A Deep Multi-Task Learning Approach for Bioelectrical Signal Analysis
Abstract
1. Introduction
- Based on the purpose and motivation behind data analysis tasks, we define two different scenarios for utilizing multi-task learning in analyzing bioelectrical signals, the consistent source-target scenario and the inconsistent source-target scenario, which is proved to enhance the transferability of the proposed schemes.
- For each scenario, we propose a method to decompose the analysis task into several subtasks and convert the original dataset to adapt to the multi-task learning. We also design the generic parameter-sharing neural networks for each scenario and illustrate the details of implementing different basic neural layers, like convolutional layers and recurrent layers.
- We conduct extensive experiments on four electrocardiograms databases. The experiment’s results demonstrate that the proposed systems can improve the analysis performance and enable the predictive models to be transferable.
2. Preliminaries
2.1. Deep Learning
2.2. Multi-Task Learning
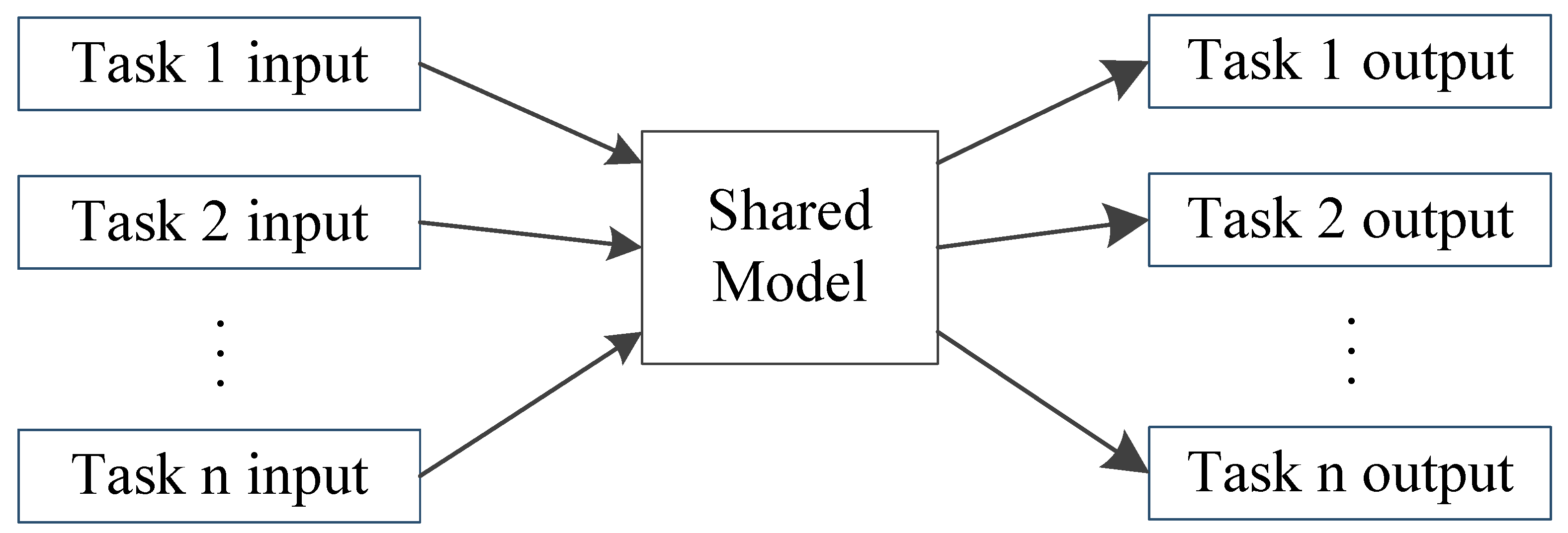
3. Deep Multi-Task Learning for Bioelectrical Signal Analysis
4. Deep Multi-Task Bioelectrical Analysis in Consistent Source-Target Scenario
4.1. Multiple Tasks and Related Datasets
4.2. Deep Parameter-Sharing Neural Networks for Consistent Source-Target Scenario
4.2.1. Parameter-Sharing Convolutional Neural Networks
4.2.2. Parameter-Sharing Recurrent Neural Networks
5. Deep Multi-Task Bioelectrical Analysis in Inconsistent Source-Target Scenario
5.1. Multiple Tasks and Related Datasets
5.2. Deep Parameter-Sharing Neural Networks in Inconsistent Source-Target Scenario
5.2.1. Parameter-Sharing Convolution Neural Networks
5.2.2. Parameter-Sharing Recurrent Neural Networks
6. Experiments on ECG Signal Analysis
- Ventricular (V): Such arrhythmias start in the heart’s lower chambers, which can be very dangerous and usually require medical care right away. There are two types of such arrhythmias: premature ventricular contraction (PVC) and ventricular escape (VE).
- Fusion (F): Continuation of fusion will lead to stroke and heart failure. There is only one type of arrhythmias: fusion of ventricular and normal (fVN).
- Supraventricular (S): It refers to the arrhythmias that need to be noticed, but not necessarily needs to be sent to the hospital immediately. There are four types: atrial premature (AP) that almost of people have experienced, aberrated atrial premature (aAP), nodal (NP), and supraventricular premature (SP).
- Normal heartbeat (N): It includes normal heart beats that was wrongly detected by the previous arrhythmia detection algorithm (NOR), and some normal arrhythmias: left or right bundle branch block (LBBB/RBBB), atrial escape (AE) and nodal (junctional) escape (NE).
6.1. Datasets Description
6.1.1. The MIT-BIH Arrhythmia Database (mitdb)
6.1.2. The MIT-BIH Long Term Database (ltdb)
6.1.3. The MIT-BIH Supraventricular Arrhythmia Database (svdb)
6.1.4. St.-Petersburg Institute of Cardiological Technics 12-Lead Arrhythmia Database (incartdb)
6.2. Data Preprocessing
7. Results
7.1. Experiments on Consistent Source-Target Scenario
7.1.1. Person-Wise MTL
7.1.2. Database-Wise MTL
7.2. Experiments on Inconsistent Source-Target Scenario
7.2.1. Person-Wise MTL
7.2.2. Database-Wise MTL
8. Discussion
8.1. Consistent Source-Target Scenario
8.2. Inconsistent Source-Target Scenario
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Theis, F.J.; Meyer-Bäse, A. Biomedical Signal Analysis: Contemporary Methods and Applications; MIT Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Choi, B.J.; Kim, J.H.; Yang, W.J.; Han, D.J.; Park, J.; Park, D.W. Parylene-based flexible microelectrode arrays for the electrical recording of muscles and the effect of electrode size. Appl. Sci. 2020, 10, 7364. [Google Scholar] [CrossRef]
- Aoyama, T.; Kohno, Y. Temporal and quantitative variability in muscle electrical activity decreases as dexterous hand motor skills are learned. PLoS ONE 2020, 15, e0236254. [Google Scholar] [CrossRef] [PubMed]
- Behadada, O.; Chikh, M.A. An interpretable classifier for detection of cardiac arrhythmias by using the fuzzy decision tree. Artif. Intell. Res. 2013, 2, 45. [Google Scholar] [CrossRef][Green Version]
- Guler, I.; Ubeyli, E.D. Multiclass support vector machines for EEG-signals classification. IEEE Trans. Inf. Technol. Biomed. 2007, 11, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Frénay, B.; De Lannoy, G.; Verleysen, M. Improving the transition modelling in hidden Markov models for ECG segmentation. In Proceedings of the ESANN, Bruges, Belgium, 22–24 April 2009. [Google Scholar]
- Chen, X.; Ji, J.; Loparo, K.; Li, P. Real-time personalized cardiac arrhythmia detection and diagnosis: A cloud computing architecture. In Proceedings of the 2017 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Orlando, FL, USA, 16–19 February 2017; pp. 201–204. [Google Scholar]
- Sze, V.; Chen, Y.H.; Yang, T.J.; Emer, J. Efficient processing of deep neural networks: A tutorial and survey. arXiv 2017, arXiv:1703.09039. [Google Scholar] [CrossRef]
- Lipton, Z.C.; Kale, D.C.; Elkan, C.; Wetzell, R. Learning to diagnose with LSTM recurrent neural networks. arXiv 2015, arXiv:1511.03677. [Google Scholar]
- Rajpurkar, P.; Hannun, A.Y.; Haghpanahi, M.; Bourn, C.; Ng, A.Y. Cardiologist-level arrhythmia detection with convolutional neural networks. arXiv 2017, arXiv:1707.01836. [Google Scholar]
- Girshick, R.; Donahue, J.; Darrell, T.; Malik, J. Rich feature hierarchies for accurate object detection and semantic segmentation. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Columbus, OH, USA, 23–28 June 2014; pp. 580–587. [Google Scholar]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Chen, J.; Samuel, R.D.J.; Poovendran, P. LSTM with bio inspired algorithm for action recognition in sports videos. Image Vis. Comput. 2021, 112, 104214. [Google Scholar] [CrossRef]
- Shoeb, A.H.; Guttag, J.V. Application of machine learning to epileptic seizure detection. In Proceedings of the 27th International Conference on Machine Learning (ICML-10), Haifa, Israel, 21–24 June 2010; pp. 975–982. [Google Scholar]
- Jambukia, S.H.; Dabhi, V.K.; Prajapati, H.B. Classification of ECG signals using machine learning techniques: A survey. In Proceedings of the 2015 International Conference on Advances in Computer Engineering and Applications, Ghaziabad, India, 19–20 March 2015; pp. 714–721. [Google Scholar]
- Woodland, P.C. Speaker adaptation for continuous density HMMs: A review. In ISCA Tutorial and Research Workshop (ITRW) on Adaptation Methods for Speech Recognition; ISCA: Sophia-Antipolis, France, 2001. [Google Scholar]
- Li, X.; Bilmes, J. Regularized adaptation of discriminative classifiers. In Proceedings of the 2006 IEEE International Conference on Acoustics Speech and Signal Processing Proceedings, Toulouse, France, 14–19 May 2006; Volume 1, p. I. [Google Scholar]
- Raina, R.; Battle, A.; Lee, H.; Packer, B.; Ng, A.Y. Self-taught learning: Transfer learning from unlabeled data. In Proceedings of the 24th International Conference on Machine Learning, Corvalis, OR, USA, 20–24 June 2007; pp. 759–766. [Google Scholar]
- Yang, J.; Yan, R.; Hauptmann, A.G. Cross-domain video concept detection using adaptive svms. In Proceedings of the 15th ACM International Conference on Multimedia, Augsburg, Germany, 25–29 September 2007; pp. 188–197. [Google Scholar]
- Blitzer, J.; McDonald, R.; Pereira, F. Domain adaptation with structural correspondence learning. In Proceedings of the 2006 Conference on Empirical Methods in Natural Language Processing, Sydney, Australia, 22–23 July 2006; pp. 120–128. [Google Scholar]
- Dai, W.; Yang, Q.; Xue, G.R.; Yu, Y. Boosting for transfer learning. In Proceedings of the 24th International Conference on Machine Learning, Corvalis, OR, USA, 20–24 June 2007; pp. 193–200. [Google Scholar]
- Ruder, S. An overview of multi-task learning in deep neural networks. arXiv 2017, arXiv:1706.05098. [Google Scholar]
- Lin, S.; Shi, C.; Chen, J. GeneralizedDTA: Combining pre-training and multi-task learning to predict drug-target binding affinity for unknown drug discovery. BMC Bioinform. 2022, 23, 367. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Chen, X.; Luo, C.; Li, P. A deep multi-task learning approach for ECG data analysis. In Proceedings of the 2018 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Las Vegas, NV, USA, 4–7 March 2018; pp. 124–127. [Google Scholar]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Zhong, G.; Yu, H. A review on the attention mechanism of deep learning. Neurocomputing 2021, 452, 48–62. [Google Scholar] [CrossRef]
- Minsky, M.; Papert, S.A.; Bottou, L. Perceptrons: An Introduction to Computational Geometry; MIT Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Rafiq, M.; Bugmann, G.; Easterbrook, D. Neural network design for engineering applications. Comput. Struct. 2001, 79, 1541–1552. [Google Scholar] [CrossRef]
- Liu, S.; Borovykh, A.; Grzelak, L.A.; Oosterlee, C.W. A neural network-based framework for financial model calibration. J. Math. Ind. 2019, 9, 9. [Google Scholar] [CrossRef]
- Hinton, G.E.; Osindero, S.; Teh, Y.W. A fast learning algorithm for deep belief nets. Neural Comput. 2006, 18, 1527–1554. [Google Scholar] [CrossRef]
- Bengio, Y. Learning deep architectures for AI. Found. Trends Mach. Learn. 2009, 2, 1–127. [Google Scholar] [CrossRef]
- Bengio, Y.; Courville, A.; Vincent, P. Representation learning: A review and new perspectives. IEEE Trans. Pattern Anal. Mach. Intell. 2013, 35, 1798–1828. [Google Scholar] [CrossRef]
- Socher, R.; Bengio, Y.; Manning, C.D. Deep learning for NLP (without magic). In Proceedings of the 50th Annual Meeting of the Association for Computational Linguistics: Tutorial Abstracts, Jeju Island, Republic of Korea, 8–14 July 2012; p. 5. [Google Scholar]
- Hu, B.; Lu, Z.; Li, H.; Chen, Q. Convolutional neural network architectures for matching natural language sentences. arXiv 2014, arXiv:1503.03244. [Google Scholar] [CrossRef]
- Medsker, L.; Jain, L. Recurrent neural networks. Des. Appl. 2001, 5, 2. [Google Scholar]
- Hammad, M.; Abd El-Latif, A.A.; Hussain, A.; Abd El-Samie, F.E.; Gupta, B.B.; Ugail, H.; Sedik, A. Deep learning models for arrhythmia detection in IoT healthcare applications. Comput. Electr. Eng. 2022, 100, 108011. [Google Scholar] [CrossRef]
- Hochreiter, S.; Schmidhuber, J. Long short-term memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Van Merriënboer, B.; Gulcehre, C.; Bahdanau, D.; Bougares, F.; Schwenk, H.; Bengio, Y. Learning phrase representations using RNN encoder-decoder for statistical machine translation. arXiv 2014, arXiv:1406.1078. [Google Scholar]
- Hasan, N.I.; Bhattacharjee, A. Deep learning approach to cardiovascular disease classification employing modified ECG signal from empirical mode decomposition. Biomed. Signal Process. Control 2019, 52, 128–140. [Google Scholar] [CrossRef]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef]
- Hu, R.; Chen, J.; Zhou, L. A transformer-based deep neural network for arrhythmia detection using continuous ECG signals. Comput. Biol. Med. 2022, 144, 105325. [Google Scholar] [CrossRef]
- Shahin, M.; Oo, E.; Ahmed, B. Adversarial Multi-Task Learning for Robust End-to-End ECG-based Heartbeat Classification. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 341–344. [Google Scholar]
- Mormont, R.; Geurts, P.; Marée, R. Multi-task pre-training of deep neural networks for digital pathology. IEEE J. Biomed. Health Inform. 2020, 25, 412–421. [Google Scholar] [CrossRef]
- Misra, I.; Shrivastava, A.; Gupta, A.; Hebert, M. Cross-stitch networks for multi-task learning. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 3994–4003. [Google Scholar]
- Zhang, Z.; Luo, P.; Loy, C.C.; Tang, X. Facial landmark detection by deep multi-task learning. In Proceedings of the European Conference on Computer Vision, Zurich, Switzerland, 6–12 September 2014; pp. 94–108. [Google Scholar]
- Collobert, R.; Weston, J. A unified architecture for natural language processing: Deep neural networks with multitask learning. In Proceedings of the 25th International Conference on Machine Learning, Helsinki, Finland, 5–9 July 2008; pp. 160–167. [Google Scholar]
- Luong, M.T.; Le, Q.V.; Sutskever, I.; Vinyals, O.; Kaiser, L. Multi-task sequence to sequence learning. arXiv 2015, arXiv:1511.06114. [Google Scholar]
- Liu, P.; Qiu, X.; Huang, X. Adversarial Multi-task Learning for Text Classification. arXiv 2017, arXiv:1704.05742. [Google Scholar]
- Liu, P.; Qiu, X.; Huang, X. Recurrent neural network for text classification with multi-task learning. arXiv 2016, arXiv:1605.05101. [Google Scholar]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. Physiobank, physiotoolkit, and physionet. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef] [PubMed]
- Cantzos, D.; Dimogianopoulos, D.; Tseles, D. ECG diagnosis via a sequential recursive time series—Wavelet classification scheme. In Proceedings of the IEEE EUROCON, Zagreb, Croatia, 1–4 July 2013. [Google Scholar]
- Bhanot, K.; Peddoju, S.K.; Bhardwaj, T. A model to find optimal percentage of training and testing data for efficient ECG analysis using neural network. Int. J. Syst. Assur. Eng. Manag. 2018, 9, 12–17. [Google Scholar] [CrossRef]







| Records | Deep NNs | STL | MTL |
|---|---|---|---|
| 1D CNN | |||
| RNN | |||
| 14,046 | LSTM | ||
| GRU | |||
| avg. | |||
| 1D CNN | |||
| RNN | |||
| 14,134 | LSTM | ||
| GRU | |||
| avg. |
| Databases | Deep NNs | STL | MTL |
|---|---|---|---|
| 1D CNN | |||
| RNN | |||
| mitdb | LSTM | ||
| GRU | |||
| avg. | |||
| 1D CNN | |||
| RNN | |||
| ltdb | LSTM | ||
| GRU | |||
| avg. | |||
| 1D CNN | |||
| RNN | |||
| svdb | LSTM | ||
| GRU | |||
| avg. | |||
| 1D CNN | |||
| RNN | |||
| incartdb | LSTM | ||
| GRU | |||
| avg. |
| Source Tasks | Target Task | Deep NNs | STL | MTL |
|---|---|---|---|---|
| 1D CNN | ||||
| RNN | ||||
| 106, 116, 210 | 223 | LSTM | ||
| GRU | ||||
| avg. | ||||
| 1D CNN | ||||
| RNN | ||||
| 116, 210, 223 | 106 | LSTM | ||
| GRU | ||||
| avg. | ||||
| 1D CNN | ||||
| RNN | ||||
| 106, 210, 223 | 116 | LSTM | ||
| GRU | ||||
| avg. | ||||
| 1D CNN | ||||
| RNN | ||||
| 106, 116, 223 | 210 | LSTM | ||
| GRU | ||||
| avg. |
| Source Tasks | Target Task | Deep NNs | STL | MTL |
|---|---|---|---|---|
| 1D CNN | ||||
| RNN | ||||
| ltdb, svdb, | mitdb | LSTM | ||
| incartdb | GRU | |||
| avg. | ||||
| 1D CNN | ||||
| RNN | ||||
| mitdb, svdb, | ltdb | LSTM | ||
| incartdb | GRU | |||
| avg. | ||||
| 1D CNN | ||||
| RNN | ||||
| mitdb, ltdb, | svdb | LSTM | ||
| incartdb | GRU | |||
| avg. | ||||
| 1D CNN | ||||
| RNN | ||||
| mitdb, ltdb, | incartdb | LSTM | ||
| svdb | GRU | |||
| avg. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medhi, J.K.; Ren, P.; Hu, M.; Chen, X. A Deep Multi-Task Learning Approach for Bioelectrical Signal Analysis. Mathematics 2023, 11, 4566. https://doi.org/10.3390/math11224566
Medhi JK, Ren P, Hu M, Chen X. A Deep Multi-Task Learning Approach for Bioelectrical Signal Analysis. Mathematics. 2023; 11(22):4566. https://doi.org/10.3390/math11224566
Chicago/Turabian StyleMedhi, Jishu K., Pusheng Ren, Mengsha Hu, and Xuhui Chen. 2023. "A Deep Multi-Task Learning Approach for Bioelectrical Signal Analysis" Mathematics 11, no. 22: 4566. https://doi.org/10.3390/math11224566
APA StyleMedhi, J. K., Ren, P., Hu, M., & Chen, X. (2023). A Deep Multi-Task Learning Approach for Bioelectrical Signal Analysis. Mathematics, 11(22), 4566. https://doi.org/10.3390/math11224566





