An Agent-Based Interpretation of Leukocyte Chemotaxis in Cancer-on-Chip Experiments
Abstract
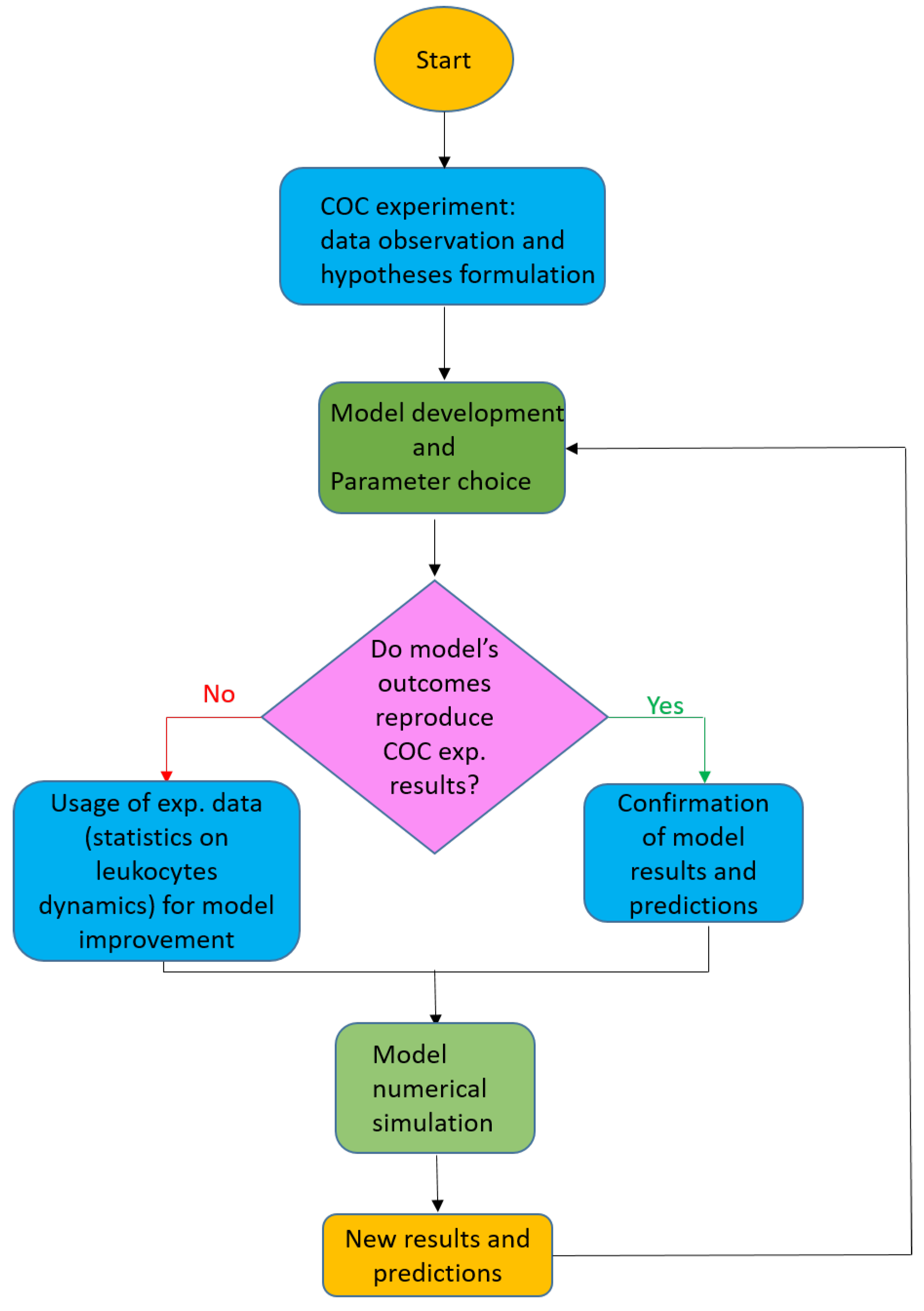
:1. Introduction
1.1. Original Contribution of the Present Paper and Sketch of the Methodology
1.2. Plan of the Paper
2. Biological Framework
Setting of the Real Experiment
3. The Hybrid Agent-Based Cellular Automata Model
3.1. The Mathematical Model
3.2. Numerical Algorithm
- Definition of chip geometry and random positioning of TCs and leukocytes in the computational domain at initial time, avoiding superpositions;
- Evolution in time and space of the chemoattractant (elimination/production/diffusion);
- Migration of the leukocytes, characterized by biased random walk.
3.2.1. Part 1: Creation of the Chip Environment
- if ;
- if ;
- if the point falls on an obstacle separating two consecutive channels;
- otherwise, i.e., if the point is free from obstacles and is not occupied by either cancer cells or Leukocytes.
3.2.2. Part 2: Production/Diffusion/Elimination of Annexin
- Linear elimination: for each pixel , ;
- Linear production: for each pixel , ;
- Diffusion among the Moore neighbors of the pixel .
3.2.3. Part 3: Leukocyte Migration
- Find the and neighboring pixels belonging to , disregarding the pixels occupied by obstacles and the pixels outside the map;
- Compute the total peripheral quantity of annexin and , summing over the neighboring cells and excluding the central one, as well as the total quantity of annexin , including the central pixel;
- Compute a threshold probability value , with , so that for small values of annexin in and around the central pixel the probability of the leukocyte moving at all is low. Pass to the next step only if , with a uniformly distributed random variable;
- Move to neighboring pixel with probability and . This is performed by computing cumulative probabilities and , generating another uniformly random variable and picking as next pixel position the pixel if , with the obvious stipulation that . Notice that even in this case (having decided that movement is possible), it may happen (with probability ) that the destination pixel is the same as the current pixel, i.e., that the leukocyte does not move at all.
4. Results
Qualitative Calibration of the Model
5. Discussion
6. Conclusions and Future Aims
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Businaro, L.; De Ninno, A.; Schiavoni, G.; Lucarini, V.; Ciasca, G.; Gerardino, A.; Belardelli, F.; Gabriele, L.; Mattei, F. Cross talk between cancer and immune cells: Exploring complex dynamics in a microfluidic environment. Lab Chip 2013, 13, 229–239. [Google Scholar] [CrossRef]
- Vacchelli, E.; Ma, Y.; Baracco, E.E.; Sistigu, A.; Enot, D.P.; Pietrocola, F.; Kroemer, G.; Stoll, G.; Boige, V.; Mulot, C.; et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science 2015, 13, 972–978. [Google Scholar] [CrossRef]
- Biselli, E.; Agliari, E.; Barra, A.; Bertani, F.R.; Gerardino, A.; De Ninno, A.; Businaro, L. Organs on chip approach: A tool to evaluate cancer-immune cells interactions. Sci. Rep. 2017, 7, 912737. [Google Scholar] [CrossRef]
- Comes, M.C.; Casti, P.; Mencattini, A.; Di Giuseppe, D.; Mermet-Meillon, F.; De Ninno, A.; Martinelli, E. The influence of spatial and temporal resolutions on the analysis of cell-cell interaction: A systematic study for time-lapse microscopy applications. Sci. Rep. 2019, 9, 6789. [Google Scholar] [CrossRef] [PubMed]
- Gori, M.; Simonelli, M.C.; Giannitelli, S.M.; Businaro, L.; Trombetta, M.; Rainer, A. Investigating nonalcoholic fatty liver disease in a liver-on-a-chip microfluidic device. PLoS ONE 2016, 11, e0159729. [Google Scholar] [CrossRef]
- Agliari, E.; Biselli, E.; De Ninno, A.; Schiavoni, G.; Gabriele, L.; Gerardino, A.; Businaro, L. Cancer-driven dynamics of immune cells in a microfluidic environment. Sci. Rep. 2014, 4, 6639. [Google Scholar] [CrossRef]
- Greenberg, J.M.; Alt, W. Stability results for a diffusion equation with functional drift approximating a chemotaxis model. Trans. Am. Math. Soc. 1987, 300, 235–258. [Google Scholar] [CrossRef]
- Keller, E.F.; Segel, L.A. Initiation of slime mold aggregation viewed as an instability. J. Theor. Biol. 1970, 26, 399–415. [Google Scholar] [CrossRef]
- Altrock, P.M.; Liu, L.L.; Michor, F. The mathematics of cancer: Integrating quantitative models. Nat. Rev. Cancer 2015, 15, 730–745. [Google Scholar] [CrossRef] [PubMed]
- Bretti, G.; Natalini, R. Numerical approximation of nonhomogeneous boundary conditions on networks for a hyperbolic system of chemotaxis modeling the physarum dynamics. J. Comput. Methods Sci. Eng. 2018, 18, 85–115. [Google Scholar] [CrossRef] [Green Version]
- Hillen, T.; Painter, K.J. A user’s guide to PDE models for chemotaxis. J. Math. Biol. 2009, 58, 183–217. [Google Scholar] [CrossRef] [PubMed]
- Checcoli, A.; Pol, J.G.; Naldi, A.; Noel, V.; Barillot, E.; Kroemer, G.; Thieffry, D.; Calzone, L.; Stoll, G. Dynamical Boolean Modeling of Immunogenic Cell Death. Front Physiol. 2020, 11, 590479. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, D.; Panetta, J.C. Modeling immunotherapy of the tumor-immune interaction. J. Math. Biol. 1998, 37, 235–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Kim, S.K.; Khawar, I.A.; Jeong, S.Y.; Chung, S.; Kuh, H.J. Microfluidic co- culture of pancreatic tumor spheroids with stellate cells as a novel 3D model for investigation of stroma- mediated cell motility and drug resistance. J. Exp. Clin. Cancer Res. 2018, 37, 4. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.L.; Seager, R.J.; Litvak, F.; Spill, F.; Sieow, J.L.; Leong, P.H.; Kumar, D.; Tan, A.S.M.; Wong, S.C.; Adriani, G.; et al. Integrated in silico and 3D in vitro model of macrophagemigration in response to physical and chemical factors in the tumor microenvironment. Integr. Biol. 2020, 12, 90–108. [Google Scholar] [CrossRef] [PubMed]
- Perthame, B. Transport Equations in Biology; Birkhäuser: Basel, Switzerland, 2007. [Google Scholar]
- Segovia-Juarez, J.L.; Ganguli, S.; Kirschner, D. Identifying control mechanisms of granuloma formation during M. tuberculosis infection using an agent-based model. J. Theor. Biol. 2004, 231, 357–376. [Google Scholar] [CrossRef]
- Yang, T.D.; Park, J.; Choi, Y.; Choi, W.; Ko, T.; Kyoung, J. Zigzag turning preference of freely crawling cells. PLoS ONE 2011, 6, e20255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, G.; Mi, Q.; Dutta-Moscato, J.; Vodovotz, Y. Agent-based models in translational systems biology. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009, 1, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Bauer, A.L.; Beauchemin, C.A.; Perelson, A.S. Agent-based modeling of host-pathogen systems: The successes and challenges. Inf. Sci. 2009, 179, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Cilfone, N.A.; Kirschner, D.E.; Linderman, J.J. Strategies for efficient numerical implementation of hybrid multi-scale agent-based models to describe biological systems. Cell. Mol. Bioeng. 2015, 8, 119–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen Edalgo, Y.T.; Zornes, A.L.; Ford Versypt, A.N. A hybrid discrete-continuous model of metastatic cancer cell migration through a remodeling extracellular matrix. Aiche J. 2019, 65, e16671. [Google Scholar] [CrossRef]
- Perfahl, H.; Hughes, B.D.; Alarcón, T.; Maini, P.K.; Lloyd, M.C.; Reuss, M.; Byrne, H.M. 3D hybrid modelling of vascular network formation. J. Theor. Biol. 2017, 414, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, F.R.; Chaplain, M.A.; Lorenzi, T. A hybrid discrete-continuum approach to model Turing pattern formation. Math. Biosci. Eng. 2020, 17, 7442–7479. [Google Scholar] [CrossRef] [PubMed]
- Othmer, H.G. Cell-Based, Continuum and Hybrid Models of Tissue Dynamics. In Mathematical Models and Methods for Living Systems; Book Series: Lecture Notes in Mathematics; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–72. [Google Scholar]
- Braun, E.C.; Bretti, G.; Natalini, R. Mass-Preserving Approximation of a Chemotaxis Multi-Domain Transmission Model for Microfluidic Chips. Mathematics 2021, 9, 688. [Google Scholar] [CrossRef]
- Braun, E.C.; Bretti, G.; Natalini, R. Parameter estimation techniques for a chemotaxis model inspired by Cancer-on-Chip (COC) experiments. Int. J. -Non-Linear Mech. 2022, 140, 103895. [Google Scholar] [CrossRef]
- Bretti, G.; De Ninno, A.; Natalini, R.; Peri, D.; Roselli, N. Estimation Algorithm for a Hybrid PDE–ODE Model Inspired by Immunocompetent Cancer-on-Chip Experiment. Axioms 2021, 10, 243. [Google Scholar] [CrossRef]
- Anbumani, S.; da Silva, A.M.; Carvalho, I.G.; Fischer, E.R.; de Souza e Silva, M.; von Zuben, A.A.G.; Cotta, M.A.; Carvalhom, H.F.; de Souza, A.A.; Janissen, R. Controlled spatial organization of bacterial growth reveals key role of cell filamentation preceding Xylella fastidiosa biofilm formation. NPJ Biofilms Microbiomes 2021, 7, 86. [Google Scholar] [CrossRef]
- Curk, T.; Marenduzzo, D.; Dobnikar, J. Chemotactic sensing towards ambient and secreted attractant drives collective behaviour of E. coli. PLoS ONE 2013, 8, e74878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kindler, O.; Pulkkinen, O.; Cherstvy, A.G.; Metzler, R. Burst statistics in an early biofilm quorum sensing model: The role of spatial colony-growth heterogeneity. Sci. Rep. 2019, 9, 12077. [Google Scholar] [CrossRef] [Green Version]
- Limoli, D.H.; Warren, E.A.; Yarrington, K.D.; Donegan, N.P.; Cheung, A.L.; O’Toole, G.A. Interspecies interactions induce exploratory motility in Pseudomonas aeruginosa. eLife 2019, 8, e47365. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, K.; Yamaguchi, Y.; Usui, S.; Nashimoto, Y.; Hanada, S.; Kiyokawa, E.; Miura, T.; Uemura, A.; Yokokawa, R.; Nishiyama, K. A new perfusion culture method with a self-organized capillary network. PLoS ONE 2020, 15, e0240552. [Google Scholar] [CrossRef] [PubMed]
- Parlato, S.; De Ninno, A.; Molfetta, R.; Toschi, E.; Salerno, D.; Mencattini, A.; Romagnoli, G.; Fragale, A.; Roccazzello, L.; Buoncervello, M.; et al. 3D Microfluidic model for evaluating immunotherapy efficacy by tracking dendritic cell behaviour toward tumor cells. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Mattei, F.; Andreone, S.; Mencattini, A.; De Ninno, A.; Businaro, L.; Martinelli, E.; Schiavoni, G. Oncoimmunology Meets Organs-on-Chip. Front. Mol. Biosci. 2021, 8, 627454. [Google Scholar] [CrossRef] [PubMed]
- Maulana, T.I.; Kromidas, E.; Wallstabe, L.; Cipriano, M.; Alb, M.; Zaupa, C.; Loskill, P.; Hudecek, M.; Fogal, B. Immunocompetent cancer-on-chip models to assess immuno-oncology therapy. Adv. Drug Deliv. Rev. 2021, 173, 281–305. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.R.; Hein, M.Y.; Cox, J.; Mann, M. A “proteomic ruler” for protein copy number and concentration estimation without spike-in standards. Mol. Cell. Proteom. 2014, 13, 3497–3506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulter, E.; Grall, D.; Cagnol, S.; Van Obberghen-Schilling, E. Regulation of cell-matrix adhesion dynamics and Rac-1 by integrin linked kinase. FASEB J. 2006, 20, 1489–1491. [Google Scholar] [CrossRef]
- Weninger, W.; Biro, M.; Jain, R. Leukocyte migration in the interstitial space of non-lymphoid organs. Nat. Rev. Immunol. 2014, 14, 232–246. [Google Scholar] [CrossRef]
- Harris, T.H.; Banigan, E.J.; Christian, D.A.; Konradt, C.; Tait Wojno, E.D.; Norose, K.; Hunter, C.A.; Wilson, E.M.; John, B.; Weninger, W.; et al. Generalized Lévy walks and the role of chemokines in migration of effector CD8+ T cells. Nature 2012, 486, 545–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Qi, S.; Jin, H.; Qi, Z.; Zhang, Z.; Fu, L.; Luo, Q. Zigzag Generalized Lévy Walk: The In Vivo Search Strategy of Immunocytes. Theranostics 2015, 5, 1275–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Parameter | Description | Units | Value | Refs. |
|---|---|---|---|---|
| discretization time step | design | |||
| D | Diffusivity of chemoattractant | calibration | ||
| production rate of chemicals | calibration | |||
| consumption rate of chemicals | calibration | |||
| threshold value for migration | - | 1000 | calibration | |
| parameter enforcing migration towards high concentration | - | 1 | calibration | |
| length of the channels | 500 | datum | ||
| horizontal size of the box | 1702 | datum | ||
| vertical size of the box | 1362 | datum | ||
| number of microchannels in the video footage | - | 31 | datum | |
| width of each microchannel | 12 | datum | ||
| width of obstacles | 33 | datum | ||
| observation time | datum | |||
| radius of leukocytes | 4 | [37] | ||
| radius of tumor cells | 10 | [38] | ||
| number of tumor cells | ∼60 | datum | ||
| normalized rate of new leukocyte accrual | calibration | |||
| number of frames of the laboratory experiment | - | 1440 | datum |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bretti, G.; De Gaetano, A. An Agent-Based Interpretation of Leukocyte Chemotaxis in Cancer-on-Chip Experiments. Mathematics 2022, 10, 1338. https://doi.org/10.3390/math10081338
Bretti G, De Gaetano A. An Agent-Based Interpretation of Leukocyte Chemotaxis in Cancer-on-Chip Experiments. Mathematics. 2022; 10(8):1338. https://doi.org/10.3390/math10081338
Chicago/Turabian StyleBretti, Gabriella, and Andrea De Gaetano. 2022. "An Agent-Based Interpretation of Leukocyte Chemotaxis in Cancer-on-Chip Experiments" Mathematics 10, no. 8: 1338. https://doi.org/10.3390/math10081338
APA StyleBretti, G., & De Gaetano, A. (2022). An Agent-Based Interpretation of Leukocyte Chemotaxis in Cancer-on-Chip Experiments. Mathematics, 10(8), 1338. https://doi.org/10.3390/math10081338







