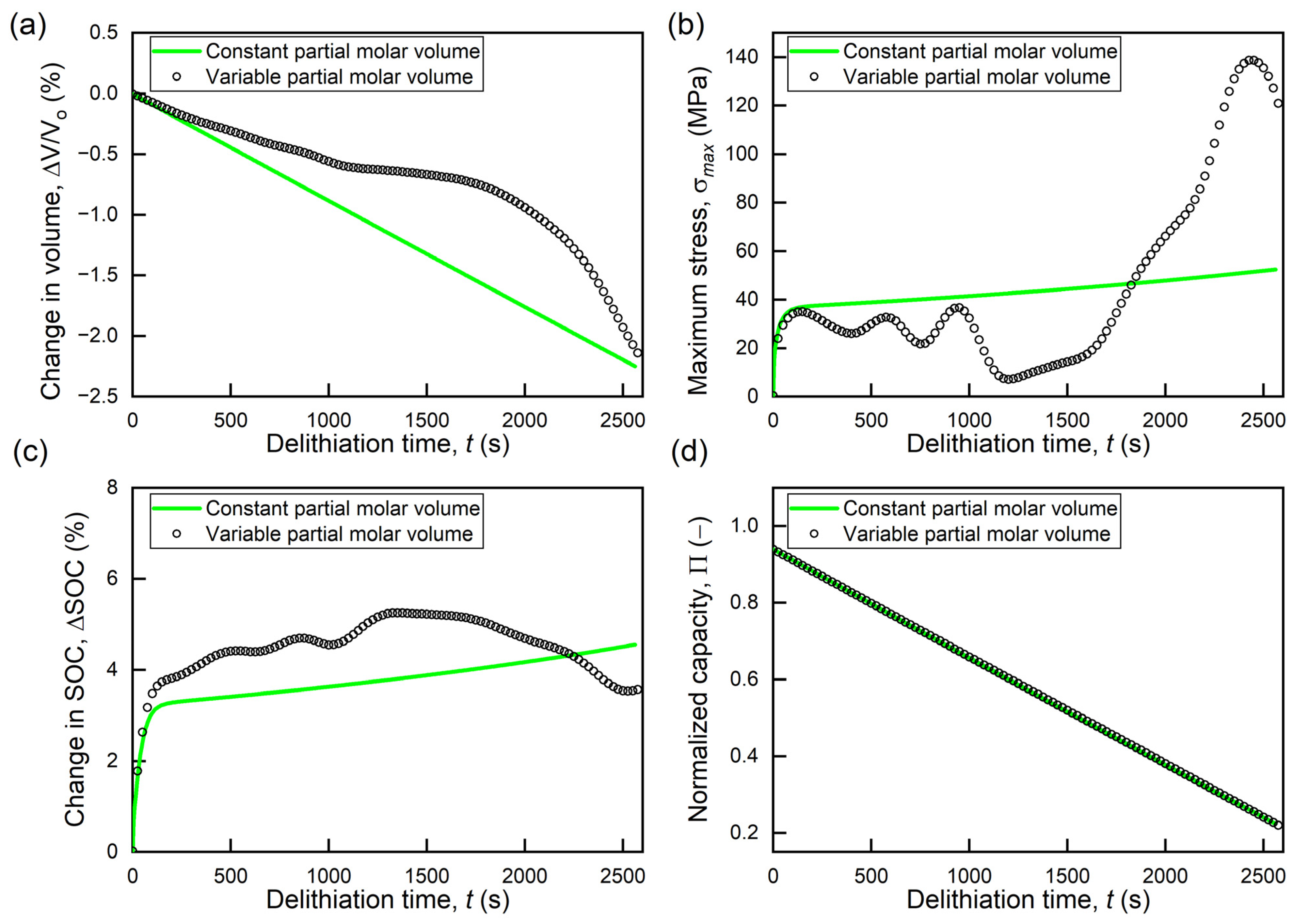
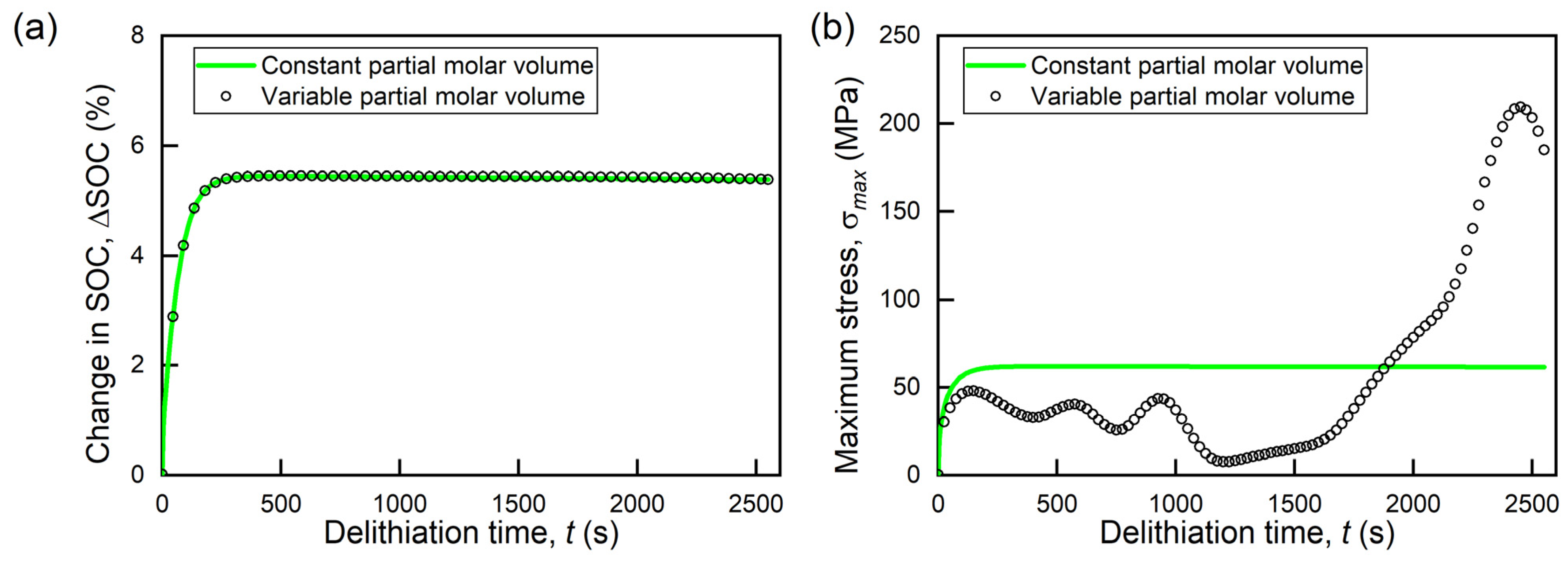
3.2. Effects of Variable Partial Molar Volume
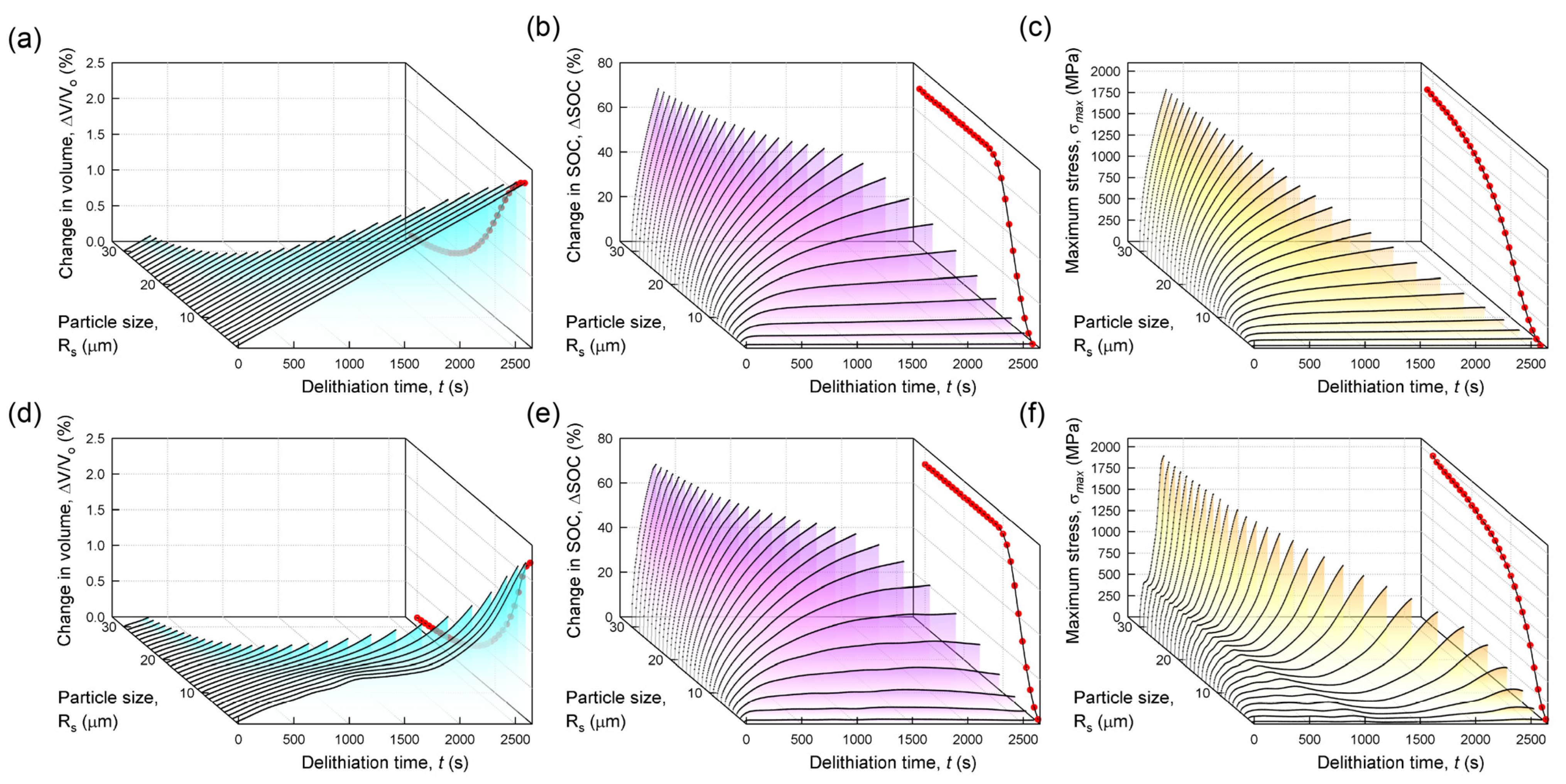
3.3. Effects of Particle Size
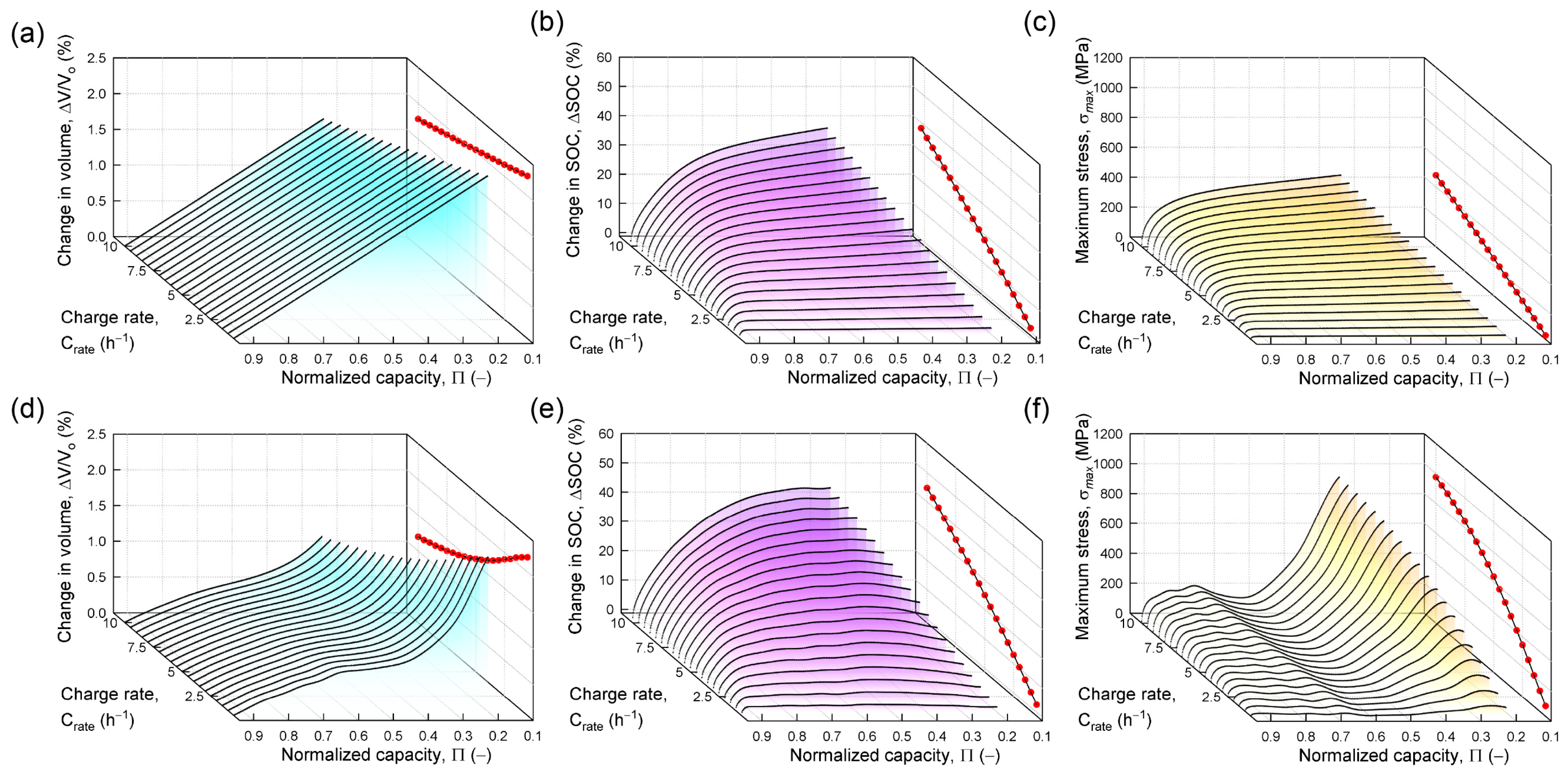
3.4. Effects of Charge Rate
4. Conclusions
- 1.
- The local volume change induced by the concentration-dependent chemical expansion of the active material significantly alters the global volume change of the active particles, which suggests that the stress increase due to the surrounding materials in electrodes will be affected by the concentration-dependent partial molar volume of the active materials.
- 2.
- The concentration-dependent partial molar volume significantly changes the stress evolution trends and SOC differences. The peak stress due to diffusion is almost three times greater for a variable partial molar volume. However, the accumulated capacity within the particle remains independent of the change in partial molar volume.
- 3.
- The trends of the maximum diffusion-induced stress in the particle correlate with variations in the time rate of change (total volume change of particle). Although changes in the partial molar volume affect the stress response of the active particles, the lithium concentration patterns and stress distributions remain the same.
- 4.
- As the particle size increases, the propensity for mechanical failure increases with the use of the concentration-dependent partial molar volume. However, the effect of changing the partial molar volume on the stress rise decreases for larger particles.
- 5.
- Faster charging with a concentration-dependent partial molar volume increases diffusion-induced stress levels compared to using a constant partial molar volume.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energ. Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Nie, L.; Chen, S.; Liu, W. Challenges and strategies of lithium-rich layered oxides for Li-ion batteries. Nano Res. 2022, 1–12. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; MacNeil, D.D.; Dahn, J.R. Layered cathode materials Li [Ni x Li (1/3− 2x/3) Mn (2/3− x/3)] O 2 for lithium-ion batteries. Electrochem. Solid-State Lett. 2001, 4, A191. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Ohzuku, T. Novel lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for advanced lithium-ion batteries. J. Power Sources 2003, 119–121, 171–174. [Google Scholar] [CrossRef]
- Iqbal, N.; Choi, J.; Lee, C.; Khan, A.; Tanveer, M.; Lee, S. A Review on Modeling of Chemo-mechanical Behavior of Particle—Binder Systems in Lithium-Ion Batteries. Multiscale Sci. Eng. 2022, 4, 79–93. [Google Scholar] [CrossRef]
- Ali, Y.; Iqbal, N.; Lee, S. Inhomogeneous stress development at the multiparticle electrode of lithium-ion batteries. Int. J. Energy Res. 2021, 45, 14788–14803. [Google Scholar] [CrossRef]
- Christensen, J.; Newman, J. Stress generation and fracture in lithium insertion materials. J. Solid State Electrochem. 2006, 10, 293–319. [Google Scholar] [CrossRef]
- Zhang, X.; Shyy, W.; Marie Sastry, A. Numerical Simulation of Intercalation-Induced Stress in Li-Ion Battery Electrode Particles. J. Electrochem. Soc. 2007, 154, A910. [Google Scholar] [CrossRef]
- Deshpande, R.; Cheng, Y.-T.; Verbrugge, M.W.; Timmons, A. Diffusion Induced Stresses and Strain Energy in a Phase-Transforming Spherical Electrode Particle. J. Electrochem. Soc. 2011, 158, A718. [Google Scholar] [CrossRef]
- Park, J.; Lu, W.; Sastry, A.M. Numerical Simulation of Stress Evolution in Lithium Manganese Dioxide Particles due to Coupled Phase Transition and Intercalation. J. Electrochem. Soc. 2011, 158, A201. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, K.; Liu, Y.; Stein, P.; Xu, B.-X. A chemo-mechanical grain boundary model and its application to understand the damage of Li-ion battery materials. Scr. Mater. 2020, 183, 45–49. [Google Scholar] [CrossRef]
- Barai, P.; Higa, K.; Ngo, A.T.; Curtiss, L.A.; Srinivasan, V. Mechanical Stress Induced Current Focusing and Fracture in Grain Boundaries. J. Electrochem. Soc. 2019, 166, A1752–A1762. [Google Scholar] [CrossRef]
- Han, S.; Park, J.; Lu, W.; Sastry, A.M. Numerical study of grain boundary effect on Li+ effective diffusivity and intercalation-induced stresses in Li-ion battery active materials. J. Power Sources 2013, 240, 155–167. [Google Scholar] [CrossRef]
- Zhao, K.; Pharr, M.; Vlassak, J.J.; Suo, Z. Fracture of electrodes in lithium-ion batteries caused by fast charging. J. Appl. Phys. 2010, 108, 073517. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.; Sastry, A.M.; Lu, W. Molecular Dynamics Simulations of SOC-Dependent Elasticity of LixMn2O4 Spinels in Li-Ion Batteries. J. Electrochem. Soc. 2013, 160, A968–A972. [Google Scholar] [CrossRef]
- Schmidt, A.P.; Bitzer, M.; Imre, Á.W.; Guzzella, L. Experiment-driven electrochemical modeling and systematic parameterization for a lithium-ion battery cell. J. Power Sources 2010, 195, 5071–5080. [Google Scholar] [CrossRef]
- Stein, P.; Xu, B. 3D Isogeometric Analysis of intercalation-induced stresses in Li-ion battery electrode particles. Comput. Methods Appl. Mech. Eng. 2014, 268, 225–244. [Google Scholar] [CrossRef]
- Singh, G.; Bhandakkar, T.K. Semianalytical study of the effect of realistic boundary conditions on diffusion induced stresses in cylindrical Lithium ion electrode-binder system. Int. J. Mech. Sci. 2019, 163, 105141. [Google Scholar] [CrossRef]
- Stein, P.; Moradabadi, A.; Diehm, M.; Xu, B.-X.; Albe, K. The influence of anisotropic surface stresses and bulk stresses on defect thermodynamics in LiCoO2 nanoparticles. Acta Mater. 2018, 159, 225–240. [Google Scholar] [CrossRef]
- Pharr, M.; Suo, Z.; Vlassak, J.J. Variation of stress with charging rate due to strain-rate sensitivity of silicon electrodes of Li-ion batteries. J. Power Sources 2014, 270, 569–575. [Google Scholar] [CrossRef]
- Ma, Z.S.; Xie, Z.C.; Wang, Y.; Zhang, P.P.; Pan, Y.; Zhou, Y.C.; Lu, C. Failure modes of hollow core–shell structural active materials during the lithiation–delithiation process. J. Power Sources 2015, 290, 114–122. [Google Scholar] [CrossRef]
- Li, J.; Fang, Q.; Wu, H.; Liu, Y.; Wen, P. Investigation into diffusion induced plastic deformation behavior in hollow lithium ion battery electrode revealed by analytical model and atomistic simulation. Electrochim. Acta 2015, 178, 597–607. [Google Scholar] [CrossRef]
- Wen, J.; Wei, Y.; Cheng, Y.-T. Stress evolution in elastic-plastic electrodes during electrochemical processes: A numerical method and its applications. J. Mech. Phys. Solids 2018, 116, 403–415. [Google Scholar] [CrossRef] [Green Version]
- Colclasure, A.M.; Smith, K.A.; Kee, R.J. Modeling detailed chemistry and transport for solid-electrolyte-interface (SEI) films in Li–ion batteries. Electrochim. Acta 2011, 58, 33–43. [Google Scholar] [CrossRef]
- Deng, J.; Wagner, G.J.; Muller, R.P. Phase Field Modeling of Solid Electrolyte Interface Formation in Lithium Ion Batteries. J. Electrochem. Soc. 2013, 160, A487–A496. [Google Scholar] [CrossRef]
- Liu, L.; Park, J.; Lin, X.; Sastry, A.M.; Lu, W. A thermal-electrochemical model that gives spatial-dependent growth of solid electrolyte interphase in a Li-ion battery. J. Power Sources 2014, 268, 482–490. [Google Scholar] [CrossRef]
- Gao, E.; Lu, B.; Zhao, Y.; Feng, J.; Song, Y.; Zhang, J. Stress-Regulated Protocols for Fast Charging and Long Cycle Life in Lithium-Ion Batteries: Modeling and Experiments. J. Electrochem. Soc. 2021, 168, 060549. [Google Scholar] [CrossRef]
- Iqbal, N.; Haq, I.U.; Lee, S. Chemo-mechanical model predicted critical SOCs for the mechanical stability of electrode materials in lithium-ion batteries. Int. J. Mech. Sci. 2022, 216, 107034. [Google Scholar] [CrossRef]
- Iqbal, N.; Lee, S. Stress-regulated pulse charging protocols via coupled electrochemical-mechanical model for the mechanical stability of electrode materials in lithium-ion batteries. J. Power Sources 2022, 536, 231376. [Google Scholar] [CrossRef]
- Iqbal, N.; Ali, Y.; Haq, I.U.; Lee, S. Progressive interface debonding in composite electrodes of Li-ion batteries via mixed-mode cohesive zone model: Effects of binder characteristics. Compos. Struct. 2021, 259, 113173. [Google Scholar] [CrossRef]
- Klinsmann, M.; Rosato, D.; Kamlah, M.; McMeeking, R.M. Modeling crack growth during Li extraction and insertion within the second half cycle. J. Power Sources 2016, 331, 32–42. [Google Scholar] [CrossRef]
- Xu, R.; Yang, Y.; Yin, F.; Liu, P.; Cloetens, P.; Liu, Y.; Lin, F.; Zhao, K. Heterogeneous damage in Li-ion batteries: Experimental analysis and theoretical modeling. J. Mech. Phys. Solids 2019, 129, 160–183. [Google Scholar] [CrossRef]
- Guo, Z.-S.; Zhang, T.; Zhu, J.; Wang, Y. Effects of hydrostatic pressure and modulus softening on electrode curvature and stress in a bilayer electrode plate. Comput. Mater. Sci. 2014, 94, 218–224. [Google Scholar] [CrossRef]
- He, Y.-L.; Hu, H.J.; Song, Y.-C.; Guo, Z.-S.; Liu, C.; Zhang, J.-Q. Effects of concentration-dependent elastic modulus on the diffusion of lithium ions and diffusion induced stress in layered battery electrodes. J. Power Sources 2014, 248, 517–523. [Google Scholar] [CrossRef]
- Qi, Y.; Hector, L.G.; James, C.; Kim, K.J. Lithium Concentration Dependent Elastic Properties of Battery Electrode Materials from First Principles Calculations. J. Electrochem. Soc. 2014, 161, F3010. [Google Scholar] [CrossRef] [Green Version]
- Sitinamaluwa, H.S.; Wang, M.C.; Will, G.; Senadeera, W.; Zhang, S.; Yan, C. Lithium concentration dependent structure and mechanics of amorphous silicon. J. Appl. Phys. 2016, 119, 245103. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Kang, Y.; Song, H.; Zhang, Q. Real-time measurements and experimental analysis of material softening and total stresses of Si-composite electrode. J. Power Sources 2019, 424, 100–107. [Google Scholar] [CrossRef]
- Yang, B.; He, Y.-P.; Irsa, J.; Lundgren, C.A.; Ratchford, J.B.; Zhao, Y.-P. Effects of composition-dependent modulus, finite concentration and boundary constraint on Li-ion diffusion and stresses in a bilayer Cu-coated Si nano-anode. J. Power Sources 2012, 204, 168–176. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, Z. Effects of electrode properties and fabricated pressure on Li ion diffusion and diffusion-induced stresses in cylindrical Li-ion batteries. Model. Simul. Mater. Sci. Eng. 2014, 22, 025016. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, S.; Zhang, K.; Zheng, B.; Lyu, L. Buckling behavior of a wire-like electrode with a concentration-dependent elastic modulus based on a deformed configuration. Eur. J. Mech.-A/Solids 2021, 85, 104111. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, K.; Wang, C.; Han, J.; Gao, H. Concentration dependent properties and plastic deformation facilitate instability of the solid-electrolyte interphase in Li-ion batteries. Int. J. Solids Struct. 2020, 198, 99–109. [Google Scholar] [CrossRef]
- Weng, L.; Xu, C.; Chen, B.; Zhou, J.; Cai, R.; Shi, Y. A comparative study on ratcheting deformation between negative Poisson’s ratio electrode and thin film electrode in Li-ion battery cyclic operation. Mech. Mater. 2020, 150, 103567. [Google Scholar] [CrossRef]
- Hong, C.S.; Qaiser, N.; Nam, H.G.; Han, S.M. Effect of Li concentration-dependent material properties on diffusion induced stresses of a Sn anode. Phys. Chem. Chem. Phys. 2019, 21, 9581–9589. [Google Scholar] [CrossRef]
- Dora, J.K.; Sengupta, A.; Ghosh, S.; Yedla, N.; Chakraborty, J. Stress evolution with concentration-dependent compositional expansion in a silicon lithium-ion battery anode particle. J. Solid State Electrochem. 2019, 23, 2331–2342. [Google Scholar] [CrossRef]
- Malavé, V.; Berger, J.R.; Martin, P.A. Concentration-Dependent Chemical Expansion in Lithium-Ion Battery Cathode Particles. J. Appl. Mech. 2014, 81, 091005. [Google Scholar] [CrossRef]
- Qian, Y.; Lu, B.; Bao, Y.; Zhao, Y.; Song, Y.; Zhang, J. Prelithiation design for suppressing delamination in lithium-ion battery electrodes. Appl. Math. Mech.-Engl. Ed. 2021, 42, 1703–1716. [Google Scholar] [CrossRef]
- Cai, X.; Guo, Z. Influence of Li Concentration-Dependent Diffusion Coefficient and Modulus Hardening on Diffusion-Induced Stresses in Anisotropic Anode Particles. J. Electrochem. Soc. 2021, 168, 010517. [Google Scholar] [CrossRef]
- Karami, A.; Nayebi, A. Kinematic hardening analysis of Li-ion battery with concentration-dependent material behaviours under cyclic charging and discharging. J. Power Sources 2020, 461, 228155. [Google Scholar] [CrossRef]
- Deshpande, R.; Qi, Y.; Cheng, Y.-T. Effects of Concentration-Dependent Elastic Modulus on Diffusion-Induced Stresses for Battery Applications. J. Electrochem. Soc. 2010, 157, A967–A971. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Y.; Zheng, B. Effects of concentration-dependent elastic modulus on Li-ions diffusion and diffusion-induced stresses in spherical composition-gradient electrodes. J. Appl. Phys. 2015, 118, 105102. [Google Scholar] [CrossRef]
- de Biasi, L.; Kondrakov, A.O.; Geßwein, H.; Brezesinski, T.; Hartmann, P.; Janek, J. Between Scylla and Charybdis: Balancing Among Structural Stability and Energy Density of Layered NCM Cathode Materials for Advanced Lithium-Ion Batteries. J. Phys. Chem. C 2017, 121, 26163–26171. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li [NixCoyMnz] O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, X.; Xia, S.; Zhang, K.; Wei, C.; Bak, S.; Shadike, Z.; Liu, X.; Yang, Y.; Xu, R.; et al. High-Voltage Charging-Induced Strain, Heterogeneity, and Micro-Cracks in Secondary Particles of a Nickel-Rich Layered Cathode Material. Adv. Funct. Mater. 2019, 29, 1900247. [Google Scholar] [CrossRef]
- Iqbal, N.; Ali, Y.; Lee, S. Chemo-mechanical response of composite electrode systems with multiple binder connections. Electrochim. Acta 2020, 364, 137312. [Google Scholar] [CrossRef]
- Takahashi, K.; Higa, K.; Mair, S.; Chintapalli, M.; Balsara, N.; Srinivasan, V. Mechanical degradation of graphite/PVDF composite electrodes: A model-experimental study. J. Electrochem. Soc. 2016, 163, A385–A395. [Google Scholar] [CrossRef] [Green Version]
- Ryu, I.; Choi, J.W.; Cui, Y.; Nix, W.D. Size-dependent fracture of Si nanowire battery anodes. J. Mech. Phys. Solids 2011, 59, 1717–1730. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, K. Electronic structure and comparative properties of LiNi x Mn y Co z O2 cathode materials. J. Phys. Chem. C 2017, 121, 6002–6010. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, J.; Cui, S.; Song, X.; Su, Y.; Deng, W.; Wu, Z.; Wang, X.; Wang, W.; Rao, M.; et al. Kinetics tuning of Li-ion diffusion in layered Li (Ni x Mn y Co z) O2. J. Am. Chem. Soc. 2015, 137, 8364–8367. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, W.; Yao, J.; Bu, L.; Li, X.; Tian, K.; Lu, H.; Quan, C.; Xu, S.; Xu, K.; et al. Suppressing structural degradation of Ni-rich cathode materials towards improved cycling stability enabled by a Li2 MnO3 coating. J. Mater. Chem. A 2020, 8, 17429–17441. [Google Scholar] [CrossRef]
- Cheng, E.J.; Hong, K.; Taylor, N.J.; Choe, H.; Wolfenstine, J.; Sakamoto, J. Mechanical and physical properties of LiNi0. 33Mn0. 33Co0. 33O2 (NMC). J. Eur. Ceram. Soc. 2017, 37, 3213–3217. [Google Scholar] [CrossRef]
- Mistry, A.; Juarez-Robles, D.; Stein, M.; Smith, K.; Mukherjee, P.P. Analysis of long-range interaction in lithium-ion battery electrodes. J. Electrochem. Energy Convers. Storage 2016, 13, 031006. [Google Scholar] [CrossRef]



| Parameters | Symbols | Units | Values | |||
|---|---|---|---|---|---|---|
| NMC-111 | NMC-523 | NMC-622 | NMC-811 | |||
| Young’s modulus 1 | GPa | 202.98 | 191.79 | 181.52 | 194.4 | |
| Diffusion coefficient 2 | m2 s−1 | 3.39 × 10−15 | 3.89 × 10−15 | 7.5 × 10−15 | 4.0 × 10−14 | |
| Specific capacity 3 | mAh g−1 | 188.75 | 194.89 | 203.18 | 213.42 | |
| Stoichiometric lithium concentration 4 | mol m−3 | 33,452 | 34,542 | 36,009 | 37,825 | |
| Minimum SOC 5 | % | 21 | 19 | 18 | 11 | |
| Maximum SOC 6 | % | 94 | 93 | 93 | 90 | |
| Poisson’s ratio 7 | - | 0.25 | ||||
| Density 8 | kg m−3 | 4750 | ||||
| Faraday’s constant | C mol−1 | 96,487 | ||||
| Absolute temperature | K | 300 | ||||
| Universal gas constant | J mol−1 K−1 | 8.314 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, N.; Choi, J.; Lee, C.; Ayub, H.M.U.; Kim, J.; Kim, M.; Kim, Y.; Moon, D.; Lee, S. Effects of Diffusion-Induced Nonlinear Local Volume Change on the Structural Stability of NMC Cathode Materials of Lithium-Ion Batteries. Mathematics 2022, 10, 4697. https://doi.org/10.3390/math10244697
Iqbal N, Choi J, Lee C, Ayub HMU, Kim J, Kim M, Kim Y, Moon D, Lee S. Effects of Diffusion-Induced Nonlinear Local Volume Change on the Structural Stability of NMC Cathode Materials of Lithium-Ion Batteries. Mathematics. 2022; 10(24):4697. https://doi.org/10.3390/math10244697
Chicago/Turabian StyleIqbal, Noman, Jinwoong Choi, Changkyu Lee, Hafiz Muhammad Uzair Ayub, Jinho Kim, Minseo Kim, Younggee Kim, Dongjae Moon, and Seungjun Lee. 2022. "Effects of Diffusion-Induced Nonlinear Local Volume Change on the Structural Stability of NMC Cathode Materials of Lithium-Ion Batteries" Mathematics 10, no. 24: 4697. https://doi.org/10.3390/math10244697
APA StyleIqbal, N., Choi, J., Lee, C., Ayub, H. M. U., Kim, J., Kim, M., Kim, Y., Moon, D., & Lee, S. (2022). Effects of Diffusion-Induced Nonlinear Local Volume Change on the Structural Stability of NMC Cathode Materials of Lithium-Ion Batteries. Mathematics, 10(24), 4697. https://doi.org/10.3390/math10244697







