Spatial Demo-Genetic Predator–Prey Model for Studying Natural Selection of Traits Enhancing Consumer Motility
Abstract
:1. Introduction
2. The Model and Simulation Scenarios
3. Results
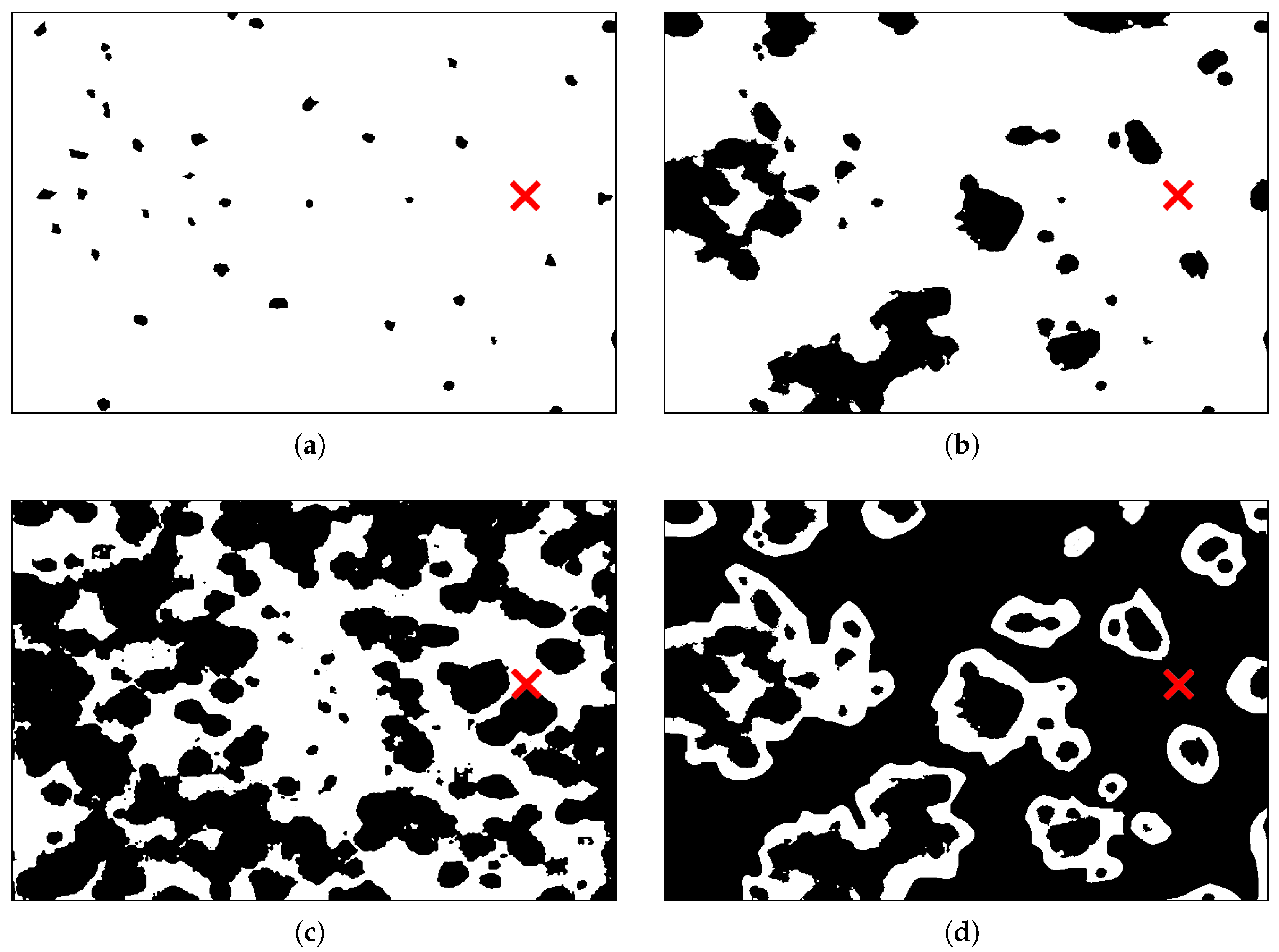
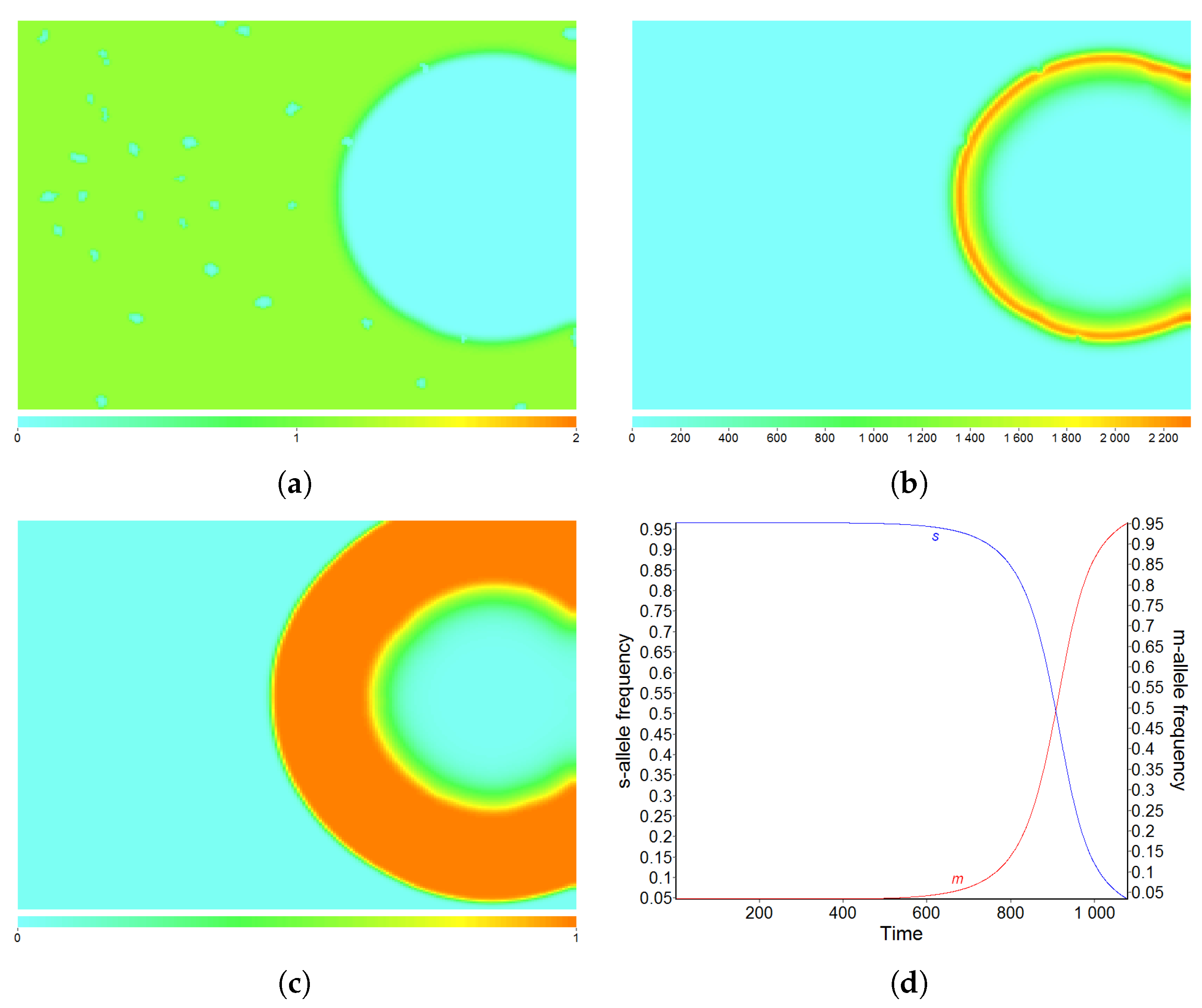
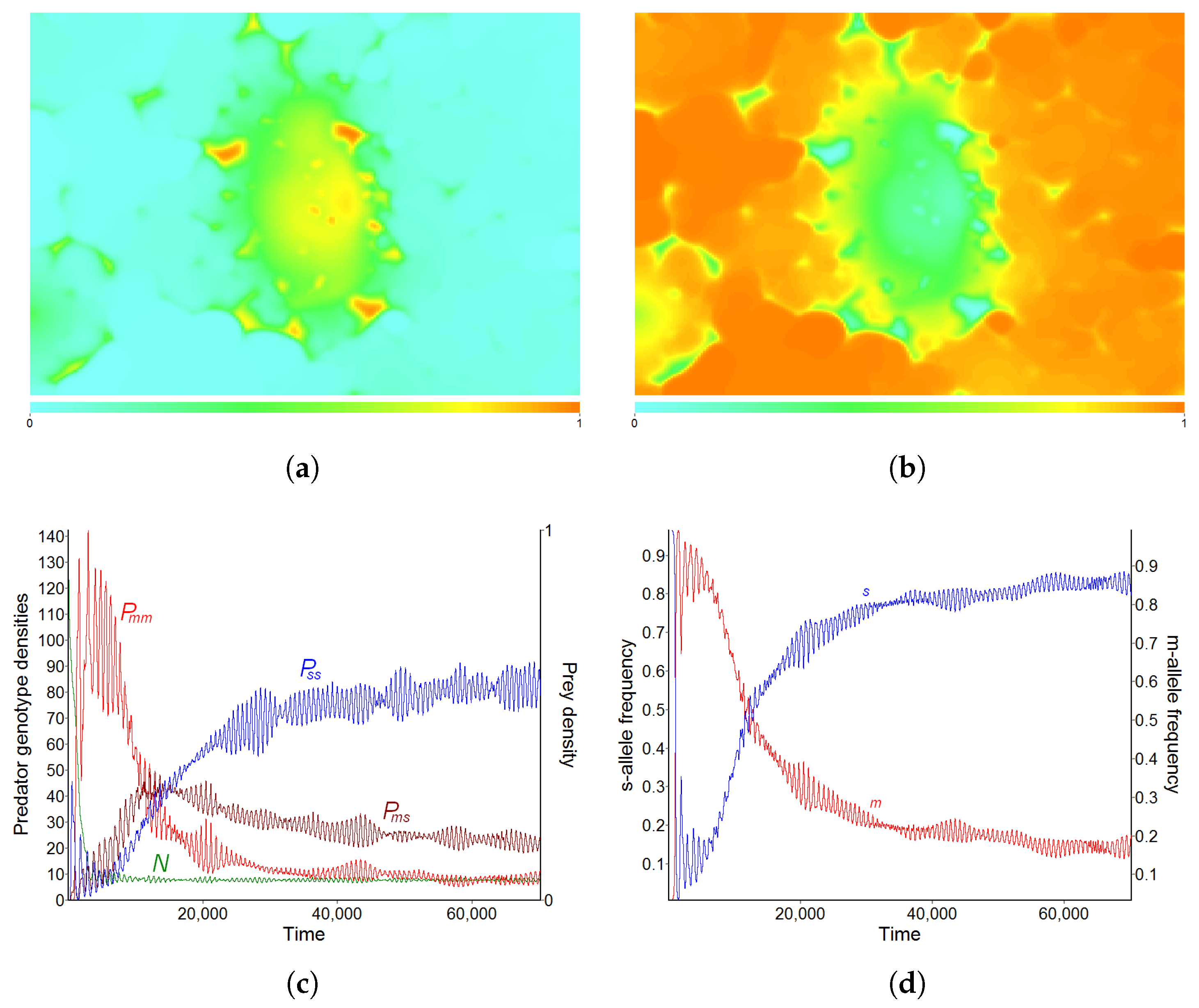
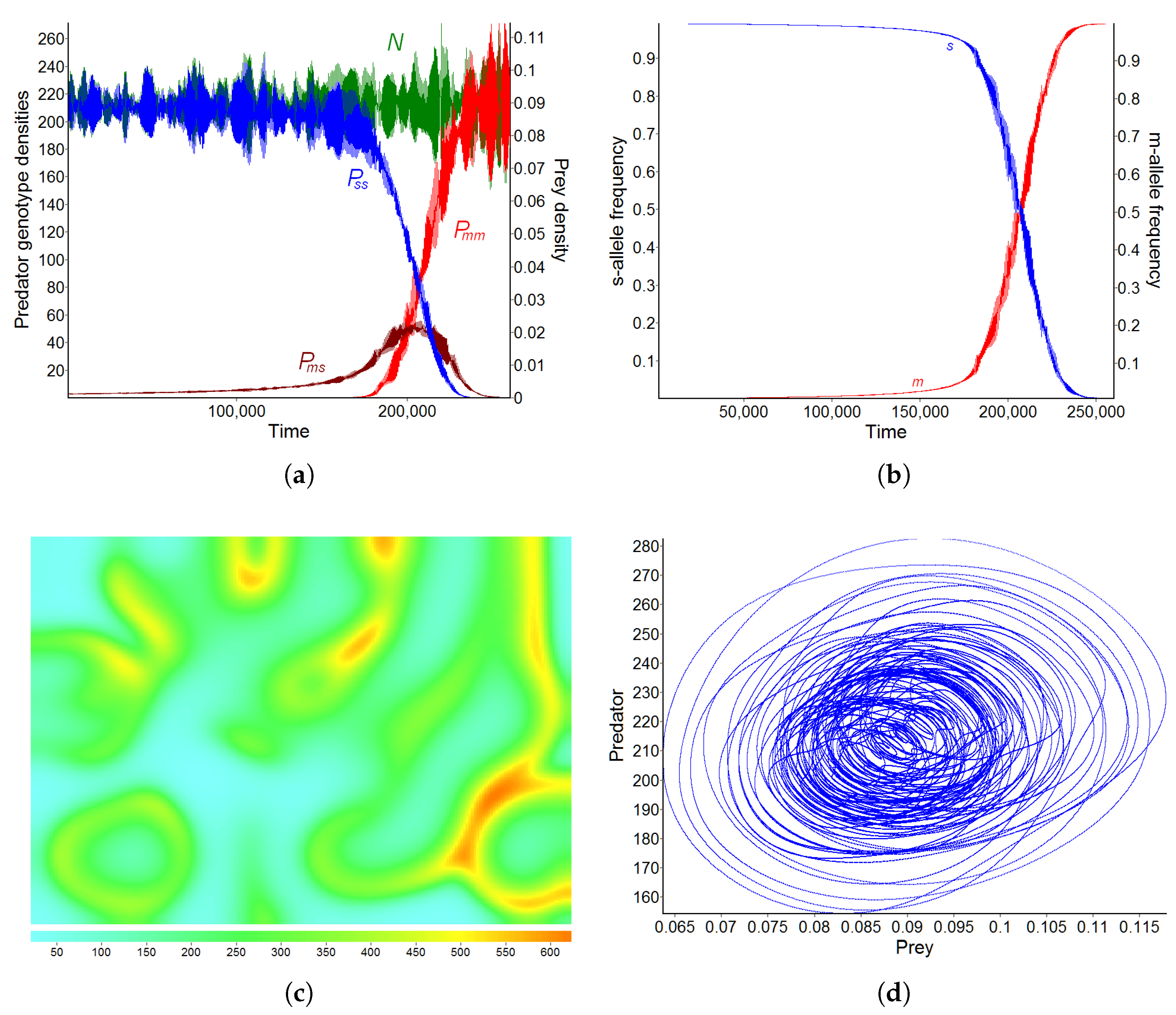
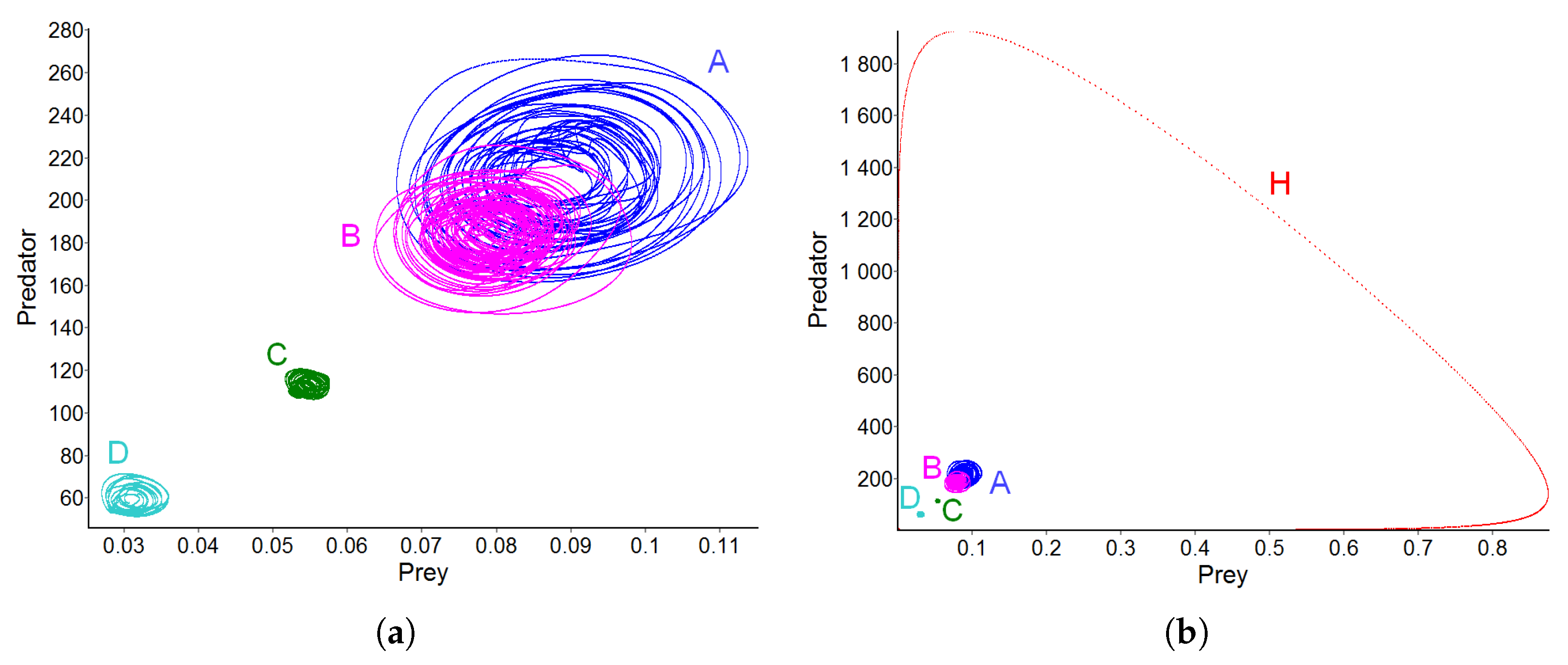
3.1. Pattern A—Weak Fragmentation of the Habitat
3.2. Pattern B—Moderate Fragmentation of the Habitat
3.3. Pattern C—Essential Fragmentation of the Habitat
3.4. Pattern D—Strong Fragmentation of the Habitat
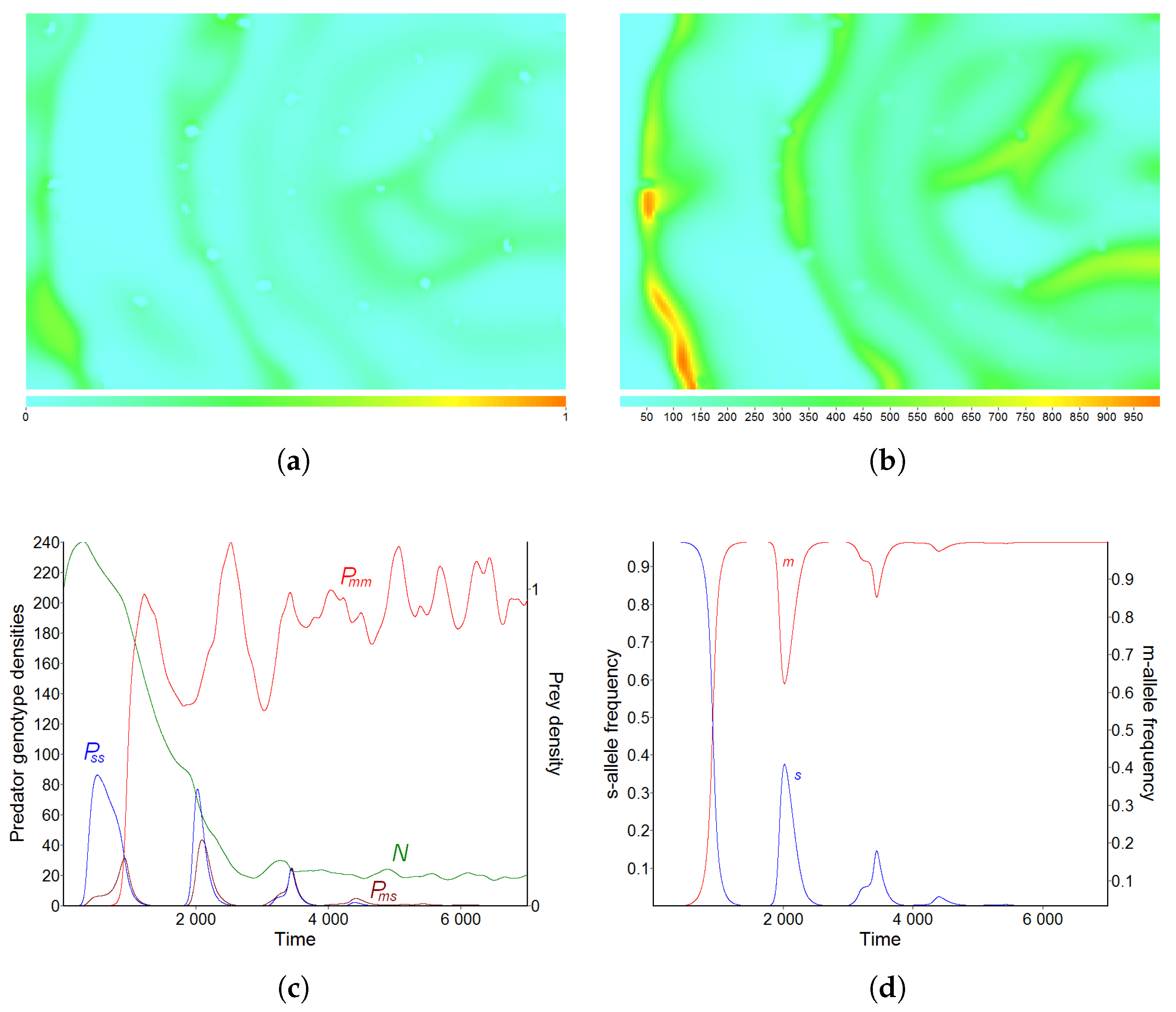
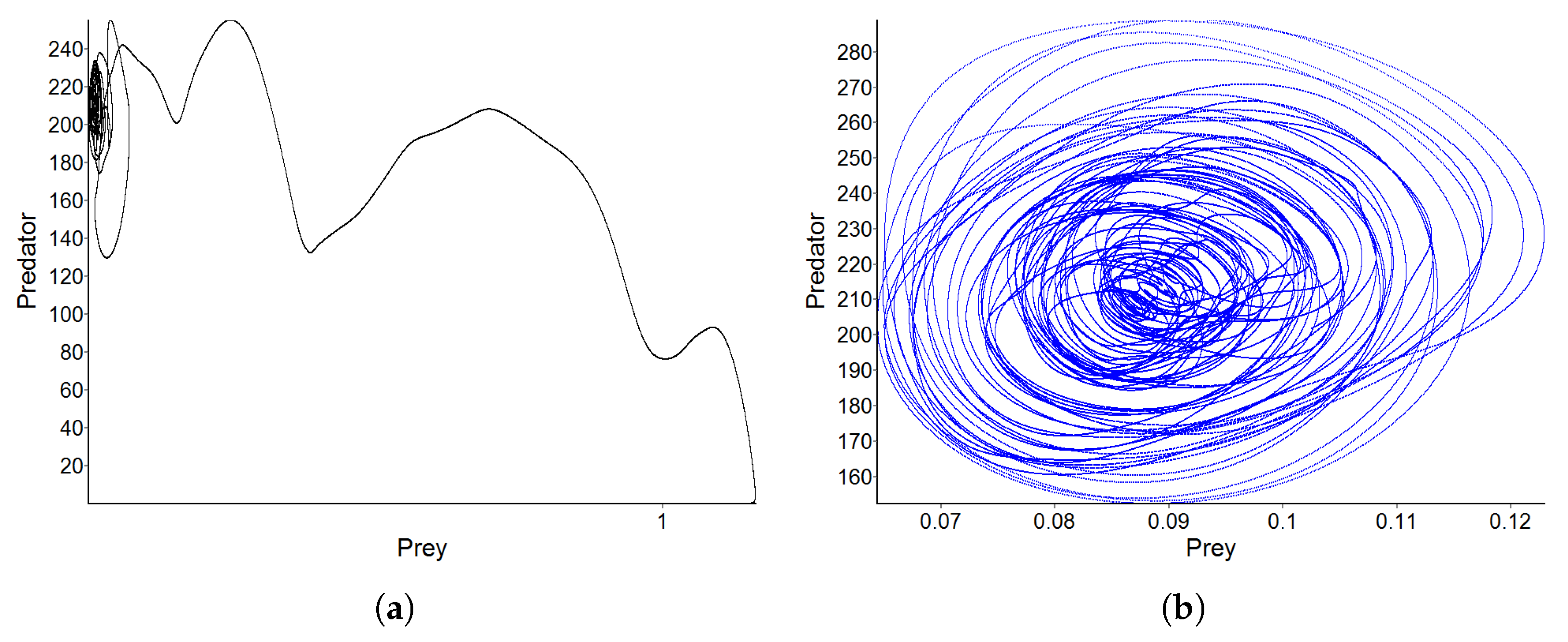
3.5. Effect of Diffusion-Generated Pattern
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RMA | Rosenzweig–MacArthur |
| PDE | Partial Differential Equation |
| ODE | Ordinary Differential Equation |
| SPW | Solitary Population Wave |
References
- Bambach, R.K.; Knoll, A.H.; Sepkoski, J.J. Anatomical and ecological constraints on Phanerozoic animal diversity in the marine realm. Proc. Natl. Acad. Sci. USA 2002, 99, 6854–6859. [Google Scholar] [CrossRef] [PubMed]
- Geritz, S.A.H.; Metz, J.A.J.; Kisdi, E.; Meszena, G. Dynamics of adaptation and evolutionary branching. Phys. Rev. Lett. 1997, 78, 2024–2027. [Google Scholar] [CrossRef] [Green Version]
- Mathias, A.; Kisdi, E.; Olivieri, I. Divergent evolution of dispersal in a heterogeneous landscape. Evolution 2001, 55, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Cosner, C. Reaction-diffusion-advection models for the effects and evolution of dispersal. Discret. Contin. Dyn. Syst. 2014, 34, 1701–1745. [Google Scholar] [CrossRef]
- Baguette, M.; Legrand, D.; Fréville, H.; Van Dyck, H.; Ducatez, S. Evolutionary ecology of dispersal in fragmented landscape. In Dispersal Ecology and Evolution; Clobert, J., Baguette, M., Benton, T.G., Bullock, J.M., Eds.; Oxford Academic: Oxford, UK, 2012; pp. 380–391. [Google Scholar] [CrossRef]
- Cheptou, P.-O.; Hargreaves, A.L.; Bonte, D.; Jacquemyn, H. Adaptation to fragmentation: Evolutionary dynamics driven by human influences. Phil. Trans. R. Soc. B 2017, 372, 20160037. [Google Scholar] [CrossRef] [Green Version]
- Dragicevic, A.; Gurtoo, A. Stochastic control of ecological networks. J. Math. Biol. 2022, 85, 7. [Google Scholar] [CrossRef]
- Lehtonen, J.; Otsuka, J. Evolutionary game theory of continuous traits from a causal perspective. Phil. Trans. R. Soc. 2023, 378, 20210507. [Google Scholar] [CrossRef]
- Arditi, R.; Tyutyunov, Y.; Morgulis, A.; Govorukhin, V.; Senina, I. Directed movement of predators and the emergence of density-dependence in predator-prey models. Theor. Popul. Biol. 2001, 59, 207–221. [Google Scholar] [CrossRef] [Green Version]
- Sapoukhina, N.; Tyutyunov, Y.; Arditi, R. The role of prey-taxis in biological control: A spatial theoretical model. Am. Nat. 2003, 162, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Tyutyunov, Y.V.; Kovalev, O.V.; Titova, L.I. Spatial demogenetic model for studying phenomena observed upon introduction of the ragweed leaf beetle in the south of Russia. Math. Model. Nat. Phenom. 2013, 8, 80–95. [Google Scholar] [CrossRef] [Green Version]
- Tyutyunov, Y.V.; Sen, D.; Titova, L.I.; Banerjee, M. Predator overcomes the Allee effect due to indirect prey–taxis. Ecol. Complex. 2019, 39, 100772. [Google Scholar] [CrossRef]
- Tyutyunov, Y.V.; Zagrebneva, A.D.; Azovsky, A.I. Spatiotemporal pattern formation in a prey-predator system: The case study of short-term interactions between diatom microalgae and microcrustaceans. Mathematics 2020, 8, 1065. [Google Scholar] [CrossRef]
- Ha, D.; Tsybulin, V.; Zelenchuk, P. How to model the local interaction in the predator–prey system at slow diffusion in a heterogeneous environment? Ecol. Complex. 2022, 52, 101026. [Google Scholar] [CrossRef]
- Morgulis, A. Waves in a hyperbolic predator–prey system. Axioms 2022, 11, 187. [Google Scholar] [CrossRef]
- Ahmed, D.A.; Hudgins, E.J.; Cuthbert, R.N.; Kourantidou, M.; Diagne, C.; Haubrock, P.J.; Leung, B.; Liu, C.; Leroy, B.; Petrovskii, S.; et al. Managing biological invasions: The cost of inaction. Biol. Invasions 2022, 24, 1927–1946. [Google Scholar] [CrossRef]
- Sen, D.; Ghorai, S.; Banerjee, M.; Morozov, A. Bifurcation analysis of the predator–prey model with the Allee effect in the predator. J. Math. Biol. 2022, 84, 7. [Google Scholar] [CrossRef]
- Han, R.; Dey, S.; Banerjee, M. Spatio-temporal pattern selection in a prey-predator model with hunting cooperation and Allee effect in prey. Chaos Solitons Fractals 2023, 171, 113441. [Google Scholar] [CrossRef]
- Chowdhury, P.R.; Petrovskii, S.; Volpert, V.; Banerjee, M. Attractors and long transients in a spatio-temporal slow–fast Bazykin’s model. Commun. Nonlinear Sci. Numer. Simul. 2023, 118, 107014. [Google Scholar] [CrossRef]
- Ginzburg, L.R. Theory of Natural Selection and Population Growth; Benjamin/Cummings: Menlo Park, CA, USA, 1983; 160p. [Google Scholar]
- Hastings, A. Population Biology: Concepts and Models; Springer Science & Business Media: New York, NY, USA, 2013; 220p. [Google Scholar]
- Svirezhev, Y.M.; Passekov, V.P. Fundamentals of Mathematical Evolutionary Genetics; Kluwer Acad. Publ.: Dordrecht, The Netherlands, 2011; 412p. [Google Scholar]
- Nonaka, E.; Svanbäck, R.; Thibert-Plante, X.; Englund, G.; Brännström, Å. Mechanisms by which phenotypic plasticity affects adaptive divergence and ecological speciation. Am. Nat. 2015, 186, E126–E143. [Google Scholar] [CrossRef]
- Patel, S.; Schreiber, S.J. Evolutionarily driven shifts in communities with intraguild predation. Am. Nat. 2015, 186, E98–E110. [Google Scholar] [CrossRef] [Green Version]
- Goodsman, D.W.; Cooke, B.; Coltman, D.W.; Lewis, M.A. The genetic signature of rapid range expansions: How dispersal, growth and invasion speed impact heterozygosity and allele surfing. Theor. Popul. Biol. 2014, 98, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nathan, L.R.; Kanno, Y.; Vokoun, J.C. Population demographics influence genetic responses to fragmentation: A demogenetic assessment of the ‘one migrant per generation’ rule of thumb. Biol. Conserv. 2017, 210, 261–272. [Google Scholar] [CrossRef]
- Zhdanova, O.; Frisman, E. Alternative attractors in an ecological-genetic model of populations with non-overlapping generations. Ecol. Conplex. 2017, 31, 135–143. [Google Scholar] [CrossRef]
- Il’ichev, V.G.; Il’icheva, O.A. Spatial adaptation of populations in ecological models. Biophysics 2018, 63, 274–281. [Google Scholar] [CrossRef]
- Kostitzin, V.A. Sur les équations diffèrentielles du problè me de la sélection mendélienne. Comptes rendus de l’Académie des Sciences 1936, 203, 156–157. [Google Scholar]
- Kostitzin, V.A. Biologie Mathématique; Librairie Armand Colin: Paris, France, 1937; 223p. [Google Scholar]
- Kostitzin, V.A. Equations diffèrentielles générales du problème de sélection naturelle. CR Acad. Sci. 1938, 206, 570–572. [Google Scholar]
- Kostitzin, V.A. Sur les coefficients mendeliens d’hérédité. Comptes Rendus De L’acad. Des. Sci. 1938, 206, 883–885. [Google Scholar]
- Tyutyunov, Y.V.; Sapoukhina, N.Y.; Senina, I.N.; Arditi, R. Explicit model for searching behavior of predator. Zhurnal Obs. Biol. 2002, 63, 137–148. (In Russian) [Google Scholar]
- Tyutyunov, Y.V.; Sapoukhina, N.Y.; Senina, I.N.; Arditi, R. Taxis as a factor stabilizing trophic system. In Surveys of Applied and Industrial Mathematics; (Series “Mathematical Methods in Ecology”); Scientific Publisher “TVP”: Moscow, Russia, 2005; Volume 12, pp. 810–814. (In Russian) [Google Scholar]
- Tyutyunov, Y.V.; Titova, L.I.; Senina, I.N. Prey-taxis destabilizes homogeneous stationary state in spatial Gause–Kolmogorov-type model for predator-prey system. Ecol. Complex. 2017, 31, 170–180. [Google Scholar] [CrossRef]
- Rosenzweig, M.L.; MacArthur, R.H. Graphical representation and stability conditions of predator-prey interactions. Am. Nat. 1963, 97, 209–223. [Google Scholar] [CrossRef]
- Allee, W. Animal Aggregations: A Study in General Sociology; Chicago University Press: Chicago, IL, USA, 1931; 431p. [Google Scholar]
- Stephens, P.A.; Sutherland, W.J. Consequences of the Allee effect for behaviour, ecology and conservation. Trends Ecol. Evol. 1999, 14, 401–405. [Google Scholar] [CrossRef]
- Zhou, S.-R.; Liu, Y.-F.; Wang, G. The stability of predator-prey systems subject to the Allee effect. Theor. Popul. Biol. 2005, 67, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Tourniaire, R.; Ferran, A.; Giuge, L.; Piotte, C.; Gambier, J. A natural flightless mutation in the ladybird. Harmon. Axyridis. Entomol. Exp. Appl. 2000, 96, 33–38. [Google Scholar] [CrossRef]
- Facon, B.; Crespin, L.; Loiseau, A.; Lombaert, E.; Magro, A.; Estoup, A. Can things get worse when an invasive species hybridizes? The harlequin ladybird Harmonia axyridis in France as a case study. Evol. Appl. 2011, 4, 71–88. [Google Scholar] [CrossRef]
- Kovalev, O.V. Spread of adventive plants of Ambrosieae tribe in Eurasia and methods of bilogical control of Ambrosia L. (Asteraceae). In Theoretical Principles of Biological Control of the Common Ragweed, Proceedings of the Zoological Institute; Kovalev, O.V., Belokobylsky, S.A., Eds.; “Nauka” Publishing House, Leningrad Branch: Leningrad, Russia, 1989; Volume 189, pp. 7–23. (In Russian) [Google Scholar]
- Kovalev, O.V. The solitary population wave, a physical phenomenon accompanying the introduction of a chrysomelid. In New Developments in the Biology of Chrysomelidae; Jolivet, P., Ed.; SPB Academic Publishing bv: The Hague, The Netherlands, 2004; pp. 91–601. [Google Scholar]
- Kovalev, O.V.; Tyutyunov, Y.V.; Arkhipova, O.E.; Kachalina, N.A.; Iljina, L.P.; Titova, L.I. On assessment of the large-scale effect of introduction of the ragweed leaf beetle Zygogramma suturalis F. (Coleoptera, Chrysomelidae) Phytocenoses South Russia. Entomol. Rev. 2015, 95, 1–14. [Google Scholar] [CrossRef]
- Kovalev, O.V. Microevolutional processes in population of Zygogramma suturalis F. (Coleoptera, Chrisomelidae) introduced from Nort America to the USSR. In Theoretical Principles of Biological Control of the Common Ragweed, Proceedings of the Zoological Institute; Kovalev, O.V., Belokobylsky, S.A., Eds.; “Nauka” Publishing House, Leningrad Branch: Leningrad, Russia, 1989; Volume 189, pp. 139–165. (In Russian) [Google Scholar]
- Hastings, A.; Byers, J.E.; Crooks, J.A.; Cuddington, K.; Jones, C.G.; Lambrinos, J.G.; Talley, T.S.; Wilson, W.G. Ecosystem engineering in space and time. Ecol. Lett. 2007, 10, 153–164. [Google Scholar] [CrossRef]
- Tyutyunov, Y.V.; Titova, L.I.; Arditi, R. A minimal model of pursuit-evasion in a predator–prey system. Math. Model. Nat. Phenom. 2007, 2, 122–738. [Google Scholar] [CrossRef]
- Morgulis, A.; Ilin, K. Indirect taxis on a fluctuating environment. Mathematics 2020, 8, 2052. [Google Scholar] [CrossRef]
- Schuster, F.L.; Levandowsky, M. Chemosensory responses of Acanthamoeba castellanii: Visual Analysis of Random Movement and Responses to Chemical Signals. J. Eukaryot. Microbiol. 1996, 43, 150–158. [Google Scholar] [CrossRef]
- Hoefler, C.D.; Taylor, M.; Jakob, E.M. Chemosensory response to prey in Phidippus audax (Araneae, Salticidae) Pardosa milvina (Araneae, Lycosidae). J. Arachnol. 2002, 30, 155–158. [Google Scholar] [CrossRef]
- Gardiner, J.M.; Atema, J. The function of bilateral odor arrival time differences in olfactory orientation of sharks. Curr. Biol. 2010, 20, 1187–1191. [Google Scholar] [CrossRef] [Green Version]
- Tyutyunov, Y.V.; Titova, L.I. Simple models for studying complex spatiotemporal patterns of animal behavior. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 140, 193–202. [Google Scholar] [CrossRef]
- Senina, I.; Lehodey, P.; Sibert, J.; Hampton, J. Integrating tagging and fisheries data into a spatial population dynamics model to improve its predictive skills. Can. J. Fish. Aquat. Sci. 2019, 77, 576–593. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, M.C.; Wisenden, B.D.; Chivers, D.P. Chemical ecology of predator-prey interactions in aquatic ecosystems: A review and prospectus. Canad. J. Zool. 2010, 33, 698–724. [Google Scholar] [CrossRef]
- Travis, J.M.J.; Dytham, C. Habitat persistence, habitat availability and the evolution of dispersal. Proc. R. Soc. B Biol. Sci. 1999, 266, 723–728. [Google Scholar] [CrossRef]
- Petrovskii, S.V.; Malchow, H. A minimal model of pattern formation in a prey-predator system. Math. Comput. Model. 1999, 29, 49–63. [Google Scholar] [CrossRef]
- Petrovskii, S.V.; Malchow, H. Wave of chaos: New mechanism of pattern formation in spatio-temporal population dynamics. Theor. Popul. Biol. 2001, 59, 157–174. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, M. Turing and non-Turing patterns in two-dimensional prey-predator models. In Applications of Chaos and Nonlinear Dynamics in Science and Engineering—Volume 4. Understanding Complex Systems; Banerjee, S., Rondoni, L., Eds.; Springer: Cham, Switzerland, 2015; pp. 257–280. [Google Scholar] [CrossRef]
- Mukherjee, N.; Ghorai, S.; Banerjee, M. Cross-diffusion induced Turing and non-Turing patterns in Rosenzweig–MacArthur model. Lett. Biomath. 2019, 6, 1–22. [Google Scholar] [CrossRef]
- Tyutyunov, Y.V.; Zagrebneva, A.D.; Surkov, F.A.; Azovsky, A.I. Derivation of density flux equation for intermittently migrating population. Oceanology 2010, 50, 67–76. [Google Scholar] [CrossRef]
- Tyutyunov, Y.; Titova, L.; Arditi, R. Predator interference emerging from trophotaxis in predator-prey systems: An individual-based approach. Ecol. Complex. 2008, 5, 48–58. [Google Scholar] [CrossRef]
- Arditi, R.; Ginzburg, L. How Species Interact. Altering the Standard View on Trophic Ecology; Oxford University Press: Oxford, UK; New York, NY, USA, 2012; 170p. [Google Scholar]
- Tyutyunov, Y.V.; Titova, L.I.; Berdnikov, S.V. A mechanistic model for interference and Allee effect in the predator population. Biophysics 2013, 58, 258–264. [Google Scholar] [CrossRef]
- Tyutyunov, Y.V.; Titova, L.I. Ratio-dependence in predator-prey systems as an edge and basic minimal model of predator interference. Front. Ecol. Evol. 2021, 9, 725041. [Google Scholar] [CrossRef]
- Arditi, R.; Ginzburg, L.R. Coupling in predator-prey dynamics: Ratio-dependence. J. Theor. Biol. 1989, 139, 311–326. [Google Scholar] [CrossRef]
- Rosenzweig, M.L. Paradox of enrichment: Destabilization of exploitation ecosystem in ecological time. Science 1971, 171, 385–387. [Google Scholar] [CrossRef]
- Luck, R.F. Evaluation of natural enemies for biological control: A behavioral approach. Trends Ecol. Evol. 1990, 5, 196–199. [Google Scholar] [CrossRef]
- Arditi, R.; Berryman, A.A. The biological control paradox. Trends Ecol. Evol. 1991, 6, 32. [Google Scholar] [CrossRef]
- Berryman, A. The theoretical foundations of biological control. In Theoretical Approaches to Biological Control; Hawkins, B., Cornell, H.V., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 3–21. [Google Scholar]
- Borrelli, J.J.; Allesina, S.; Amarasekare, P.; Arditi, R.; Chase, I.; Damuth, J.; Holt, R.D.; Logofet, D.O.; Novak, M.; Rohr, R.P.; et al. Selection on stability across ecological scales. Trends Ecol. Evol. 2015, 30, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Edmonds, C.A.; Lillie, A.S.; Cavalli-Sforza, L.L. Mutations arising in the wave front of an expanding population. Proc. Natl. Acad. Sci. USA 2004, 101, 975–979. [Google Scholar] [CrossRef]
- Hallatschek, O.; Nelson, D.R. Gene surfing in expanding populations. Theor. Popul. Biol. 2008, 73, 158–170. [Google Scholar] [CrossRef] [Green Version]
- Lehe, R.; Hallatschek, O.; Peliti, L. The rate of beneficial mutations surfing on the wave of a range expansion. PLoS Comput. Biol. 2012, 8, e1002447. [Google Scholar] [CrossRef] [Green Version]
- Roques, L.; Garnier, J.; Hamel, F.; Klein, E.K. Allee effect promotes diversity in traveling waves of colonization. Proc. Natl. Acad. Sci. USA 2012, 109, 8828–8833. [Google Scholar] [CrossRef] [PubMed]
- Hunt, G.; Rabosky, D.L. Phenotypic evolution in fossil species: Pattern and process. Annu. Rev. Earth Planet Sci. 2014, 42, 421–441. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyutyunov, Y.V. Spatial Demo-Genetic Predator–Prey Model for Studying Natural Selection of Traits Enhancing Consumer Motility. Mathematics 2023, 11, 3378. https://doi.org/10.3390/math11153378
Tyutyunov YV. Spatial Demo-Genetic Predator–Prey Model for Studying Natural Selection of Traits Enhancing Consumer Motility. Mathematics. 2023; 11(15):3378. https://doi.org/10.3390/math11153378
Chicago/Turabian StyleTyutyunov, Yuri V. 2023. "Spatial Demo-Genetic Predator–Prey Model for Studying Natural Selection of Traits Enhancing Consumer Motility" Mathematics 11, no. 15: 3378. https://doi.org/10.3390/math11153378
APA StyleTyutyunov, Y. V. (2023). Spatial Demo-Genetic Predator–Prey Model for Studying Natural Selection of Traits Enhancing Consumer Motility. Mathematics, 11(15), 3378. https://doi.org/10.3390/math11153378





